Enhancing the thermal stability of a single-chain Fv fragment by in vivo global fluorination of the proline residues†
Selvakumar
Edwardraja
a,
Sokalingam
Sriram
a,
Raghunathan
Govindan
a,
Nediljko
Budisa
b and
Sun-Gu
Lee
*a
aDept. of Chemical Engineering, Pusan National University, Busan, Republic of Korea. E-mail: sungulee@pusan.ac.kr; Fax: +82 51 512 8563; Tel: +82 51 510 3680
bDept. of Biocatalysis, Institute of Chemistry, Technical University of Berlin, Juni 124 Berlin, Germany. E-mail: budisa@biocat.tu-berlin.de; Fax: +49 30 314 79 651; Tel: +49 30 314 23 661
First published on 19th November 2010
Abstract
Single-chain Fv (scFv) format protein is a commonly used analytical tool for diagnostic and therapeutic applications. The usage of scFv antibody fragments in therapeutic applications has demonstrated that they need to have high thermostability. Many rational or irrational methods have been described erstwhile to engineer or improve the stability of scFv proteins by modifications of natural amino acid. Here we have demonstrated an alternate strategy to efficiently improve the thermostability of scFvs by non-canonical amino acid. Previously, fluoroprolines have been proven as a choice to tune the stability of many polypeptides and few globular proteins. Hence we exploited the usage of fluoroproline to enhance the thermal stability of scFv by replacing the natural proline on the framework regions of scFv that influence the folding or stability. To demonstrate our approach, a bacterial cytoplasmic foldable and humanized anti-c-Met scFv (hu-MscFv) was used. The hu-MscFv proline sites were successfully incorporated with (2S,4R)-4-fluoroproline without affecting its structure and function by the in vivo residue specific global replacement method which exploits bacterial auxotrophic system. The time-dependent temperature effect on the activity of fluorinated hu-MscFv revealed its increased stability at 40 °C along with improved half-life than the hu-MscFv with natural proline. Further model based structure analysis on hu-MscFv with fluoroproline indicated that the fluorine atoms were able to establish new favourable dipole interactions with neighbouring polar groups in their local micro environments that rationalizes its improved thermostability. Moreover the scFv sequence based statistical analysis strongly supports the fact that this method can be applied to any target scFv, since they contain high frequency conserved proline sites in their framework regions.
Introduction
ScFv is the most advantageous format of antibody fragments that complements the binding function of an intact antibody. In addition, the scFv proteins can be easily generated from existing, well-characterized monoclonal antibodies, which allows for their rapid design and characterization.1,2 They are applicable in therapies, diagnostics and so on, and need to be stable for their efficient usage.3 However, their stability is not so high compared to intact IgG molecules, which limits their applicability.4–6 In particular, insufficient thermal stability of scFvs often precludes their use as therapeutic or diagnostic agents.7ScFv is constructed by linking two variable domains of IgG, i.e.VH and VL. Each variable domain is composed of a highly conserved framework region (FR) that primarily affects the folding/stability, and a hyper-variable complementarity determining region (CDR) that plays a major a role in the binding activity.8,9 There have been lots of studies devoted to engineering the scFv proteins for stability improvement,9,10 which can be categorized into two ways. The first way is the rational11–15 or irrational engineering16–20 of FR sequences in the target scFv. The second way of stabilization is the grafting of CDR sequences of the target scFv into stable FR sequences.21–23 Indeed both methods have been successfully applied to improve the biophysical stabilities of scFv and helped us overcome the problems caused by the poor stability of scFv.
Here we propose an alternative strategy for enhancing the stability of scFv proteins. The strategy is based on the approach that fluorinates proline residues of the target protein and the following features of the approach tempted us to test it for stabilizing scFv. First, fluorination of proline residues induces stereoelectronic effects that can affect the folding/stability of polypeptides. A number of reports have demonstrated that it is possible to tune or enhance the stability of polypeptide structures by exploiting the effect.24–26 For instance, hyperstable collagen could be synthesized by introducing fluoroproline into the sequence. Second, fluoroprolines such as (2S,4R)-4-fluoroproline ((4R)-FPro) and (2S,4S)-4-fluoroproline ((4S)-FPro) are efficiently recognized by the natural translation system of Escherichia coli. Thus we can synthesize recombinant protein with (4R)-FPro or (4S)-FProin vivo on a large scale.27,28 In fact, most studies on the fluoroproline effect have focused on peptide sequences rather than globular proteins partly due to the difficulty in fluorination of globular proteins. However, a recent report has clearly shown that (4R)-FPro and (4S)-FPro were very efficiently incorporated into the proline sites of recombinant protein by the residue specific substitution method which exploits auxotrophic strain.26 Moreover, the report demonstrated that folding efficiency of green fluorescent protein (GFP) could be tuned with the fluoroprolines, which specially encouraged us to apply the approach for scFv. Third, the in vivo residue specific substitution method using existing residues on the target protein allows us to engineer proteins without any modifications of primary sequences.29,30 Thus we can employ the approach not only to engineer protein with its original sequences but also to further modify the sequences engineered by traditional protein engineering methods.
