Library synthesis and cytotoxicity of a family of 2-phenylacrylonitriles and discovery of an estrogen dependent breast cancer lead compound†
Mark
Tarleton
a,
Jayne
Gilbert
b,
Mark J.
Robertson
a,
Adam
McCluskey
*a and
Jennette A.
Sakoff
b
aChemistry, School of Environmental & Life Science, The University of Newcastle, University Drive Callaghan, NSW 2308, Australia. E-mail: Adam.McCluskey@newcastle.edu.au; Fax: +61 249 215472; Tel: +61 249 216486
bDepartment of Medical Oncology, Calvary Mater Hospital, Edith & Platt Streets, Waratah, NSW 2298, Australia. E-mail: Jennette.Sakoff@newcastle.edu.au
First published on 15th November 2010
Abstract
In our efforts to prevent highly toxic compounds progressing through our anti-parasitic drug development program, we serendipitously discovered a family of 2-phenylacrylonitriles with excellent growth inhibition of a panel of ten human cancer cell lines. Focused library approaches facilitated the identification of a simple pharmacophore, comprising two terminal aromatic moieties linked via a conjugated cyano (acrylonitrile) moiety. Efforts that perturbed this pharmacophore resulted in a significant drop in growth inhibition. Multiple libraries led to the discovery of two key lead compounds. The first, (Z)-2-(3,4-dichlorophenyl)-3-(4-methoxyphenyl)acrylonitrile (31) exhibits broad spectrum growth inhibition with GI50 values of 0.52–3 μM (HT29 and BE2-C cancer cell lines respectively; average = 1.6 μM). Of greater note is (Z)-2-(3,4-dichlorophenyl)-3-(4-nitrophenyl)acrylonitrile (28), a 0.127 ± 0.043 μM growth inhibitor of the estrogen receptor positive (ER+ve) human breast cancer cell line, MCF-7. Analogue 28 displays up to 543 fold selectivity towards MCF-7 cells compared with nine other non-breast derived cancer cell lines. Further screening of 28 against one human, ER−ve breast cancer cell line (MDA-MB231) and one normal non-tumourigenic breast epithelial cell line (MCF-10A) returned poor growth inhibition values of 34 ± 2 and 16 ± 4μM, demonstrating ca. ∼268 and∼126 fold preference for the MCF-7 estrogen dependent breast cancer cells.
Introduction
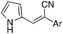
Over the past decade, our group has invested considerable effort in the development of highly focused compound libraries in the search for new biologically active molecules. We have an active interest in the development of anti-cancer and anti-parasitic agents.1 In this latter regard, we were initially keen to ensure the internal validity of our anti-parasitic screening approach, and as such were keen to include anti-parasitic agents of known activity. Our preference was for a small library of agents with divergent activities and ease of synthetic access. The (Z)-2-phenyl-3-(1H-pyrrol-2-yl)acrylonitrile derivatives reported by Ali et al., fulfilled both of these criteria (Fig. 1).2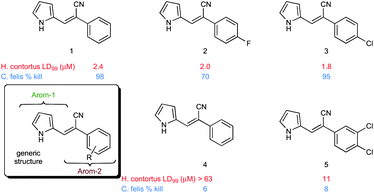 | ||
| Fig. 1 Generic structural representation, and selected examples, of the compounds reported by Ali et al. as active against Haemonchus contortus (H. contortus) and Ctenocephalides felis (C. felis).2 | ||
One additional requirement that we imposed on our development of anti-parasitic agents was a toxicity pre-filter to ensure that the ultimate end user would not be subjected to highly toxic agents that would be applied in a non-ideal environment, e.g. rural farming communities. We thus chose to routinely examine the cytotoxicity of all new compounds in our anti-parasite program via cytotoxicity screening against a panel of ten human cancer cell lines: HT29 and SW480 (colon carcinoma), MCF-7 (breast carcinoma), A2780 (ovarian carcinoma), H460 (lung carcinoma), A431 (skin carcinoma), DU145 (prostate carcinoma), BEC-2 (neuroblastoma), SJ-G2 (glioblastoma), MIA (pancreatic carcinoma). The first series of compounds examined in this panel of cell lines were the known (Z)-2-phenyl-3-(1H-pyrrol-2-yl)acrylonitriles (1–5, Fig. 1).2 To our surprise, these analogues were highly cytotoxic (see below). Intrigued by these findings we set about developing a series of highly focused libraries in an effort to improve on the cell death and cell line specificity that we noted. Our efforts are reported herein.
