Biological evaluation of imidazolium- and ammonium-based salts as HIV-1 integrase inhibitors
Kasthuraiah
Maddali†
a,
Vineet
Kumar†
b,
Christophe
Marchand
a,
Yves
Pommier
*a and
Sanjay V.
Malhotra
*b
aLaboratory of molecular pharmacology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892, USA. E-mail: pommier@nih.gov; Tel: +1-301-496-5944
bLaboratory of Synthetic Chemistry, SAIC Frederick Inc., National Cancer Institute at Frederick, 1050 Boyles St., Frederick, MD 21702, USA. E-mail: malhotrasa@mail.nih.gov; Tel: +1-301-846-5141
First published on 20th December 2010
Abstract
Ammonium- and imidazolium-based ionic salts were studied for their application as inhibitors of HIV-1 integrase (IN). These compounds were active in the inhibition of both the 3′-processing (3′-P) and strand transfer (ST) steps of the integration reaction. A correlation of activity with chain length of the alkyl substituent in both classes of ionic salts was observed.
Introduction
The replication of human immunodeficiency virus type 1 (HIV-1a), which is responsible for AIDS is prevented by four classes of chemotherapeutic agents i.e.reverse transcriptase inhibitors (RTI), protease inhibitors (PI), integrase inhibitors (INI) and fusion inhibitors. The widespread combination therapy including RTI and PI is often referred as HAART (highly active antiretroviral therapy), which effectively inhibits HIV replication to such an extent that the virus becomes imperceptible in the blood.1–3 However, this therapy fails to eliminate viruses that are already integrated in the host genome or that persist in cellular and anatomical “reservoirs”. Furthermore, prolonged drug exposure during treatment selects for drug resistance, which reduces therapeutically available options for the patients.4 Due to these considerations and the toxicity of a number of antiretroviral agents, the discovery for additional targets has been accelerated for HIV drugs development. Among them, HIV integrase (IN) has been studied intensely over the past 15 years.5–7 IN has recently been fully validated as a therapeutic target with the first FDA approved IN inhibitor raltegravir.8 As HIV integrase is critical for HIV replication, it remains a key viral target for the design and discovery of new anti-HIV agents.IN is encoded at the 3′-end of the HIV polgene and is a relatively small (32 kDa) viral protein. It catalyzes the incorporation of HIV DNA into host chromosomal DNA (cDNA).6,9–13 The process begins in cytoplasm, where the viral cDNA (produced by reverse transcription) is assembled with IN, followed by the specific endonucleolytic cleavage of two nucleotides from each 3′-end of double-stranded viral DNA. This step produces recessed viral DNA ends and is referred as 3′-processing (3′-P). The next step occurs in the nucleus and is identified as strand transfer (ST), which corresponds to the ligation of the viral 3′-OH DNA ends (generated by 3′-processing) to the 5′-phosphate of an acceptor DNA (physiologically a host chromosome). The ST step, occurring in the nucleus, is partitioned from the 3′-processing step in the cytoplasm. Finally, the integration process is completed after gap repair of the viral and host DNA functions. Although IN has been regarded as a potentially attractive target for anti-HIV drug development for over 15 years, the discovery of new clinically applicable inhibitors has been challenging.6,14,15 A range of natural and synthetic compounds have been identified as inhibitors of recombinant IN enzyme in biochemical assays. Polyhydroxylated aromatics and diketo-compounds were among the first inhibitors identified.5,16–18 However, those early polyhydroxylated derivatives were later demonstrated to inhibit viral entry or to be too toxic to be pursued as therapeutic IN inhibitors.19 Later, many structurally diverse compounds have been reported,6,20,21–31 but so far only the β-diketo acids27,28,32,33 and pyrimidones34–37 and their related compounds represent the most selective and biologically-validated inhibitors of IN.