The availability of proline sites in target proteins is important in order to apply the approach using fluoroprolines. We analyzed the proline profile of scFv protein sequences from the Kabat database,31 showing that approximately 90% of scFv sequences contain at least seven or more proline frequencies in their composition (Fig. 1). Further analyses on scFv sequences for proline distribution and conservation revealed the presence of 10 conserved proline sites, in which eight sites (VH9, VH14, VH41, VL8, VL15, VL40, VL44, VL59) were present in FR sequences and two sites (VH52a and VL95) were in CDR (Fig. S1, ESI†). These statistical analyses indicated the abundance of proline residues in FR that plays a critical role in the folding and stability of scFv, which favored our strategy as a fruitful one to be carried out.
 | ||
| Fig. 1 Statistical calculation of scFv sequences from Kabat database for proline frequencies. Plot shows the percentage of scFv sequences with their proline frequencies. Almost 90% of scFv sequences contain prolines with the frequencies of 7 or above indicated as black bars and fewer frequencies as gray bars. | ||
Scheme 1 outlines our strategy of single step stabilization of scFv proteins with fluoroprolines. To demonstrate it, an engineered anti-c-Met scFv (hu-MscFv)32 was employed as a target scFv sequence. The hu-MscFv recognizing c-Met, a receptor of hepatocyte growth factor/scattering factor, is expected to be used in clinical treatment or imaging of many cancer cells.33 Previously, we generated the hu-MscFv sequence by grafting the CDR of anti-c-Met scFv from rabbit into the consensus derived human FR (huFR) for the production in E. colicytoplasm as well as for humanization, and predicted its tertiary structure via homology modeling method.32 From the sequence and modeled structure of hu-MscFv, we could identify the distribution, cis/trans state, conservation, solvent accessibility and secondary structure of proline residues in the scFv protein (Table 1). The hu-MscFv sequence lacks proline residue in its CDR and contains a total of eight proline residues in its FR sequence, among which seven prolines are distributed in the conserved proline sites and only one proline exists at the non-conserved site. These properties of hu-MscFv made it as an ideal candidate to demonstrate our strategy. In this study, we introduced (4R)-FPro or (4S)-FPro into the target scFv, investigated the thermal stability of scFv with fluoroproline, and performed the structural analysis of the fluorinated protein.
 | ||
| Scheme 1 Schematic representation of the approach for stability improvement of scFv proteins with fluoroprolines. The proline conserved sites are indicated with the numerical values in parentheses for the relative frequency of proline on the VH and VL domains that were examined from the Kabat database (Fig. S1, ESI†). As a single step stabilization of scFv, the available conserved proline residues in the target scFvs can be easily replaced with fluoroprolines by residue specific substitution method in the cytoplasm of E. coli, to enhance the stability of the target scFv. | ||
| Chain | Kabat position | FR a/CDRb | Amino acid position | cis/trans isomerism | Evolutionary significance | Solvent accessibility | Secondary structure/motifsc |
|---|---|---|---|---|---|---|---|
| a FR: framework region. b CDR: complementary determining region. c Analysed by PDBSum Generate online tool. | |||||||
| Heavy | 14 | FR | 30 | trans | Conserved | Exposed | Loop/β turn |
| Heavy | 41 | FR | 57 | trans | Conserved | Exposed | Loop/β turn/β hairpins |
| Light | 8 | FR | 162 | cis | Conserved | Buried | Loop |