Results and discussion
Our drug development paradigm is a simple one, relying on the application of robust reliable chemistries in the synthesis of highly focused compound libraries that are then subjected to rapid biological screening. This approach allows for the development of multiple library generations in a short period of time. The original synthesis of (Z)-2-phenyl-3-(1H-pyrrol-2-yl)acrylonitrile derivatives reported by Ali et al., fits well within our paradigm.2 Library access was readily accomplished, in excellent yields, by the simple condensation of pyrrole-2-carboxaldehyde (Arom-1 in Fig. 1) with a family of phenylacetonitrile derivatives (Scheme 1).3–5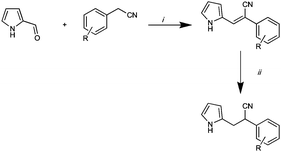 | ||
| Scheme 1 Reagents and conditions: (i) H2O, 40% PhCH2NMe3(OH), 50 °C, 5 h; (ii) 0.05M solution in acetone, ThalesNano H-cube™, 10% Pd/C catalyst at 1 mL min−1 at 50 °C and 50 bar H2 pressure. | ||
In our initial investigations, Library A comprised the five analogues shown in Fig. 1, retaining the pyrrole moiety with variations of the aromatic substituent (Arom-2 in Fig. 1). Library A was screened for growth inhibition against our panel of ten human cancer cell lines (Table 1).
| Ar (compound) | HT29 a | SW480 a | MCF-7 b | A2780 c | H460 d | A431 e | DU145 f | BE2-C g | SJ-G2 h | MIAi |
|---|---|---|---|---|---|---|---|---|---|---|
| a HT29 and SW480 (colon carcinoma). b MCF-7 (breast carcinoma). c A2780 (ovarian carcinoma). d H460 (lung carcinoma). e A431 (skin carcinoma). f DU145 (prostate carcinoma). g BEC-2 (neuroblastoma). h SJ-G2 (glioblastoma). i MIA (pancreatic carcinoma). | ||||||||||

|
41 ± 6 | 26 ± 4 | 17 ± 1 | 25 ± 2 | 81 ± 11 | 57 ± 5 | > 100 | 61 ± 7 | 46 ± 7 | 81 ± 11 |
|
31 ± 4 | 24 ± 2 | 15 ± 1 | 25 ± 0 | 52 ± 2 | 37 ± 3 | 59 ± 10 | 43 ± 7 | 30 ± 4 | 63 ± 5 |

|
18 ± 1 | 22 ± 0 | 4.0 ± 0.5 | 18 ± 0 | 19 ± 1 | 23 ± 3 | 37 ± 3 | 21 ± 1 | 20 ± 1 | 38 ± 2 |

|
25 ± 1 | 24 ± 1 | 18 ± 1 | 18 ± 1 | 32 ± 0 | 27 ± 1 | 26 ± 1 | 22 ± 1 | 24 ± 1 | 34 ± 1 |
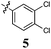
|
15 ± 1 | 23 ± 1 | 0.56 ± 0.03 | 16 ± 0 | 5.7 ± 0.7 | 3.2 ± 0.1 | 41 ± 8 | 25 ± 1 | 20 ± 1 | 46 ± 7 |
With the exception of the parent phenyl substituted (1), which is a modest inhibitor of cell growth, all Library A members displayed excellent levels of growth inhibition across all cell lines examined. Two Library A members stand out with the 4-chlorophenyl (3) and 3,4-dichlorophenyl (5) analogues returning the best growth inhibition with average GI50 values of 22 μM and 19 μM respectively. Both analogues 3 and 5 also displayed a considerable degree of specificity for the breast cancer cell line MCF-7 with GI50 values of 4.0 ± 0.5 and 0.56 ± 0.03 μM respectively. Introduction of the second chlorine atom (3 → 5) also notably enhanced cell death in the H460 (lung carcinoma) and A431 (skin carcinoma) cell lines (GI50's = 5.7 ± 0.7 and 3.2 ± 0.1 μM respectively). Thus the type and number of electronegative moieties attached to the phenyl ring appears to have a role in the growth inhibition of key cancer cell lines.