Surfactants like quaternary ammonium salts are well known for their microcidal and spermicidal activities, e.g.benzalkonium chloride is a popular microbicide used in European and Canadian spermicidal products. Also it has shown potential as an anti-HIV agent and could act as a topical agent in the protection of cynomolgus macaques against cervicovaginal transmission of simian immunodeficiency virus (SIV).38–40 Hydroxylated quaternary ammonium surfactants have been studied for their potential spermicidal and anti-HIV activities.41,42 Recently, long chain quaternary ammonium cationic surfactant have been shown to activate the catalytic switch of SRP RNA.43 Those studies motivated us to explore the potential anti-HIV activity of a new class of compounds, i.e. ionic liquids. Ionic liquids have grown rapidly as tunable materials with unique properties and limitless applications in a wide variety of disciplines, making ionic liquid as a new and broad area of research.44–50 There has been a phenomenal growth in the past two decades, resulting in few thousands of publications on ionic liquids covering different research fields including synthesis, materials, and specialty chemicals. However, a limited number of studies have been devoted to their toxicity and safety.51–55 There are well known examples where pharmaceutically active cations and anions combine together and the resulting salt exhibits therapeutic effects of both of its components.56 Therefore, it is logical to think that ionic liquids could be designed as promising anti-cancer, anti-viral and other therapeutic agents. These “Therapeutic Ionic Liquids” potentially offer different properties. If therapeutic response is seen then the major advantage of ionic liquids would be in managing/tuning their toxicity while tailoring the physico-chemical and pharmacological properties necessary for desired therapeutic application. Recently, we have explored the potential of anti-cancer activities of ionic liquids on National Cancer Institute's 60 human tumor cell lines.57 In this report; we present the studies of inhibitory effect of some representative examples of ammonium- and imidazolium-based ionic liquids on HIV-integrase using recombinant HIV-1 integrase and activity gel based assays. This is the first report where the ionic liquids have been explored as HIV-1 integrase inhibitors.
Results and discussion
We first took representative examples of different classes of ionic liquids (ILs) (1–7, Table 1) available commercially and screened them for 3′-P and ST inhibition using gel based assays. The IC50 values were calculated using dose response curves and are given in Table 1. The phosphonium, pyrrolidinium, pyridinium and guanidinium ILs (4, 5, 6 and 7, respectively) were found to be inactive (entry 4–7, Table 1) for both 3′-P and ST inhibition. Ammonium-based IL 1 was found inactive for 3′-P inhibition; however it was active for ST inhibition with an IC50 value of 6.6 μM (entry 1, Table 1). Interestingly, the imidazolium-based IL with C-8 alkyl chain substitution (2) was inactive for both 3′-P and ST, while C-18 alkyl chain substitution (3) produced high activity for inhibition of both 3′-P and ST with IC50 1.6 and 1.5 μM, respectively (entry 2 and 3, Table 1).| Entry | Compd# | Structure | IC50/μM | |
|---|---|---|---|---|
| 3′-P | ST | |||
| 1 | 1 |