| Light | 15 | FR | 169 | trans | Conserved | Exposed | Loop/β turn |
| Light | 40 | FR | 195 | trans | Conserved | Exposed | Loop/β turn/β hairpins |
| Light | 44 | FR | 199 | trans | Conserved | Exposed | β strand |
| Light | 59 | FR | 218 | trans | Conserved | Exposed | Loop |
| Light | 80 | FR | 239 | trans | Non-conserved | Exposed | Helix |
Results
Expression and activity analysis of hu-MscFv with proline and fluoroprolines
Our previous study on expression systems for the production of proteins with unnatural modifications revealed that the E. coli native promoter based pQE expression system is more efficient than the T7 promoter based pET expression system.34 Hence we constructed pQE60 based expression system through PCR amplification and cloning of hu-MscFv gene from the pET24ma-hu-MscFv32 for the current study, which resulted in pQE60-T7-hu-MscFv with N-terminus T7-tag and 6×His-tag in its C-terminus (Fig. S2, ESI†). The pQE60-T7-hu-MscFv construct was transferred into the proline auxotroph E. coli strain BL21(DE3)pLysS (KC1325), which was used as a expression host for the incorporation of proline, 4S-FPro and 4R-FPro into the hu-MscFv in the cytoplasm of E. coli that produced NP-hu-MscFv, 4S-FP-hu-MscFv and 4R-FP-hu-MscFv respectively. Details are described in material and methods section.The total cell protein profile clearly showed that the expression levels of 4S-FP-hu-MscFv and 4R-FP-hu-MscFv were almost comparable to the expression level of scFv with natural proline NP-hu-MscFv (Fig. S3, ESI†). This result suggests that 4S-FPro and 4R-FPro are efficiently recognized by E. colitranslation system as previously reported.26 The expression profiles of soluble and insoluble fractions from each variant were analyzed by western blot (Fig. 2a). It showed that the folding level of hu-MscFv was significantly affected by the incorporated proline analogues. Almost 95% of the 4S-FP-hu-MscFv was not able to fold properly thereby found mostly in the insoluble fractions, which suggested that the incorporation of 4S-FPro into the hu-MscFv proline sites negatively affected the folding of the protein. On the other hand, the 4R-FP-hu-MscFv was favored to fold properly to the same level as NP-hu-MscFv even with the incorporation of 4R-FPro (Fig. 2a).
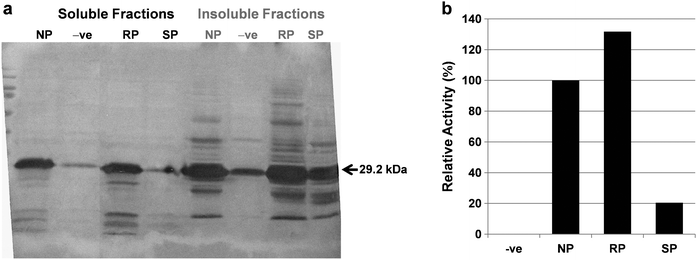 | ||
| Fig. 2 Expression and activity confirmation analysis of fluorinated hu-MscFvs. (a) Western blotting of soluble and insoluble fractions of BL21(DE3)pLysS (KC1325) for the NP-hu-MscFv, 4S-FP-hu-MscFv and 4R-FP-hu-MscFv. Lane NP, NP-hu-MscFv; lane −ve, negative control (hu-MscFv over expressed without proline or its analogue); lane RP, 4R-FP-hu-MscFv; lane SP, 4S-FP-hu-MscFv. (b) The relative activities from soluble fractions of BL21(DE3) pLysS(KC1325) for the NP-hu-MscFv, 4S-FP-hu-MscFv and 4R-FP-hu-MscFv were analyzed by ELISA. Lane −ve, negative control (hu-MscFv over expressed without proline or its analogue); lane NP, NP-hu-MscFv; lane RP, 4R-FP-hu-MscFv; lane SP, 4S-FP-hu-MscFv. The activity of NP-hu-MscFv was taken to be 100%. | ||
The binding activities of hu-MscFv variants were also examined using the soluble fractions of the respective variants. The analysis confirmed that the folded variant 4R-FP-hu-MscFv was active and not affected significantly by the replacement of prolines with 4R-FPro (Fig. 2b). As expected, there was no significant amount of activity signal from the soluble fraction of 4S-FP-hu-MscFv, which again ensured that the folded or active form of 4S-FP-hu-MscFv in the soluble fractions was very limited. These results indicated that the 4R-FPro had no negative influence on the folding and activity of hu-MscFv.