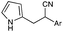
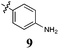
Based on the biological data from Library A it appeared that an extended conjugated system with one end possessing a high level of electronegative atoms was beneficial to activity. Working on this hypothesis we utilized 5 as the lead compound in the design and subsequent synthesis of Library B, which comprised the same five compounds as in Library A lacking only the alkene double bond of the acrylonitrile moiety, which we believed would have an adverse effect on cell death. Library B analogues were accessed via simple flow hydrogenation approaches (see experimental) (Scheme 1). We note that these conditions resulted in reduction of the –NO2 to –NH2 (9). The growth inhibition screening data for Library B analogues are shown in Table 2.
| Ar (compound) | HT29 a | SW480 a | MCF-7 b | A2780 c | H460 d | A431 e | DU145 f | BE2-C g | SJ-G2 h | MIAi |
|---|---|---|---|---|---|---|---|---|---|---|
| a HT29 and SW480 (colon carcinoma). b MCF-7 (breast carcinoma). c A2780 (ovarian carcinoma). d H460 (lung carcinoma). e A431 (skin carcinoma). f DU145 (prostate carcinoma). g BEC-2 (neuroblastoma). h SJ-G2 (glioblastoma). i MIA (pancreatic carcinoma). | ||||||||||
|
(39 ± 8) | (40 ± 14) | (47 ± 9) | (76 ± 10) | (15 ± 7) | (20 ± 4) | (7 ± 5) | (43 ± 6) | (27 ± 5) | (30 ± 5) |
|
73 ± 7 | 78 ± 8 | 65 ± 5 | 69 ± 4 | 88 ± 12 | 89 ± 11 | 91 ± 9 | 75 ± 3 | 86 ± 11 | 90 ± 10 |
|
37 ± 7 | 53 ± 3 | 26 ± 2 | 26 ± 3 | 58 ± 2 | 58 ± 1 | 60 ± 5 | 45 ± 6 | 52 ± 5 | 58 ± 6 |
|
67 ± 18 | 70 ± 5 | 37 ± 7 | 29 ± 3 | 64 ± 21 | 78.0 ± 7 | 90 ± 10 | 65 ± 9 | 87 ± 11 | 82 ± 11 |

|
(51 ± 4) | (34 ± 2) | (84 ± 1) | (63 ± 1) | (55 ± 1) | (44 ± 1) | (24 ± 3) | (42 ± 3) | (33 ± 4) | (20 ± 2) |
The effect of conjugation removal from Library B is quite evident, with a significant reduction in growth inhibition across all compounds and cell lines examined, supporting our hypothesis. The unconjugated analogues 7–9 exhibited a three fold potency reduction when compared with their conjugated counterparts, analogues 2–4, with average GI50 values of 80 (7), 47 (8) and 67 μM (9). A more significant potency reduction was noted for the dichloro analogue (10), with this analogue being essentially inactive with an average growth inhibition of 45% at 100 μM drug concentration. It is also of note, that none of these Library B analogues exhibit any degree of cell line specificity, again differing from their conjugated counterparts, which display breast carcinoma cell line specificity (MCF-7). This strongly suggests a role for conjugation from the phenyl ring through to the pyrrole moiety, i.e.conjugation that spans the entire analogue. To further evaluate the conjugation requirement we evaluated two additional focused libraries.
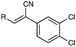
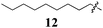
Within Library C we reintroduced the acrylonitrile moiety, but removed the Arom-1pyrrole ring, removing the potential for full extension of the conjugated pharmacophore from Arom-1 to Arom-2 (Fig. 1). Library C comprised two analogues retaining the active 3,4-dichlorophenyl pharmacophore from Library A, replacing Arom-1 with a simple alkyl chain (C4 and C9). Synthesis was effected as shown in Scheme 1, replacing pyrolle-2-carboxyaldehyde with pentanal and decanal. With both analogues a significant reduction in growth inhibition relative to 5 was observed (Table 3). The C9-12 was essentially inactive with minimal growth inhibition noted even at 100 μM drug concentration, whilst the C4-11 was two fold less potent than the lead, 5.
| Ar (compound) | HT29 a | SW480 a | MCF-7b | A2780c | H460 d | A431 e | DU145 f | BE2-C g | SJ-G2 h | MIAi |
|---|---|---|---|---|---|---|---|---|---|---|
| a HT29 and SW480 (colon carcinoma). b MCF-7 (breast carcinoma). c A2780 (ovarian carcinoma). d H460 (lung carcinoma). e A431 (skin carcinoma). f DU145 (prostate carcinoma). g BEC-2 (neuroblastoma). h SJ-G2 (glioblastoma). i MIA (pancreatic carcinoma). | ||||||||||
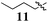
|
53 ± 0 | 68 ± 2 | 49 ± 2 | 24 ± 1 | 100 | 88 ± 1 | 87 ± 3 | 73 ± 14 | 70 ± 6 | 95 ± 4 |
|
(40 ± 3) | (53 ± 6) | (79 ± 4) | (72 ± 14) | (26 ± 5) | (29 ± 4) | (28 ± 12) | (48 ± 3) | (21 ± 1) | (22 ± 5) |
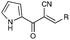
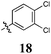
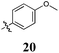
To confirm the pivotal nature of both Arom-1 and Arom-2 along with the nature of the conjugated system, we developed Library D in which the aromatic moieties were further separated by the introduction of a carbonyl spacer moiety (Scheme 2). Access to Library D was effected by treatment of pyrrole with cyanoacetic acid affording 3-oxo-3-(1H-pyrrol-2-yl)propanenitrile 13 in good yield. The required carbonyl spacer was introduced via a Knoevenagel condensation,8–13 with selected aldehydes (see Table 4 for details). These analogues, 14–20 were specifically designed such that the final products largely mirrored the analogues found in Libraries A–C.
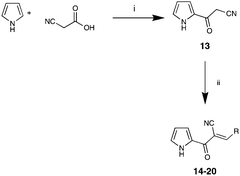 | ||
| Scheme 2 Reagents and conditions. (i) Ac2O, 35 min; (ii) RCHO (see table for details), piperidine (cat), EtOH reflux 2 h. | ||
| R1 | HT29 a | SW480 a | MCF-7b | A2780 c | H460 d | A431 e | DU145 f | BE2-C g | SJ-G2 h | MIAi |
|---|---|---|---|---|---|---|---|---|---|---|
| a HT29 and SW480 (colon carcinoma). b MCF-7 (breast carcinoma). c A2780 (ovarian carcinoma). d H460 (lung carcinoma). e A431 (skin carcinoma). f DU145 (prostate carcinoma). g BEC-2 (neuroblastoma). h SJ-G2 (glioblastoma). i MIA (pancreatic carcinoma). | ||||||||||
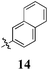
|
< 10 | < 10 | 12 ± 5 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 |
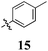
|
14 ± 3 | 14 ± 3 | 39 ± 6 | 19 ± 11 | 11 ± 4 | < 10 | < 10 | 11 ± 3 | 20 ± 4 | 27 ± 2 |
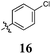
|
54 ± 9 | 68 ± 15 | 84 ± 17 | 75 ± 27 | 38 ± 5 | 31 ± 8 | 32 ± 10 | 63 ± 15 | 53 ± 9 | 59 ± 13 |
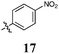
|
< 10 | < 10 | 19 ± 16 | 19 ± 16 | < 10 | < 10 | < 10 | 19 ± 5 | < 10 | < 10 |
|
< 10 | < 10 | 14 ± 2 | < 10 | < 10 | < 10 | < 10 | 14 ± 4 | 10 ± 4 | < 10 |

|
< 10 | < 10 | 15 ± 5 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | 11 ± 2 |
|
< 10 | < 10 | 22 ± 8 | < 10 | 24 ± 20 | 13 ± 8 | < 10 | < 10 | < 10 | < 10 |
As can be seen from the data presented in Table 4 relating to Library D, all analogues are essentially inactive, strongly suggesting the pivotal nature of the conjugated pharmacophore. Whilst the carbonyl spacer moiety does not directly block the conjugated system, it does allow for system elongation and the electron deficient nature of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O also perturbs the electron flow, disrupting the flow of electron density from Arom-1 to Arom-2. Regardless, it is clear that for activity no disruption of the conjugated system is permitted.
O also perturbs the electron flow, disrupting the flow of electron density from Arom-1 to Arom-2. Regardless, it is clear that for activity no disruption of the conjugated system is permitted.
Having established the pivotal nature of the Arom-1, Arom-2 and conjugated moieties we next turned our attention to modifications of Arom-1 with the synthesis of Library E. Library E retained the 3,4-dichlorophenyl moiety identified in Library A as the most active pharmacophore. Synthesis of Library E was as per Scheme 1, replacing pyrrole-2-carboxaldehyde with a family of aromatic aldehydes (see Scheme 3 and Table 5 for details).
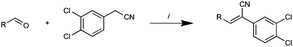 | ||
| Scheme 3 RCHO (see table for details), piperidine (cat), EtOH reflux 2 h. | ||
| Ar (compound) | HT29 a | SW480 a | MCF-7 b | A2780 c | H460 d | A431 e | DU145 f | BE2-C g | SJ-G2 h | MIAi |
|---|---|---|---|---|---|---|---|---|---|---|
| a HT29 and SW480 (colon carcinoma). b MCF-7 (breast carcinoma). c A2780 (ovarian carcinoma). d H460 (lung carcinoma). e A431 (skin carcinoma). f DU145 (prostate carcinoma). g BEC-2 (neuroblastoma). h SJ-G2 (glioblastoma). i MIA (pancreatic carcinoma). j Not soluble in testing medium. | ||||||||||
|
(56 ± 1) | (67 ± 1) | (79 ± 1) | (62 ± 2) | (36 ± 4) | (71 ± 1) | (36 ± 3) | (47 ± 12) | (72 ± 9) | (61 ± 3) |
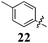
|
1.6 ± 0.09 | 2.3 ± 0.03 | 2.2 ± 0.1 | 2.0 ± 0.0 | 2.1 ± 0.1 | 1.8 ± 0.1 | 2.2 ± 0.2 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.1 |
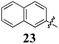
|
1.6 ± 0.2 | 2.3 ± 0.1 | 1.5 ± 0.2 | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.2 | 2.7 ± 0.4 | 1.9 ± 0.0 | 2.3 ± 0.0 | 2.1 ± 0.1 |
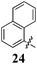
|
- j | - j | - j | - j | - j | - j | - j | - j | - j | - j |
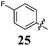
|
9.3 ± 2.4 | 5.6 ± 0.6 | 6.5 ± 1.0 | 9.5 ± 1 | 10 ± 0 | 10 ± 0 | 18 ± 2 | 13 ± 2 | 16 ± 1 | 11 ± 1 |
|
2.5 ± 0.3 | 5.0 ± 1.4 | 4.3 ± 0.9 | 4.0 ± 0.6 | 4.8 ± 1.0 | 4.3 ± 0.6 | 12 ± 4 | 4.1 ± 0.8 | 6.1 ± 0.6 | 4.0 ± 0.6 |
|
20 ± 1 | 22 ± 1 | 16 ± 0 | 20 ± 1 | 21 ± 2.0 | 20 ± 0 | 26 ± 2 | 21 ± 1 | 22 ± 1 | 21 ± 1 |
|
3.1 ± 1.8 | 8.4 ± 3.3 | 0.127 ± 0.043 | 12 ± 1 | 69 ± 2 | 7.1 ± 0.1 | 18 ± 3 | 8.9 ± 0.7 | 14 ± 1 | 27 ± 4 |
|
8.4 ± 0.2 | 8.9 ± 0.5 | 7.2 ± 0.2 | 11 ± 1 | 40 ± 6 | 16 ± 3 | 55 ± 7 | 12 ± 2 | 12 ± 1 | 15 ± 1 |
|
30 ± 3 | 36 ± 5 | 23 ± 3 | 20 ± 1 | 26 ± 2 | 21 ± 1 | 45 ± 11 | 22 ± 1 | 21 ± 2 | 28 ± 2 |

|
0.52 ± 0.05 | 1.4 ± 0.1 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0 | 0.6 ± 0.1 | 1.4 ± 0.1 | 2.7 ± 2.0 | 1.5 ± 0.2 | 0.7 ± 0.0 |
|
18 ± 1 | 27 ± 1 | 25 ± 1 | 18 ± 1 | 33 ± 3 | 25 ± 1 | 47 ± 11 | 22 ± 1 | 21 ± 2 | 38 ± 7 |
|
18 ± 1 | 18 ± 2 | 15 ± 1 | 18 ± 1 | 9.4 ± 1.6 | 26 ± 1 | 24 ± 1 | 16 ± 3 | 22 ± 1 | 22 ± 2 |
|
—j | —j | —j | —j | —j | —j | —j | —j | —j | —j |
|
>100 | 90 ± 6 | 28 ± 14 | 93 ± 3 | >100 | 88 ± 6 | >100 | >100 | 95.0 | >100 |
Library E analogues were evaluated for their growth inhibition capabilities against ten human cancer cell lines, these data are shown in Table 5.
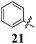
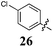
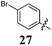
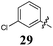
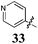
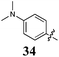
Replacement of the pyrrole Arom-1 moiety with a simple phenyl ring results in 21, an analogue which displayed only modest growth inhibition at 100 μM drug concentrations. The equivalent 4-methylphenyl, 22, and the 2-naphthyl, 23, analogue are both a highly potent broad spectrum agents returning GI50 values in the 1–2.3 μM range against all the cancer cell lines examined. This suggests the presence of a hydrophobic binding pocket. Introduction of a halogen to the phenyl ring of 21 afforded 4-F (25), 4-Cl (26) and 4-Br (27) (GI50s 3–26 μM) with increased growth inhibition relative to 21 but a decrease relative to 22 and 23. In this series the 4-Br 27 was least potent (GI50s 16–26 μM) with the 4-Cl 26 the most potent (GI50s 3–12 μM). This was clearly an effect of increasing electronegativity at this point of the molecule, which suggested that the introduction of a 4-F substituent would show an increase in cytotoxicity. However, the 4-F 25 displayed a slight reduction in potency. The potency reduction with 25 (GI50s 5.6–18 μM) was most probably due to the dual electron withdrawing and inductive effects known to be associated with F substituents. The 3-Cl analogue 29 was significantly less potent than the corresponding 4-Cl 26 with GI50s 7–55 μM, showing a preference for these moieties to be in the 4-position. Collectively, both the nature and positioning of the electron-withdrawing moiety is crucial for good levels of growth inhibition. While Library E showed an overall increase in growth inhibition across all cell types, selectivity towards and against specific tumour types was also apparent. In this regard, analogue 29 displayed good activity against all cell lines except H460 (lung carcinoma) and DU145 (prostate carcinoma) cell lines with GI50 values of 40 ± 6 and 55 ± 7 μM respectively. This level of activity, against H460 and DU145, is five times lower than against the other cell lines examined.
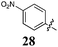
Furthermore, the introduction of the 4-NO2 with 28 had the simple effect of increasing potency against MCF-7 carcinoma cells whilst effecting a reduction in potency against the other cancer cell lines. Analogue 28 displayed an impressive level of potency and selectivity for the breast carcinoma cell line, MCF-7 with a GI50 = 0.127 ± 0.043 μM, which is 543 times more potent against the H460 (GI50 = 69 ± 2 μM) lung carcinoma cell line and 25 times more potent against the next best examined (HT29; GI50 = 3 ± 1.8 μM).
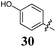
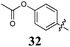
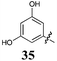
Of the other variations in the Arom-1 moiety examined, only the introduction of a 4-OCH3 moiety, 31, had any noteworthy impact on growth inhibition. The 4-OCH3 analogue (31) is the most potent broad spectrum analogue generated in this study with the majority of the GI50 values being at or below 1 μM. Interestingly neither the free 4-OH (30) nor the ethyl ester (32) showed any noteworthy inhibition or selectivity suggesting that the potency enhancements were a combination of the presence of the oxygen lone pairs of electrons and the additional steric component afforded by the introduction of a methyl group.
Returning to analogues 5 and 28 we were intrigued with the level of specificity of these analogues with the inhibition of the MCF-7 breast carcinoma cell line. As this cell line is known to be estrogen dependent and over expresses the estrogen receptor (ER+ve) we chose to examine the effect of 5 and 28 on one ER negative (ER−ve) breast carcinoma cell line (MDA-MB231) together with one normal breast cell line (MCF10A) that was derived from non-malignant breast epithelial tissue. The growth inhibition results presented in Table 6 clearly show that 5 and 28 are up to 268 fold more potent in the estrogen dependent MCF-7 cells (ER+ve) compared with the estrogen independent carcinoma cell line MDA-MB-231 (ER−ve) and up to 126 fold more potent in MCF-7 cells than in the non-tumourigenic breast epithelial cell line MCF10A. The ability to specifically target estrogen dependent tumour cells, while having little or no effect on normal breast cells, or on cells derived from other tumour types is a significant and unique finding of this study.
Conclusions
Our efforts to minimize progression of highly toxic compounds into our anti-parasitic drug development program led to the synthesis of a series of known (Z)-2-phenyl-3-(1H-pyrrol-2-yl)acrylonitrile derivatives (Fig. 1, 1–5). To our surprise these analogues displayed high levels of growth inhibition against our panel of ten human cancer cell lines. Subsequent focused library development identified Arom-1 to Arom-2conjugation as important for activity, with efforts to modify this feature having a significant effect on the levels of growth inhibition observed. The requirement for both aromatic rings was confirmed on examination of the aliphatic analogues 11 (GI50 = 24–100 μM) and 12 (inactive). The parent pyrrole moiety as Arom-1 was not required to maintain good levels of growth inhibition with activity increased by the introduction of simple substituted benzene analogues. Of particular note was the introduction of a 4-OCH3 substituent in analogue 31 which afforded the most active broad spectrum analogue with an average GI50 = 1.6 μM, and modest selectivity for the HT29 human colon carcinoma cell line (GI50 = 0.52 ± 0.05 μM). More interestingly, the introduction of a 4-NO2 substituent with analogue 28 afforded up to 543-fold MCF-7 (human breast carcinoma) cell line specificity relative to cell lines derived from other tumour types. Examination of two additional human breast cell lines: MDA-MB-231 (ER−ve carcinoma) and MCF-10A (normal epithelial), demonstrated that 28 preferentially inhibited the growth of MCF-7 cells (268 and 126 fold respectively Although somewhat less potent, a similar preference was also noted for analogue 5. The retention of the 3,4-dichlorophenyl (Arom-2) moiety appears to be important for imparting the observed selectivity for breast cancer cell lines compared to the other nine cancer cell lines examined. The MCF-7 human breast carcinoma cell line is estrogen dependent while the MDA-MB-231 line is not suggesting that hormonal status may play a role in the improved efficacy of 28 and 5. The exact nature of this phenomenon is currently under investigation and will be reported in due course.Acknowledgements
MT thanks the University of Newcastle for the award of a PhD scholarship. This work was supported by the Australian Research Council.References
- B. E. Campbell, A. McCluskey and R. B. Gasser, Biotechnol. Adv., 2010 DOI:10.1016/j.biotechadv.2010.08.008.
- A. Ali, M. Bliese, J.-A. M. Rasmussen, R. M. Sargent, S. Saubern, D. G. Sawutz, J. S. Wilkie, D. A. Winkler, K. N. Winzenberg and R. C. J. Woodgate, Bioorg. Med. Chem. Lett., 2007, 17, 993–997 CrossRef CAS.
- G. Alberghina, M. E. Amato, F. A. Bottino, A. Corsaro and S. Fisichella, J. Heterocycl. Chem., 1986, 23, 1747–1752 CrossRef CAS.
- W. Herz and J. Brasch, J. Org. Chem., 1958, 23, 711–714 CrossRef CAS.
- K. N. Winzenberg, S. Saubern and D. G. Sawutz; PCT Int. Appl. WO 06/055565, 2006.
- A. M. Bergman, V. W. Ruiz van Haperen, G. Veerman, C. M. Kuiper and G. J. Peters, Clin Cancer Res., 1996, 2, 521–530 CAS.
- J. A. Sakoff and S. P. Ackland, Cancer Chemother. Pharmacol., 2000, 46, 477–487 CrossRef CAS.
- K. Wang, D. Dabin Kim and A. Dömling, J. Comb. Chem., 2010, 12, 111–118 CrossRef CAS.
- M. Radi, L. Botta, G. Casaluce, M. Bernardini and M. Botta, J. Comb. Chem., 2010, 12, 200–205 CrossRef CAS.
- A. McCluskey, P. J. Robinson, T. Hill, J. L. Scott and J. K. Edwards, Tetrahedron Lett., 2002, 43, 3117–3120 CrossRef CAS.
- T. Inokuchi and H. Kawafuchi, J. Org. Chem., 2006, 71, 947–953 CrossRef CAS.
- F. S. Prout, F. A. Abdel-Latif and M. R. Kamal, J. Chem. Eng. Data, 1963, 8, 597–599 CrossRef CAS.
- S. V. Ryabukhin, A. S. Plaskon, D. M. Volochnyuk, S. E. Pipko, A. N. Shivanyuk and A. A. Tolmachev, J. Comb. Chem., 2007, 9, 1073–1078 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section. See DOI: 10.1039/c0md00147c |
| This journal is © The Royal Society of Chemistry 2011 |