|
>100 | 6.6 |
| 2 | 2 |
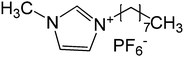
|
>100 | >100 |
| 3 | 3 |

|
1.6 ± 0.15 | 1.53 ± 0.22 |
| 4 | 4 |

|
>100 | >100 |
| 5 | 5 |

|
>100 | >100 |
| 6 | 6 |

|
>100 | >100 |
| 7 | 7 |

|
>100 | >100 |
We next tested imidazolium-based ILs having alkyl substitutions of different chain lengths for 3′-P and ST inhibition. The ILs having short alkyl chain substitution of C-4, C-6 and C-10 were found to be inactive irrespective of the counter anions (entry 1–4, Table 2). The efforts of introducing ether functionality in the shorter alkyl chain did not result in any improvement in the bioactivity (entry 11, Table 2). However, as the chain length of the alkyl substitution increased to C-12 through C-18, the bioactivity increased towards both 3′-P and ST inhibition as can be seen from their IC50 values (entry 3, Table 1 and entry 5–9, Table 2). The ILs 14 (with C-16 alkyl chain length), 15, 16 and 3 (with C-18 alkyl chain length) showed the best results with complete inhibition and least IC50 values for both 3′-P and ST which can be seen by their phosphorimager images and concentration-response curves (Fig. 1). Fig. 2 shows the effect of alkyl chain length of imidazolium ILs on the IN activity by comparing the dose response curves of 3′-P and ST inhibition. The results indicate the IN inhibitory activity of imidazolium ILs is directly proportional to the length of the alkyl chain. As mentioned earlier, ILs with alkyl chain length from C-4 to C-10 do not show any significant inhibition, both for 3′-P and ST, even at higher concentration (>333 μM). When the alkyl chain length was increased to C-12 (12) and C-14 (13), inhibition was observed for 3′-P and ST, which can also be seen from their IC50 values (entry 5 and 6, Table 2). Further increase in chain length to C-16 (14) and C-18 (15) caused even higher bioactivity shown by lower IC50 values and complete inhibition for both 3′-P and ST reactions. Surprisingly, IL 17 (with C-18 alkyl substitution and (C2F5)3F3P counter anion) did not show any activity for 3′-P inhibition; however it showed moderate ST inhibition with IC50 value of 42 μM. This suggests that the combination of proper cation and anion is very crucial for their biological activities.
| Entry | Compd# |
|
IC50/μM | ||
|---|---|---|---|---|---|
| n | X | 3′-P | ST | ||
| 1 | 8 | 3 | (CF3SO2)2N | >333 | >333 |
| 2 | 9 | 5 | (CF3SO2)2N | >333 | 250 |
| 3 | 10 | 9 | Cl | >333 | >333 |
| 4 | 11 | 9 | BF4 | >333 | >333 |
| 5 | 12 | 11 | Cl | 72.0 ± 3.5 | 36.6 ± 4.5 |
| 6 | 13 | 13 | Cl | 55.2 ± 3.0 | 20.2 ± 1.6 |
| 7 | 14 | 15 | Cl | 2.5 ± 0.3 | 1.3 ± 0.2 |
| 8 | 15 | 17 | Cl | 1.7 ± 0.2 | 1.1 ± 0.1 |
| 9 | 16 | 17 | PF6 | 1.7 ± 0.2 | 1.4 ± 0.2 |
| 10 | 17 | 17 | (C2F5)3F3P | >333 | 42 ± 4 |
| 11 | 18 |
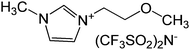
|
>333 | >333 | |
| 12 | 19 |
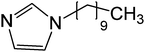
|
>333 | >333 | |
| 13 | 20 |

|
>333 | >333 | |
 | ||
| Fig. 1 Comparison of HIV-1 integrase inhibition by compounds 14, 15, 16 and 3 in a gel-based assay. (a) Phosphorimager image showing a representative experiment. 21mer, 19mer, and STP correspond to the DNA substrate, the 3′-P product, and the ST products, respectively. (b) Concentration response curves for HIV-1 integrase inhibition for ST and 3′-P (derived from densitometric analysis of the gels presented in part a). | ||
 | ||
| Fig. 2 Effect of alkyl chain length of imidazolium ILs on ST and 3′-P inhibition shown by comparison of their concentration response curves (no. of carbons of alkyl chain are given in parenthesis on the legends). | ||
Similar results were obtained when we studied the effect of chain length of alkyl substitution on the IN inhibition activity of ammonium-based ILs. The results for 3′-P and ST inhibition of ammonium ILs with different alkyl chain lengths are summarized in Table 3. The ammonium ILs with small alkyl chains up to C-4i.e.21 and 23–26 were completely inactive for both 3′-P and ST inhibition (entry 1 and 3-6, Table 3). On the other hand, the ILs with C-8 alkyl chains i.e.27 and 28 were the most active ammonium ILs as shown by lowest 3′-P and ST IC50 values (entry 7 and 8, Table 3). Complete inhibition for both reactions was observed (see phosphorimager images and concentration response curves in Fig. 3). Surprisingly, the tetramethyl ammonium IL 22 [with C-1 alkyl substitution and (C2F5)3F3P counter anion] showed limited IN inhibition with IC50 values 138 and 38.5 μM for 3′-P and ST inhibition, respectively.
| Entry | Compd# | Structure | IC50/μM | |
|---|---|---|---|---|
| 3′-P | ST | |||
| 1 | 21 |
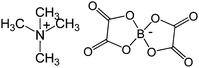
|
>333 | 310 |
| 2 | 22 |

|
138 ± 10 | 38.5 ± 2.7 |
| 3 | 23 |

|
>333 | >333 |
| 4 | 24 |

|
>333 | >333 |
| 5 | 25 |

|
>333 | >333 |
| 6 | 26 |

|
>333 | 307 |
| 7 | 27 |

|
6.80 ± 1.1 | 2.80 ± 0.55 |
| 8 | 28 |
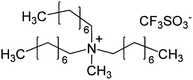
|
9.0 ± 1.0 | 6.12 ± 1.2 |
| 9 | 29 |
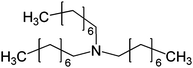
|
>333 | >333 |
| 10 | 30 |

|
>333 | >333 |
 | ||
| Fig. 3 Comparison of HIV-1 integrase inhibition by IL 27 and 28, (a) Phosphorimager image showing a representative experiment. 21mer, 19mer, and STP correspond to the DNA substrate, the 3′-P product, and the ST products, respectively. (b) Concentration response curves for HIV-1 integrase inhibition for ST and 3′-P (derived from densitometric analysis of the gels presented in part a). | ||
It is known in the literature that comparing a neutral compound with its positively or negatively charged analogues changes the biological activity drastically. For example, it has been observed that among the compounds with alkyl chain having neutral, positively charged or negatively charged head groups, only the ones with positive charge could activate the signal sequence of SRP (signal recognition particle) RNA.43 The non-ionic derivatives of imidazole with C-10 and C-18 alkyl chain lengths viz. 19 and 20, respectively were tested for IN inhibition and both were completely ineffective for 3′-P and ST inhibition (entry 12 and 13, Table 2). Similar results were observed for the non-ionic tertiary amines 29 and 30 that showed no activity for IN inhibition (entries 9 and 10, Table 3); this is contrary to their quaternary analogues that showed the maximum inhibition in this series. This observation suggests that just by converting a non-ionic compound to its salt counterpart can completely change biological activity and that tuning the proper cation/anion combination is required to obtain highly potent drug candidates.
Effect of ILs 3, 14 and 15 on precleaved substrates
We next investigated the direct effects of the compounds on ST without any interference from 3′-P inhibition. The most active ILs 3, 14 and 15 were tested in a gel based assay using a precleaved substrate, in which the terminal GT dinucleotide, 3′ to the conserved CA dinucleotide, has been removed to mimic that retroviral DNA ST substrate.55 All three compounds inhibited the ST step with IC50's between 3.5 and 5.6μM, indicating ILs are true ST inhibitors (Fig. 4). Those ST IC50 values were 3-5-fold higher than with the full-length substrate, indicating greater inhibition in the presence of full-length substrate (compare IC50 values in Fig. 4 with Table 1 and 3). These results demonstrate that the inhibitory effect of ILs on ST due to a dual inhibition of ST and 3′-P. | ||
| Fig. 4 The effect of ILs 14, 15 and 3 on the precleaved HIV LTR DNA substrate. | ||
Conclusion
The anti-HIV activity of ammonium- and imidazolium-based ionic liquids has been evaluated for the first time by studying their IN inhibitory effect using in vitro gel-based assays. The results show that increasing chain length of the alkyl substitution causes significant improvement in IN inhibition, both for imidazolium and ammonium-based ILs. Imidazolium ILs 3, 14, 15 and 16 and ammonium ILs 27 and 28 were found to be dual IN inhibitors inhibiting both 3′-P and ST reactions. Further investigation into the mechanism of action and toxicity studies of these compounds with more extensive screening may lead to their potential utility as antiviral drugs.Experimental section
Chemistry
Biological assay
Compounds 1–30 were tested for their ability to inhibit HIV-1 integrasein vitro using a gel-based assay as reported previously.23 Briefly, 400 nM recombinant integrase was mixed with 20 nM [32P]-radiolabeled DNA substrate in a buffer containing 50 mM MOPS, pH 7.2, 7.5 mM MgCl2, 14.3 mM 2-mercaptoethanol, and the drug of interest or 10% DMSO. Reactions were incubated at 37 °C for 1 h and quenched by the addition of an equal volume of gel loading dye (formamide with 0.25% bromophenol blue, 0.25% xylene cyanol and 5 mM EDTA). Reaction products were separated on a 20% polyacrylamide denaturing sequencing gel. Dried gels were exposed overnight and visualized using a Typhoon 8600 (GE Healthcare, Piscataway, NJ). Densitometry analyses were performed using ImageQuant software from GE Healthcare.Acknowledgements
The authors would like to thank the National Cancer Institute (NCI) Developmental Therapeutics Program. This project has been funded in whole or in part with federal funds from the NCI, National Institutes of Health, under Contract No. HSN261200800001E. KM and YP are supported by the Center for Cancer Research, NIH Intramural Program of the National Cancer Institute. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.References
- D. D. Richman, Nature, 2001, 410, 995–1001 CrossRef CAS.
- G. R. Painter, M. R. Almond, S. Mao and D. C. Liotta, Curr. Top. Med. Chem., 2004, 4, 1035–1044 CrossRef CAS.
- G. R. Painter, L. T. Rimsky, P. A. Furman, D. C. Liotta, R. F. Schinazi, J. B. Quinn, In Frontiers in Viral Hepatitis, Publisher: Elsevier, Amsterdam, Netherlands, 2003, 451–484 Search PubMed.
- J. Cohen, Science, 2002, 296, 2320–2324 CrossRef CAS.
- M. R. Fesen, K. W. Kohn, F. Leteurtre and Y. Pommier, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 2399–2403 CrossRef CAS.
- Y. Pommier, A. A. Johnson and C. Marchand, Nat. Rev. Drug Discovery, 2005, 4, 236–248 CrossRef CAS.
- R. Dayam, R. Gundla, L. Q. Al-Mawsawi and N. Neamati, Med. Res. Rev., 2008, 28, 118–154 CrossRef CAS.
- T. H. Evering and M. Markowitz, Drugs Today, 2007, 43, 865–877 CrossRef CAS.
- E. Asante-Appiah and A. M. Skalka, Adv. Virus Res., 1999, 52, 351–69 CAS.
- D. Esposito and R. Craigie, Adv. Virus Res., 1999, 52, 319–333 CAS.
- F. Dyda, A. B. Hickman, T. M. Jenkins, A. Engelman, R. Craigie and D. R. Davies, Science, 1994, 266, 1981–1986 CrossRef CAS.
- Y. Wu and J. W. Marsh, Science, 2001, 293, 1503–1506 CrossRef CAS.
- A. S. Espeseth, P. Felock, A. Wolfe, M. Witmer, J. Grobler, N. Anthony, M. Egbertson, J. Y. Melamed, S. Young, T. Hamill, J. L. Cole and D. J. Hazuda, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 11244–11249 CrossRef CAS.
- D. J. Hazuda and S. D. Young, Adv. Antiviral Drug Des., 2004, 4, 63–77 Search PubMed.
- R. Dayam, J. Deng and N. Neamati, Med. Res. Rev., 2006, 26, 271–309 CrossRef CAS.
- M. R. Fesen, Y. Pommier, F. Leteurtre, S. Hiroguchi, J. Yung and K. W. Kohn, Biochem. Pharmacol., 1994, 48, 595–608 CrossRef CAS.
- M. Cushman and P. Sherman, Biochem. Biophys. Res. Commun., 1992, 185, 85–90 CrossRef CAS.
- S. Carteau, J.-F. Mouscadet, H. Goulaouic, F. Subra and C. Auclair, Arch. Biochem. Biophys., 1993, 305, 606–610 CrossRef CAS.
- W. Pluymers, N. Neamati, C. Pannecouque, V. Fikkert, C. Marchand, T. R. Burke Jr., Y. Pommier, D. Schols, E. De Clercq, Z. Debyser and M. Witvrouw, Mol. Pharmacol., 2000, 58, 641–648 CAS.
- E. De Clercq, J. Med. Chem., 2005, 48, 1297–1313 CrossRef CAS.
- A. Mazumder, H. Uchida, N. Neamati, S. Sunder, M. Jaworska-Maslanka, E. Wickstrom, F. Zeng, R. A. Jones, R. F. Mandes, H. K. Chenault and Y. Pommier, Mol. Pharmacol., 1997, 51, 567–575 CAS.
- M. Taktakishvili, N. Neamati, Y. Pommier, S. Pal and V. Nair, J. Am. Chem. Soc., 2000, 122, 5671–5677 CrossRef CAS.
- D. J. Hazuda, P. Felock, M. Witmer, A. Wolfe, K. Stillmock, J. A. Grobler, A. Espeseth, L. Gabryelski, W. Schleif, C. Blau and M. D. Miller, Science, 2000, 287, 646–650 CrossRef CAS.
- J. S. Wai, M. S. Egbertson, L. S. Payne, T. E. Fisher, M. W. Embrey, L. O. Tran, J. Y. Melamed, H. M. Langford, J. P. Guare Jr., L. Zhuang, V. E. Grey, J. P. Vacca, M. K. Holloway, A. M. Naylor-Olsen, D. J. Hazuda, P. J. Felock, A. L. Wolfe, K. A. Stillmock, W. A. Schleif, L. J. Gabryelski and S. D. Young, J. Med. Chem., 2000, 43, 4923–4926 CrossRef CAS.
- C. Marchand, X. Zhang, G. C. G. Pais, K. Cowansage, N. Neamati, T. R. Burke Jr. and Y. Pommier, J. Biol. Chem., 2002, 277, 12596–12603 CrossRef CAS.
- D. J. Hazuda, S. D. Young, J. P. Guare, N. J. Anthony, R. P. Gomez, J. S. Wai, J. P. Vacca, L. Handt, S. L. Motzel, H. J. Klein, G. Dornadula, R. M. Danovich, M. V. Witmer, K. A. A. Wilson, L. Tussey, W. A. Schleif, L. S. Gabryelski, L. Jin, M. D. Miller, D. R. Casimiro, E. A. Emini and J. W. Shiver, Science, 2004, 305, 528–532 CrossRef CAS.
- J. A. Grobler, K. Stillmock, B. Hu, M. Witmer, P. Felock, A. S. Espeseth, A. Wolfe, M. Egbertson, M. Bourgeois, J. Melamed, J. S. Wai, S. Young, J. Vacca and D. J. Hazuda, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 6661–6666 CrossRef CAS.
- Y. Goldgur, R. Craigie, G. H. Cohen, T. Fujiwara, T. Yoshinaga, T. Fujishita, H. Sugimoto, T. Endo, H. Murai and D. R. Davies, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13040–13043 CrossRef CAS.
- C. Pannecouque, W. Pluymers, B. Van Maele, V. Tetz, P. Cherepanov, E. De Clercq, M. Witvrouw and Z. Debyser, Curr. Biol., 2002, 12, 1169–1177 CrossRef CAS.
- G. C. G. Pais, X. Zhang, C. Marchand, N. Neamati, K. Cowansage, E. S. Svarovskaia, V. K. Pathak, Y. Tang, M. Nicklaus, Y. Pommier and T. R. Burke Jr., J. Med. Chem., 2002, 45, 3184–3194 CrossRef CAS.
- M. Sato, T. Motomura, H. Aramaki, T. Matsuda, M. Yamashita, Y. Ito, H. Kawakami, Y. Matsuzaki, W. Watanabe, K. Yamataka, S. Ikeda, E. Kodama, M. Matsuoka and H. Shinkai, J. Med. Chem., 2006, 49, 1506–1508 CrossRef CAS.
- V. Nair, G. Chi, R. Ptak and N. Neamati, J. Med. Chem., 2006, 49, 445–447 CrossRef CAS.
- V. Nair, V. Uchil and N. Neamati, Bioorg. Med. Chem. Lett., 2006, 16, 1920–1923 CrossRef CAS.
- G. Cecere, M. D. R. R. Ferreira, P. Jones, P. Pace, A. Petrocchi, V. Summa, WO2006103399, 2006.
- E. D. Jones, N. Vandergraaff, G. Le, N. Choi, W. Issa, K. Macfarlane, N. Thienthong, L. J. Winfield, J. A. V. Coates, L. Lu, X. Li, X. Feng, C. Yu, D. I. Rhodes and J. J. Deadman, Bioorg. Med. Chem. Lett., 2010, 20, 5913–5917 CrossRef CAS.
- E. Nizi, M. V. Orsale, B. Crescenzi, G. Pescatore, E. Muraglia, A. Alfieri, C. Gardeli, S. A. H. Spieser and V. Summa, Bioorg. Med. Chem. Lett., 2009, 19, 4617–4621 CrossRef CAS.
- C. Gardelli, E. Nizi, E. Muraglia, B. Crescenzi, M. Ferrara, R. Orvieto, P. Pace, G. Pescatore, M. Poma, M. del Rosario Rico Ferreira, R. Scarpelli, C. F. Homnick, M. Ikemoto, A. Alfieri, M. Verdirame, F. Bonelli, O. Gonzalez Paz, M. Taliani, E. Monteagudo, S. Pesci, R. Laufer, R. Felock, K. A. Stillmock, D. Hazuda, M. Rowley and V. Summa, J. Med. Chem., 2007, 50, 4953–4975 CrossRef CAS.
- J. J. Merianos. Quaternary Ammonium Anitmicrobial Compounds. In Disinfection, Sterilization, and Preservation, Block, S. S., Ed; 4th ed, Lea and Febiger: Malvern, Pennsylvania; 1991, pp. 225–255 Search PubMed.
- K. Furuse, C. Ishizeki and S. Iwahara, J. AIDS, 2000, 24, 147–153 Search PubMed.
- C. Tevi-Benissan, M. Makuva, A. Morelli, M. C. Georges-Courbot, M. Matta, A. Georges and L. Belec, Protection of cynomolgus macaque against cervicovaginal transmission of SIVmac251 by the spermicide benzalkonium chloride, J. AIDS, 2000, 24, 147–153 Search PubMed.
- Y.-L. Wong, C. L. Curgman, G. F. Doncel, M. P. Hubieki, T. C. Dudding, P. S. Salve and R. D. Gandour, Tetrahedron, 2002, 58, 45–54 CrossRef CAS.
- Y.-L. Wong, M. P. Hubieki, C. L. Curgman, G. F. Doncel, T. C. Dudding, P. S. Salve and R. D. Gandour, Bioorg. Med. Chem., 2002, 10, 3599–3608 CrossRef CAS.
- N. Bradshaw, S. B. Neher, D. S. Booth and P. Walter, Science, 2009, 323, 127–130 CrossRef CAS.
- J.-C. Plaquevent, J. Levillain, F. Guillen, C. Malhiac and A.-C. Gaumont, Chem. Rev., 2008, 108, 5035–5060 CrossRef CAS.
- P. Dominguez de Maria, Angew. Chem., Int. Ed., 2008, 47, 6960–6968 CrossRef.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015–2050 CrossRef CAS.
- V. Kumar, C. E. Olsen, S. J. C. Schaffer, V. S. Parmar and S. V. Malhotra, Org. Lett., 2007, 9, 3905–3908 CrossRef CAS.
- S. V. Malhotra, V. Kumar and V. S. Parmar, Curr. Org. Synth., 2007, 4, 370–380 Search PubMed.
- H. Zhao and S. V. Malhotra, Aldrichimica Acta, 2002, 35, 75–83 CAS.
- W. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef.
- J. Ranke, S. Stolte, R. Stormann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS.
- P. Stepnowski, A. C. Skladanowski, A. Ludwiczak and E. Laczynska, Hum. Exp. Toxicol., 2004, 23, 513–517 CrossRef CAS.
- R. F. M. Frade, A. Matias, L. C. Branco, C. A. M. Afonso and C. M. M. Duarte, Green Chem., 2007, 9, 873–877 RSC.
- X. Wang, C. A. Ohlin, Q. Lu, Z. Fei, J. Hu and P. J. Dyson, Green Chem., 2007, 9, 1191–1197 RSC.
- J. Ranke, A. Muller, U. Bottin-Weber, F. Stock and S. Stolte, Ecotoxicol. Environ. Saf., 2007, 67, 430–438 CrossRef CAS.
- F. Pfannkuch; H. Rettig; P. H. Stahl. In Handbook of Pharmaceutical Salts: Properties, Selection, and Use; Wermuth, C. G.; Stahl, P. H. (Eds), Wiley-VCH, Weinheim, Germany, 2002, 130 Search PubMed.
- V. Kumar and S. V. Malhotra, Bioorg. Med. Chem. Lett., 2009, 19, 4643–4646 CrossRef CAS.
- J. Marinello, C. Marchand, B. T. Mott, A. Bain, C. J. Thomas and Y. Pommier, Biochemistry, 2008, 47, 9345–9354 CrossRef CAS.
- Q. Liu, M. H. A. Janssen, F. Van Rantwijk and R. A. Sheldon, Green Chem., 2005, 7, 39–42 RSC.
- S. Khabnadideh, Z. Rezaei, A. Khalafi-Nezhad, R. Bahrinajafi, R. Mohamadi and A. A. Farrokhroz, Bioorg. Med. Chem. Lett., 2009, 13, 2863–2865.
Footnote |
| † Both authors have equal contribution in this work. |
| This journal is © The Royal Society of Chemistry 2011 |