Confirmation of proline analogue incorporation
Both NP-hu-MscFv and 4R-FP-hu-MscFv proteins were expressed and purified from the cytoplasm of BL21(DE3)pLysS (KC1325). The purities of both proteins were checked by SDS-PAGE analysis and we confirmed that the purity level was >90% (Fig. S4, ESI†). The purified hu-MscFvs with natural proline or 4R-FPro were analyzed with randomly selected four different proline sites (VHPro8, VHPro40, VHPro44 and VHPro59) by LC-ESI-MS/MS (Fig. S5–S7, ESI†) to verify that 4R-FPro was incorporated into the proline sites of the hu-MscFv.The trypsin digested peptides containing proline sites (L.VLTQS![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) ATL.S/VHPro8, W.YQQK
ATL.S/VHPro8, W.YQQK![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) GQA
GQA![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) RYL.I/VHPro40 and VHPro44, and Y.TKGTGV
RYL.I/VHPro40 and VHPro44, and Y.TKGTGV![[P with combining low line]](https://www.rsc.org/images/entities/char_0050_0332.gif) ARF.S/VHPro59) were ionized and analyzed by ion-trap MS/MS. The hu-MscFv with 4R-FPro had most abundant three different fragment ions m/z (947.53022, 1484.76447 and 1051.57890) peptides. Similarly, the same three fragment ion peptides of NP-hu-MscFv with natural prolines were observed at m/z 929.53022, 1448.76447 and 1033.57890 respectively. The mass shift (+18, +36 and +18 Da) clearly indicated that 4R-FPro (133.12 Da) was incorporated instead of prolines (115.13 Da) in the selected four different proline sites of 4R-FP-hu-MscFv. The incorporation efficiency of 4R-FPro was estimated to be approximately more than 90% on the basis of the results of SDS-PAGE (Fig. S3, ESI†) and the HPLC-MS-MS, as already demonstrated in the previous reports.26,35
ARF.S/VHPro59) were ionized and analyzed by ion-trap MS/MS. The hu-MscFv with 4R-FPro had most abundant three different fragment ions m/z (947.53022, 1484.76447 and 1051.57890) peptides. Similarly, the same three fragment ion peptides of NP-hu-MscFv with natural prolines were observed at m/z 929.53022, 1448.76447 and 1033.57890 respectively. The mass shift (+18, +36 and +18 Da) clearly indicated that 4R-FPro (133.12 Da) was incorporated instead of prolines (115.13 Da) in the selected four different proline sites of 4R-FP-hu-MscFv. The incorporation efficiency of 4R-FPro was estimated to be approximately more than 90% on the basis of the results of SDS-PAGE (Fig. S3, ESI†) and the HPLC-MS-MS, as already demonstrated in the previous reports.26,35
Specific activity analysis
The purified scFvs were also analyzed using ELISA to compare their specific binding activities (Fig. 3). The result showed that the specific binding activity of NP-hu-MscFv was retained in the 4R-FP-hu-MscFv. Although the generated 4R-FP-hu-MscFv may not be completely homogeneous due to the limitation of residue-specific incorporation methods,27 the results suggest that the multiple site incorporation of 4R-FPro may support proper native fold hu-MscFv without significantly affecting its activity. | ||
| Fig. 3 Specific activity analysis of hu-MscFv with natural proline or its analogues. The relative specific activities of purified NP-hu-MscFv (hu-MscFv Proline) and 4R-FP-hu-MscFv (hu-MscFv 4R-FPro) were determined by ELISA assay against cMet. The specific activity of NP-hu-MscFv was taken to be 100%. Values are the average of duplicate experiments and represented as mean ± standard deviation. | ||
Thermostability of 4R-FP-hu-MscFv
To examine the effect of 4R-FPro presence in the proline sites of hu-MscFv on the thermostability of the protein, we first compared the thermostability of 4R-FP-hu-MscFv with that of NP-hu-MscFv at various temperatures. The proteins were incubated at different temperatures from 30 °C to 90 °C for 30 minutes and their binding activities were evaluated. Fig. 4 clearly showed that there was a significant difference in the activity between NP-hu-MscFv and 4R-FP-hu-MscFv at 40 °C. We could observe that both proteins were completely inactivated at 50 °C and higher temperatures. The improved thermostability of 4R-FP-hu-MscFv at 40 °C was further confirmed by measuring its time-dependent stability (Fig. 5). The estimated half life (T1/2) for NP-hu-MscFv and 4R-FP-hu-MscFv was 59 and 240 minutes respectively. These results indicate that the replacement of proline residues in hu-MscFv with 4R-FPro can improve the thermostability of scFv, although the effective range is limited to the temperatures less than 50 °C. | ||
| Fig. 4 Effect of temperature on the stability of hu-MscFv with fluoroproline. Percentage of relative binding activities of NP-hu-MscFv (N-Pro) and 4R-FP-hu-MscFv (R-Pro) were determined against c-Met by ELISA after incubating the samples in different temperatures (30, 40, 50, 60, 70, 80 and 90 °C) for 30 min. The binding activity of control (without any incubation) was taken to be 100%. | ||
 | ||
| Fig. 5 Time-dependent activity profile of hu-MscFvs. Percentage of relative binding activities of NP-hu-MscFv (N-Pro) and 4R-FP-hu-MscFv (R-Pro) were determined against cMet by ELISA after incubating the samples at 40 °C for different time durations (30, 45, 60 and 120 min). The binding activity of control (without any incubation) was taken to be 100%. Each value represents the mean of triplicates. Values are the average of triplicate experiments and represented as mean ± standard deviation. | ||
Modeling of anti-c-Met scFv with 4R-FPro and dipole interaction analysis
It has been shown with X-ray crystal structures that the fluorine atom in fluoroprolines is able to establish new interactions with the atoms of the nearby amino acids in the tertiary conformation.26 It was also described that the replacement of H with F atom in proline residues provides large dipole moments to the pyrrolidine rings because of the highly polar C–F bonds. This may promote strong dipole interactions in the local environments with polar groups such as amides, hydroxy or carbonyl groups. Hence we believed that the incorporated 4R-FPro would favor the formation of new interactions with other neighboring amino acids that may result in their improved thermal stability.To investigate the above point, the model structure of the 4R-FP-hu-MscFv was generated from the previously modeled hu-MscFv.32 The structural replacement of hydrogen atom into fluorine atom in the Cγ-(C4) position of proline residues was successfully done with the Discovery Studio 2.0 (Accelrys Inc., San Diego, CA, USA). It is always necessary to have energy minimization for the relaxation of structure after any modification. Hence the modified structure was prepared and energy was minimized as described in the Materials and Methods section. The energy minimization cycles were done with Steepest descent and followed by Conjugate gradient method, and both exited by satisfying the RMS gradient tolerance less than 0.1 kcal mol−1 Å−1, which indicated the structure attained a local minimum conformation. This energy minimized structure was further used for structural analysis (Fig. S8, ESI†).
The structural analysis for the identification of dipole interactions between the fluorine atom of 4R-FPro and the other polar groups (amide, hydroxyl or carbonyl) in the modeled structure of 4R-FP-hu-MscFv revealed the establishment of ten new interactions (Fig. 6). Among the identified ten interactions three show stereoelectronic repulsive position, in which two repulsions may be considered to be nullified by the favorable interactions of the neighbor atoms and remaining seven interactions are located in the distance to show favorable interactions with their local environment polar groups. These results indicate that the newly generated dipole interactions may induce the enhancement of the thermal stability.
 | ||
| Fig. 6 Calculated dipole interactions in the modelled structure of 4R-FP-hu-MscFv. Fluorines are cyan, the new interactions are shown in green line except three repulsive interactions, which are indicated in black line. Four interactions are found in fluorinated VH domain: (a) VH(4R)FPro14 interacts favourably with the backbone –NH– of both VHSer113 (3.95 Å) and VHAla84 (3.84 Å) but with backbone –CO– of VHVal111 (3.90 Å) sterically located as repulsive, which may be nullified with the other two favourable interactions on the neighbouring residues. (b) The VH(4R)FPro41 has the most closer interaction with the –OH– group of VHThr87 (2.78 Å). Remaining six interactions were found in the fluorinated VL domain. (c) The VL(4R)FPro8 shows strong repulsive interaction with the backbone carbonyl of VLThr10 (3.31 Å). (d) The 4R-FPro of VL(4R)FPro44 has two favourable interactions with the side chain –NH– groups (E22: 3.60 Å and E21: 3.71 Å) and one repulsive steric contact with the side chain –CO(E1)– group of VLGln38 (3.21 Å), which may be nullified with other two favourable interactions. (e) The final two favourable interactions are identified between the VL(4R)FPro59 and the side chain –NH(H12)– and –NH(H22)– groups of VLArg61 in the measured distance of 3.26 Å and 3.21 Å respectively. Sums of 10 interactions are newly created by the fluorine atoms in 4R-FP-hu-MscFv that are absent in NP-hu-MscFv. | ||
Discussion
In this paper, we have proposed a simple approach of fluoroproline based engineering for the thermal stability improvement of scFv and demonstrated it with hu-MscFv. To the best of our knowledge, this study is the first that demonstrated the stability improvement of scFvviaprotein engineering method using a non-canonical amino acid. The approach, i.e.fluorination of proline residues in FR of scFv, may be applicable to other scFv proteins because the sequences and structures of FR are highly conserved.13 The level of proline frequency, conservation and distribution also greatly supports the possibility and generality of the approach without restricting to a specific group of scFvs.22 There are still some drawbacks in the approach proposed here. For example, proline auxotroph is required to produce the fluorinated scFvs, which may limit the usage of various hosts for the preparation of such unnatural scFvs. Another problem that we met in our study was the precipitation of the fluorinated scFv at high concentration presumably due to the increased hydrophobicity of scFv surface modified by fluoroproline. Despite such limitations, we expect that the approach which does not require the modification of primary sequences of target protein can be compatibly and combinatorially used with the traditional scFv engineering methods. We also expect that, in addition to the previous study on GFP,26 this successful example may further stimulate the studies on the global fluorination of proline residues of globular proteins.Proline is a unique amino acid due to the existence of cis/transpeptidyl proline isomeric conformations on protein structures, which plays a key role as a rate limiting element for protein folding.36,37 Especially it was found to have correlation with the puckering effect in its pyrrolidine ring conformations as Cγ-exo or Cγ-endo.38,39 Previously these properties were structurally tuned with 4R-FPro and 4S-FPro that favor the Cγ-exo puckering/trans conformation and Cγ-endo puckering/cis transformation, respectively.35 These stereoelectronic effects have generally influence on the folding rate of protein depending on the peptidyl proline isomeric conformations on the protein structure. We could also observe this effect in our study. As shown in Fig. 2, only 4R-FPro favored the folding of hu-MscFv in vivo and 4S-FPro substantially disfavored it, which may be related to the isomeric conformation on hu-MscFv. According to our modeled structure of hu-MscFv, there are seven trans-prolines and only one cis-proline site which may prefer 4R-FPro and 4S-FPro, respectively. Therefore the folding of hu-MscFv with 4R-FPro might have a negative influence on the VL8 cis-proline site, but the in vivo soluble expression levels of scFv with 4R-FPro or natural proline were not different. This result indicates that the cis-proline residue in hu-MscFv may not be so sensitive to the stereoelectronic effect caused by incorporation of fluoroproline, which can be supported by the previous report showing that the VL8 proline site is insensitive to mutation.40 On the other hand, the negative effect of 4S-FPro incorporation on the folding of hu-MscFv suggests that there might be very sensitive sites in the seven trans-proline residues against the stereoelectronic effect.
We attempted the analysis of dipole interactions to understand the enhanced thermal stability of scFv, which allowed us to identify the newly formed seven favorable dipole interactions from the replaced fluorine atom in 4R-FPro. In fact, there would be other intramolecular interactions that can be newly generated from the introduced 4R-FPro and have influence on the stabilization of hu-MscFv. For example, the fluorination of the buried proline residues may increase the hydrophobic interactions in the protein core, which can synergetically stabilize the protein with dipole interactions.40 However, we presume that the newly formed dipole interactions are the major source for the stabilization of hu-MscFv rather than the hydrophobic effect because seven residues are surface-exposed and only one is buried among the eight proline residues in the protein (Table 1).
Experimental
Materials
PCR reagents, T4 DNA ligase and restriction endonucleases were purchased from Promega (Madison, WI, USA). The vector pQE60L and pREP4 plasmid were purchased from Qiagen. The host bacterium E. coli strain XL1-blue (Stratagene, CA, USA) was used for plasmid DNA preparation in this study. The proline auxotroph E. coli BL21(DE3)pLysS (KC1325) strain, kindly provided by Prof. Laszlo N. Csonka, was used as the host for the expression of hu-MscFv. E. colicells with plasmids were grown aerobically in Luria-Bertani (LB) broth (Difco Laboratories, Detroit, Michigan, USA) or on LB agar plate, supplemented with appropriate antibiotics for the selection of transformants. Natural amino acids were obtained from Sigma (St. Louis, MO, USA) and 4R-FPro and 4S-FPro synthetic amino acids purchased from Bachem AG (Bubendorf, Switzerland). The isopropyl-D-thiogalactopyranoside (IPTG) was purchased from Sigma chemicals (St. Louis, MO, USA). The nickel-nitrilo-triacetic acid (Ni–NTA) affinity column was purchased from Qiagen (Valencia, CA, USA). The ready-to-use HRP substrate containing 3,3′,5,5′-tetramethylbenzidine (TMB) was purchased from Sigma.Construction of plasmids and strains
The anti-c-Met scFv gene for pQE60 (pQE60-T7-hu-MscFv) was constructed by cloning the gene amplified by two primers (5′-CCATGGCTAGCATGACTGGTGGACAGCAAATG-3′ and 5′-AGATCTTTATTAATGGTGATGATGGTGGTGACGTTTGATCT-3′) using pET24ma-hu-MscFv as template, into the pQE60-L with NcoI and BglII restriction enzymes. The T7 tag sequence was added to the N-terminal of the scFv gene and the expressed scFv antibodies could be detected by a HRP-labeled anti-T7 tag monoclonal antibody in Western blotting or enzyme linked immunosorbent assay (ELISA). We fused hexahistidine at the C-terminus of the scFv sequence to purify the fragment antibodies simply through a Ni–NTA column.Expression of hu-MscFv with proline and its analogues
The constructed pQE60-T7-hu-MscFv was transformed into E. coli BL21(DE3)pLysS (KC1325), for the production of recombinant natural and unnatural recombinant hu-MscFv. pREP4 plasmid bearing lacIgene was also co-transformed to further facilitate the tight control of protein expression. The M9 minimal medium was supplemented with 0.4 (w/v) of glucose, 0.1 mM of CaCl2, 1.0 mM of MgSO4, 35 μg mL−1 of thiamine and 19 amino acids (0.35 mM) and used as a stock. The cells were grown in the prepared M9 minimal medium stock with the addition of 0.05 mM natural proline, until its depletion from the culture in the mid-log growth phase (OD600 0.5–0.8) as described elsewhere.41Briefly 1 mM of natural proline, 4R-FPro, and 4S-FPro were added into separate culture flasks and immediately induced with 0.05 mM IPTG for the cytoplasmic expressions of NP-hu-MscFv, 4R-FP-hu-MscFv, and 4S-FP-hu-MscFv respectively, for 5 h at 25 °C. Finally the OD600 was measured and cells were harvested by centrifugation for 10 min (5000 rpm) at 4 °C and stored at −70 °C until use. Lysis of the cells was carried out by using BugBuster protein extraction kit (Novagen). Briefly, collected cell pellet corresponding to 1 mL of 1 OD cells were resuspended in 100 μL of lysis buffer, incubated at room temperature for 20 min, and centrifuged for 45 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C. The supernatant was saved as a soluble fraction, and the pellet was resuspended in 8 M urea for insoluble fraction. The amount of the soluble fraction as well as insoluble fraction was analyzed by SDS-PAGE (12% acrylamide gel) and Western blotting as described previously.32
000 rpm at 4 °C. The supernatant was saved as a soluble fraction, and the pellet was resuspended in 8 M urea for insoluble fraction. The amount of the soluble fraction as well as insoluble fraction was analyzed by SDS-PAGE (12% acrylamide gel) and Western blotting as described previously.32
Protein purification
To purify the over-expressed NP-hu-MscFv and 4R-FP-hu-MscFv proteins from the soluble fraction of cell extracts, they were incubated with 5 mg Ni–NTA HisBind Resin (Novagen) for 3 h at 4 °C. The resin was loaded into column, washed with 2 × 4 mL washing buffer (50 mM phosphate buffer, pH 8.0, 300 mM NaCl, and 10 mM imidazole) and the target protein was eluted with 1 mL elution buffer (50 mM phosphate buffer, pH 8.0, 300 mM NaCl, and 250 mM imidazole). The imidazole was removed by diafiltration. Concentrations of purified protein samples were determined using a UV/VIS spectrophotometer, at 280 nm with calculated extinction coefficient of 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 M−1 cm−1 from the hu-MscFv sequences for both scFvs.
580 M−1 cm−1 from the hu-MscFv sequences for both scFvs.
LC-MS and LC-MS/MS analyses
The purified and concentrated samples of hu-MscFv expressed with natural proline or 4R-FPro were loaded into the SDS-PAGE and the protein bands were excised and used for nano-LC-ESI-MS/MS analysis as previously described.42 The coomassie stained protein bands were excised and destained using a solution made of 25 mM ammonium bicarbonate in 50 mM acetonitrile. The dried gel piece was digested overnight using trypsin and GluC followed by desalting on a C18-based zip-tip (Millipore, Billerica, MA). The eluted peptide solution was dried, re-dissolved in aqueous solution with 0.1 (v/v) formic acid, and injected into the nano-LC-MS/MS, which is a linear ion-trap MS, LTQ (Thermo Fisher, San Jose, CA) combined with nano-flow LC (Dionex, Sunnyvale, CA). The data from mass spectrometry were analyzed using the SEQUEST program.Analysis of 4R-FP-hu-MscFv binding activity
To confirm and quantify the binding activity of 4R-FP-hu-MscFv against c-Met, enzyme-linked immunosorbent assay (ELISA) was performed as described previously.32 Briefly, 10 μg of soluble fraction of cell extracts for confirmation or the purified and diluted to 10 ng of scFv proteins for quantification were added to the plate coated with c-Met and incubated at room temperature for 1 h. After washing with PBS containing 0.05 Tween 20 (pH 7.2), HRP-conjugated anti His-tag antibody (Roche) was added to the plate and incubated at room temperature for 1 h. After washing, bound fusion proteins were detected with ABTS. The binding activity of scFv was determined by subtracting the A405 nm of background binding to ovalbumin from the values obtained against c-Met. The obtained A405 values by using purified proteins were used to calculate specific activity.Thermostability of 4R-FP-hu-MscFv
To investigate the thermal stability of hu-MscFvs, the scFv protein samples were incubated at different temperatures, ranging from 30 to 90 °C for 30 min. To determine the half life time (T1/2) for hu-MscFvs activity, samples were incubated for different time durations at constant temperature of 40 °C. After incubation the protein samples were chilled on ice, and then their residual activity was determined by enzyme-linked immunosorbent assay (ELISA) against c-Met as described above. The binding activity of scFv was determined by subtracting the A405 nm of background binding to ovalbumin from the values obtained against c-Met.Modeling of hu-MscFv with 4R-FPro
The modeled structure of the variable domains of hu-MscFv was readily available from our previous study of hu-MscFv,32 which was modeled using templates with high sequence identity and structural resolution. The quality of the model was also checked with different evaluation methods and confirmed for its high reliability. Hence the same model was used for the structural replacement of H into F atom in the Cγ-(C4) position of proline residues. The modifications were carried out using the Discovery Studio 2.0 (Accelrys Inc., San Diego, CA, USA). The modified structure was prepared for energy minimization by typing with automatic assignment of Momany & Rone CHARMm force field. Steepest descent method that is usually preferred for relaxation of small changes in the structure was used for initial minimization and followed by Conjugate gradient method for better convergence of structure to attain local minimum. In the solvent conditions, to specify the use of a distance-dependent dielectric constant the Implicit Solvent Model parameter value was changed to Implicit Distance-Dependent Dielectrics and also the Solvent Dielectric Constant value was set as 4 to mimic at a crude level the screening effects of solvent. All the other parameter values were set to default as recommended in the software. The energy minimization cycles were carried out until the RMS gradient tolerance was satisfied as less than 0.1 kcal mol−1 Å−1.Analysis of dipole interactions
The Discovery Studio 2.0 (Accelrys Inc., San Diego, CA, USA) was used for the identification of dipole interaction details between the Fluorine atom of 4R-FPro residues and the nearby other polar groups (amide, hydroxyl or carbonyl) in the modeled structure of 4R-FP-hu-MscFv. To identify the interactions the calculations were done within 0.4 nm distance from the Fluorine atoms to other polar groups.Nomenclature
The residue numbering proposed by Kabat et al.31 was adopted throughout this article.Acknowledgements
This research was supported by the Basic Science Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0017001).Notes and references
- E. Furrer, M. Berdugo, C. Stella, F. Behar-Cohen, R. Gurny, U. Feige, P. Lichtlen and D. M. Urech, Invest. Ophthalmol. Visual Sci., 2009, 50, 771–778.
- M. Ottiger, M. A. Thiel, U. Feige, P. Lichtlen and D. M. Urech, Invest. Ophthalmol. Visual Sci., 2009, 50, 779–786.
- L. Presta, Curr. Opin. Struct. Biol., 2003, 13, 519–525 CrossRef CAS.
- S. Ewert, T. Huber, A. Honegger and A. Pluckthun, J. Mol. Biol., 2003, 325, 531–553 CrossRef CAS.
- E. Garber and S. J. Demarest, Biochem. Biophys. Res. Commun., 2007, 355, 751–757 CrossRef CAS.
- D. Rothlisberger, A. Honegger and A. Pluckthun, J. Mol. Biol., 2005, 347, 773–789 CrossRef.
- J. Willuda, A. Honegger, R. Waibel, P. A. Schubiger, R. Stahel, U. Zangemeister-Wittke and A. Pluckthun, Cancer Res., 1999, 59, 5758–5767 CAS.
- A. Honegger, Therapeutic Antibodies, Springer, Berlin, Heidelberg, 2008, vol. 181, pp. 47–68 Search PubMed.
- A. Worn and A. Pluckthun, J. Mol. Biol., 2001, 305, 989–1010 CrossRef CAS.
- S. Ewert, A. Honegger and A. Pluckthun, Methods, 2004, 34, 184–199 CrossRef CAS.
- S. Ewert, A. Honegger and A. Pluckthun, Biochemistry, 2003, 42, 1517–1528 CrossRef CAS.
- E. Monsellier and H. Bedouelle, J. Mol. Biol., 2006, 362, 580–593 CrossRef CAS.
- B. Steipe, Methods Enzymol., 2004, 388, 176–186 CAS.
- B. Steipe, B. Schiller, A. Pluckthun and S. