Interactions of polysulfanes with components of red blood cells†
Thomas
Schneider
a,
Lalla A.
Ba
a,
Khairan
Khairan
a,
Clemens
Zwergel
a,
Nguyen Duc
Bach
b,
Ingolf
Bernhardt
b,
Wolfgang
Brandt
c,
Ludger
Wessjohann
c,
Marc
Diederich
d and
Claus
Jacob
*a
aDivision of Bioorganic Chemistry, School of Pharmacy, Saarland University, D-66123, Saarbruecken, Germany. E-mail: c.jacob@mx.uni-saarland.de; Fax: +49 681 302 3464; Tel: +49 681 302 3129
bDivision of Biophysics, Department of Biology, Saarland University, D-66123, Saarbruecken, Germany
cLeibniz Institute of Plant Biochemistry, Department of Bioorganic Chemistry, Weinberg 3, D-06120, Halle, Saale, Germany
dLaboratoire de Biologie Moléculaire et Cellulaire de Cancer, Hôpital Kirchberg 9, rue Edward Steichen, L-2540, Luxembourg, Luxembourg
First published on 19th January 2011
Abstract
Traditionally, the activity of most polysulfanes has been associated with the redox behaviour of the sulfur-sulfur bond. Here we show that polysulfanes, such as diallyltri- and tetrasulfide, also interact with cellular membranes and certain metalloproteins. Together, multiple interactions with various biological targets may explain best the biological activity of such compounds.
Polysulfanes (RSxR, R ≠ H, x ≥ 3) form a class of compounds which also includes several interesting natural products.‡ The latter occur, for instance, in garlic, onions, shallots and certain mushrooms.1Diallyltrisulfide (DATS§) and diallyltetrasufide (DATTS) from garlic exhibit a wide spectrum of therapeutically interesting biological activities, including antimicrobial properties and cytotoxicity against certain cancer cells. Unfortunately, the underlying chemical and biochemical causes of this activity are often little understood. During the last decade, it has become apparent that many polysulfanes undergo complex redox transformations in vitro and in vivo: these compounds act as specific oxidants which S-thiolate and hence inhibit proteins and enzymes. In addition, their reduced forms, i.e. the perthiols (RSxH, R ≠ H, x ≥ 2), are able to generate superoxide radical anions (O2˙−) in the presence of glutathione (GSH) and certain metal ions (Fig. 1).2
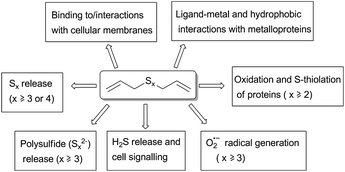 | ||
| Fig. 1 Chemical properties and reactivity of polysulfanes which may explain aspects of their biological activity. While some reactions necessarily require the presence of longer sulfur-sulfur chains, others, such as ligand-metal and hydrophobic interactions, increase gradually from the monosulfide (DAS) to the tetrasulfide (DATTS). Please note that this scheme is neither final nor complete. | ||
While these redox transformations represent one major feature of polysulfane chemistry, they may not cover the full breadth of polysulfane reactivity. In theory, various other interactions with biomolecules are possible, some of which have hardly been considered so far. The chemical structure of polysulfanes such as DATS and DATTS, for instance, points towards a high lipophilicity, specific membrane and protein interactions and the ability to coordinate to certain metal ions. Besides redox activity, we have therefore investigated two alternative, biochemically relevant modes of action: (a) hydrophobic interactions with proteins and/or membranes; and (b) metal–ligand and hydrophobic interactions with key metalloproteins.
In order to study these effects, we have established a simple assay based on the haemolysis of red blood cells (RBCs).3 This test system has several advantages compared to conventional cell-based assays.
Firstly, RBCs do not divide: any effects observed therefore are due to damage to existing cells rather than a combination of cytostatic and cytotoxic effects. Secondly, RBCs provide an ideal system to study interactions with cellular membranes. And finally, RBCs are rich in haemoglobin (Hb), which in addition to membrane interactions also allows us to study effects of polysulfanes on a (prominent) metalloprotein.¶
Not unexpectedly, DATS and DATTS cause the haemolysis of RBCs in a concentration dependent manner. As Fig. 2a illustrates in the case of DATS, significant haemolysis occurs at concentrations of around 50 to 100 μM. Similar results have been observed for the other polysulfanes, all of which cause significant haemolysis at a concentration of 50 μM and above (Fig. 2b). Interestingly, the extent of haemolysis increases with increasing length of the sulfur-sulfur chain. These findings are in good agreement with the literature, where a number of reports mention cytotoxic effects of diallylsulfides on mammalian cells, which increase with increasing sulfur chain length. Such activities usually reach a maximum with compounds DATS and DATTS and show IC50 values between 20 and 200 μM.4,5
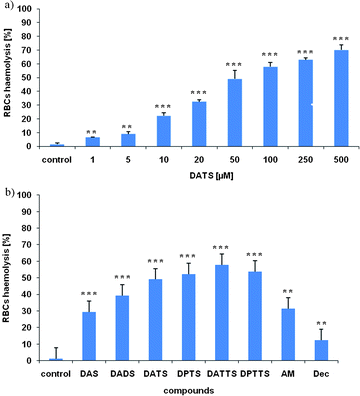 | ||
| Fig. 2 Haemolysis of RBCs depends on the concentration of sulfanes and increases with increasing length of the sulfur-sulfur chain. Haemolysis of RBCs in the presence of DATS after 30 min incubation is concentration dependent (a). To compare the different sulfanes among each other and with a series of controls, the extent of haemolysis of RBCs was measured in the absence and presence of 50 μM of each sulfane (b).§ Data depicted as mean ± SD (n = 3).||. | ||
Nonetheless, the results obtained in RBCs somewhat differ from most (but not all) literature reports.
In the RBCs assay, diallylmonosulfide (DAS) and diallyldisulfide (DADS) are also active, albeit to a lesser extent. While the activity of DADS may be explained by a redox process (thiol/disulfide exchange with subsequent S-thiolation of proteins and enzymes, Fig. 1), DAS is clearly redox inactive under these conditions. The gradual increase of activity from DAS to DATTS, as observed by us in RBCs, is in line with some previous studies, such as a recent, landmark study by Tsuchiya and Nagayama in 2008.6 The activity of DAS, however, cannot be explained fully by conventional redox hypotheses.
Furthermore, RBCs do not contain the kind of redox signalling networks present in other cells. Oxidatively induced signalling and leakage of mitochondria with subsequent apoptosis, for instance, is not feasible in RBCs. The effects of (poly)sulfanes on RBCs therefore may require an additional or alternative explanation besides traditional redox processes.
We have therefore turned our attention to the lipophilicity of the various diallylsulfides. The lipophilicity of these compounds, measured by a HPLC method and expressed as logP-values, increases gradually from DAS (logP = 2.41) to DATTS (logP = 3.12) (Table 1).7–9 This rather high lipophilicity of the diallylsulfanes may enable extensive interactions with biological targets, such as cellular membranes and hydrophobic areas in proteins. We have therefore studied the effects of the various sulfanes on the membranes of RBCs, using exposure of phosphatidylserine (PS) to the outer layer of the cell membrane as a measure of effects on—and changes associated with—the cell membrane.
| Compound | logP (found) | logP (calc.) | pKi (calc. for Hb) | pKi (calc. for HDAC 4) |
|---|---|---|---|---|
| DAS | 2.41 ± 0.18 | 2.23 ± 0.21 | 6.1 | 4.0 |
| DADS | 2.72 ± 0.15 | 2.52 ± 0.91 | 6.4 | 4.0 |
| DATS | 2.98 ± 0.19 | 2.64 ± 0.86 | 6.7 | 3.9 |
| DPTS | 3.24 ± 0.17 | 3.13 ± 0.82 | 6.6 | 4.4 |
| DATTS | 3.12 ± 0.16 | 2.88 ± 1.00 | 6.8 | 4.8 |
| DPTTS | 3.45 ± 0.14 | 3.38 ± 0.97 | 7.2 | 4.9 |
| AM | 1.45 ± 0.12 | 1.39 ± 0.19 | 4.2 | 3.8 |
| Dec | 4.71 ± 0.17 | 4.61 ± 0.48 | 6.8 | 4.7 |
PS exposure was measured by FACS using AnnexinV as a specific stain (Fig. 3).10–12 It occurs at sulfane concentrations similar to the ones required for RBCs haemolysis and gradually increases from DAS to DATTS (Fig. 3). In many respects, PS exposure therefore reflects the activities and trends observed in RBCs haemolysis. It may also explain the rather ‘unusual’ activity of allylmercaptan (AM): the latter causes significant haemolysis (Fig. 2b), yet is clearly not an oxidizing, S-thiolating agent on its own or able to generate O2˙− (AM may, however, form disulfide bonds with cysteine residues in proteins in the presence of oxidizing agents or by reacting with already existing disulfide bonds in proteins). As in the case of DAS, the activity of AM cannot be rationalized easily by a traditional redox hypothesis, yet its pronounced activity in the FACS analysis of PS exposure fits in with membrane interactions.
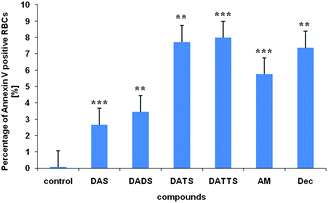 | ||
| Fig. 3 PS exposure in the RBCs cell membrane after 20 min incubation with 50 μM sulfane.§ See text for experimental details. Data are depicted as mean ± SD (n = 3)|| Please note that the membranes of haemolysed cells have also been affected by the sulfanes tested yet those cells do no longer show up in this assay. The percentages of Annexin V positive cells on the one hand and haemolysed cells on the other therefore differ. | ||
PS exposure in RBCs usually is either the result of direct membrane disruption (e.g. by an exogenic agent) or a consequence of Ca2+ influx and increased phospholipid scramblase activity.13 We have therefore investigated the Ca2+ status in the presence of sulfanes, using FACS and Fluo-4 as a stain for Ca2+. Not surprisingly, DATS causes a significant influx of Ca2+ ions into the RBCs (Fig. 4) which may in turn activate the phospholipid scramblase and hence cause PS exposure.
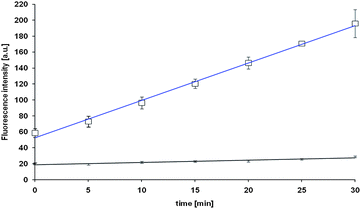 | ||
| Fig. 4 Uptake of Ca2+ in RBCs in the presence of 50 μM of DATS (blue) and in the absence of DATS as control (black). See text for experimental details. Data are depicted as mean ± SD (n = 3).||. | ||
A similar influx of Ca2+ was found for 1,9-decadiene (Dec), a control compound which also increases PS exposure. While Ca2+ influx may explain the exposure of PS, other, more direct effects on the cell membrane should not be ruled out: the activity of DATTS, for instance, may (also) be due to its rather unusual, helical structure, which predestines this molecule for membrane disruption (Fig. 5).14–16
 | ||
| Fig. 5 The most stable, helical conformation of DATTS was modeled using the PLANTS program. All dihedral angles have a value of −90.0°. This helical structure is 114 kJ mol−1 more stable compared to the all-trans conformation of DATTS. Please note that the all-trans form of Dec (the carbon analogue of DATTS) in contrast is the most stable form of this hydrocarbon. | ||
Importantly, Ca2+ influx and PS exposure are cellular processes which in some cell types may serve as initial trigger for wider signalling pathways, including apoptotic cascades.
A complete chain of intracellular events is therefore emerging, which could connect the initial interaction of polysulfanes with their (membrane) targets to apoptotic cell deathvia a set of complex cellular signalling events. In our studies with RBCs, there appears to be a rather strong interaction with the cell membrane, which on one hand may explain increased PS exposure in intact cells and, on the other hand, form the basis for (subsequent) haemolysis, i.e. widespread lysis of the RBCs and Hb release. Both processes have been observed in our assays, and may be causally connected. In any case, the activity of the various sulfanes tested is often surprisingly similar in both assays.
Nonetheless, it may be rather naïve to assign the whole biological chemistry of polysulfanes to hydrophobic interactions with cell membranes. The carbon analogue of DATTS, i.e. Dec, is also highly lipophilic (logP = 4.71), causes significant Ca2+ influx and PS exposure, yet barely induces haemolysis in RBCs. Indeed, our previous studies all point towards a surprisingly low toxicity of Dec compared to the one associated with DATS and DATTS.
It is therefore likely that the phospholipid bilayer is not the (only) target of these compounds. The cell membrane is a highly complex structure which also contains a plethora of different proteins, including numerous transporters (pumps, channels, carriers). Interactions of polysulfanes with (metallo-)proteins at or near the cell membrane should therefore not be ruled out. In RBCs, Hb is distributed all over the cell, i.e. it is also found near the inner membrane surface and near membrane carriers. Hb may therefore serve as an ‘indicator’ for such metalloprotein interactions.
Indeed, there is some evidence for polysulfane-metalloprotein interactions. When DATS and DATTS are added to a suspension of RBCs, an almost immediate change of colour from lighter to darker red can be observed. While it is difficult to quantify and explain this colour change in whole cells, significant changes in the UV/VIS spectra of isolated human Hb (and indeed of other haem proteins, such as myoglobin and cytochrome c) occur when sulfanes are added (Fig. 6). These changes, which include decreases in the Soret band, are most pronounced for DATS and DATTS, pointing towards a particularly strong interaction of these polysulfanes with the metal site. CD spectra recorded for Hb in the absence and presence of various sulfanes confirm changes to the Hb protein in the presence of DAS, DADS, DATS and DATTS. Spectral changes particularly affect the region of the haem signal, supporting an interaction at or near the metal site (data not shown). Interestingly, similar changes are also observed for AM and inorganic sulfide anions (S2−). It therefore appears that not only reduced thiols, but also the sulfur atoms in a polysulfane chain are able to interact with metal ions, such as iron. Indeed, such interactions have been discussed a few years ago by Ralf Steudel and colleagues.17
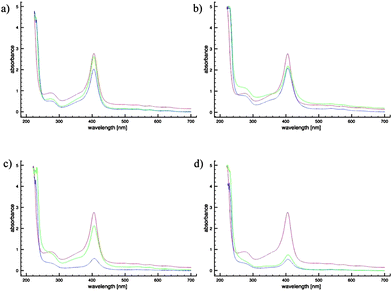 | ||
| Fig. 6 UV/Vis spectra of 150 μM Hb in physiological solution in the absence (red) and presence of 100 μM (green) and 300 μM (blue) DAS (a), DADS (b), DATS (c), or DATTS (d). Please note that the effects observed for DAS and DADS are rather small and may, at least in part, be due to light scattering. | ||
We have therefore modelled binding of the various (poly)sulfanes to Hb in a computer model using the PLANTS docking program (Fig. 7).18–20 The binding constants and geometries calculated for these interactions, albeit clearly of a preliminary nature, lend support to the notion that (a) all sulfanes studied are able to interact with Hb with reasonable, gradually increasing binding constants pKi of 6.1 (DAS), 6.4 (DADS), 6.7 (DATS) and 6.8 (DATTS), (b) binding occurs at or near the metal site in Hb, (c) the binding constants of DAS, DADS, DATS and DATTS are significantly higher than the one calculated for AM (4.2), indicating that binding is due to a combination of sulfur-metal and hydrophobic interactions and (d) binding of Dec also occurs (pKi = 6.8), yet at a slightly different position and without the assistance of metal interactions (Table 1).
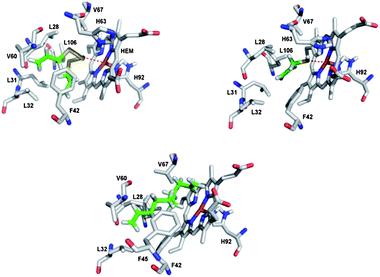 | ||
| Fig. 7 Modelled binding of DATTS, AM and 1,9-decadiene to Hb using the PLANTS docking program (the binding site of the Hb monomer is shown).22 While DATTS, in a twisted conformation, binds rather strongly by a combination of ligand-metal and hydrophobic interactions, AM binds more weakly to the iron ion without any major hydrophobic interactions and 1,9-decadiene binds exclusively via hydrophobic interactions at a site further away from the iron ion. | ||
It is therefore possible that metalloproteins form (additional) targets for polysulfanes. Besides direct interactions with the phospholipid bilayer, such interactions could even explain the Ca2+ influx and subsequent PS exposure observed in this study. Indeed, polysulfanes not only bind to Hb, but also to other proteins, as calculated for human histone deacetylase (HDAC) 4, a zinc enzyme involved in the control of gene expression (Table 1). Ultimately, this type of polysulfane-metalloprotein interaction needs to be studied in more detail, especially binding specificity for certain (metallo-)proteins, structural aspects of binding and the possibility of simultaneous binding via two or more sulfur atoms.21
In conclusion, our studies have shed new light on the unique combination of physical and chemical properties of polysulfanes, which include redox activity, O2˙− radical generation, hydrophobic interactions with membranes and combined ligand-metal/hydrophobic interactions with certain proteins. Recent studies by Benavides and colleagues have even provided some evidence that such sulfanes may release H2S under certain conditions.23 Together, rather than in isolation, these properties may explain the widespread and often selective biological activity associated with polysulfanes. Redox activity and radical generation, for instance, may shift the intracellular redox state in cancer cells to more oxidizing potentials, ultimately inducing cell death.24,25 Similarly, interactions with metalloenzymes and inhibition of various hydrophobic metalloproteins may affect certain cells more dramatically than others. Related considerations apply to changes in the composition of the cell membrane and membranes of cellular organelles.
While our studies have focussed on the ‘simple’ model of RBCs, the resulting biochemical changes may also take place in other cell types. Furthermore, while Hb and certain HDACs may interact quite strongly with some of these lipophilic sulfur compounds, other cellular components, such as hydrophobic proteins and (parts of) the tubulin network (where such lipophilic compounds may become enriched) should also be considered.
Ultimately, the RSxR scaffold and its various biochemical targets, which may include cellular membranes and hydrophobic pockets as well as metalloproteins and redox-sensitive proteins, provide strong leads for future studies in the area of selective cytotoxicity and drug development.
Acknowledgements
The authors acknowledge Saarland University, the Interreg IVA Programme (Corena-Network; 35GR11051) and the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement 215009 RedCat for financial support.Notes and references
- R. Steudel, Chem. Rev., 2002, 102, 3905–3945 CrossRef CAS.
- R. Munday, J. S. Munday and C. M. Munday, Free Radical Biol. Med., 2003, 34, 1200–1211 CrossRef CAS.
- M. Avron and N. Shavit, Anal. Biochem., 1963, 6, 549–554 CrossRef CAS.
- C. Busch, C. Jacob, A. Anwar, T. Burkholz, L. A. Ba, C. Cerella, M. Diederich, W. Brandt, L. Wessjohann and M. Montenarh, Int. J. Oncol., 2010, 36, 743–749 CAS.
- C. Cerella, C. Scherer, S. Cristofanon, E. Henry, A. Anwar, C. Busch, M. Montenarh, M. Dicato, C. Jacob and M. Diederich, Apoptosis, 2009, 14, 641–654 Search PubMed.
- H. Tsuchiya and M. Nagayama, J. Biomed. Sci., 2008, 15, 653–660 CrossRef CAS.
- C. Yamagami and N. Takao, Chem. Pharm. Bull., 1992, 40, 925–929 CAS.
- C. Yamagami and N. Takao, Chem. Pharm. Bull., 1993, 41, 694–698 CAS.
- J. J. Irwin and B. K. Shoichet, J. Chem. Inf. Model., 2005, 45, 177–182 CrossRef CAS.
- J. Dachary-Prigent, J. M. Freyssinet, J. M. Pasquet, J. C. Carron and A. T. Nurden, Blood, 1993, 81, 2554–2565 CAS.
- G. Koopman, C. P. Reutelingsperger, G. A. Kuijten, R. M. Keehnen, S. T. Pals and M. H. van Oers, Blood, 1994, 84, 1415–1420 CAS.
- F. A. Kuypers, R. A. Lewis, M. Hua, M. A. Schott, D. Discher, J. D. Ernst and B. H. Lubin, Blood, 1996, 87, 1179–1187 CAS.
- P. A. Lang, S. Kaiser, S. Myssina, T. Wieder, F. Lang and S. M. Huber, Am. J. Physiol. Cell Physiol., 2003, 285, C1553–C1560 CAS.
- P. Hohenberg and W. Kohn, Phys. Rev. B, 1964, 136, 864–871 Search PubMed.
- W. Kohn and L. J. Sham, Phys. Rev. A, 1965, 140, 1133–1138 Search PubMed.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- Y. Steudel, M. W. Wong and R. Steudel, Chem.–Eur. J., 2005, 11, 1281–1293 CrossRef CAS.
- O. Korb, T. Stutzle and T. E. Exner, in Ant Colony Optimization and Swarm Intelligence, Proceedings, ed. M. Dorigo, L. M. Gambardella, M. Birattari, A. Martinoliand T. Stutzle, Springer-Verlag Berlin, Germany 2006, pp. 247–258 Search PubMed.
- O. Korb, in 4th German Conference on Chemoinformatics, ed. F. Oellien, Chem Cent J, Goslar, Germany, 2008, p. O10. Search PubMed.
- O. Korb, T. Stutzle and T. E. Exner, J. Chem. Inf. Model., 2009, 49, 84–96 CrossRef CAS.
- H. Nian, B. Delage, J. T. Pinto and R. H. Dashwood, Carcinogenesis, 2008, 29, 1816–1824 CrossRef CAS.
- H. M. Berman, J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov and P. E. Bourne, Nucleic Acids Res., 2000, 28, 235–242 CrossRef CAS.
- G. A. Benavides, G. L. Squadrito, R. W. Mills, H. D. Patel, T. S. Isbell, R. P. Patel, V. M. Darley-Usmar, J. E. Doeller and D. W. Kraus, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17977–17982 CrossRef CAS.
- V. Jamier, L. A. Ba and C. Jacob, Chem.–Eur. J., 2010, 16, 10920–10928 CrossRef CAS.
- M. Doering, L. A. Ba, N. Lilienthal, C. Nicco, C. Scherer, M. Abbas, A. A. P. Zada, R. Coriat, T. Burkholz, L. Wessjohann, M. Diederich, F. Batteux, M. Herling and C. Jacob, J. Med. Chem., 2010, 53, 6954–6963 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of compounds, in vitro assays, structure modeling. See DOI: 10.1039/c0md00203h |
| ‡ In biochemical publications, polysulfanes are sometimes slightly incorrectly referred to as ‘polysulfides’. |
| § Abbreviations: DAS, diallylsulfide; DADS, diallyldisulfide; DATS, diallyltrisulfide; DATTS, diallyltetrasulfide; DPTS, dipropyltrisulfide; DPTTS, dipropyltetrasulfide; AM, allylmercaptan; Dec, 1,9-decadiene. |
| ¶ To avoid any misunderstandings, we must point out that this study does not aim at the development of any drugs or agents able to lyse RBCs. |
| || The differences between the measurements were examined using Student's t test, a value p < 0.05 was considered statistically significant. Symbols: *: p < 0.05; **: p < 0.01; ***: p < 0.001 versus controls. |
| This journal is © The Royal Society of Chemistry 2011 |
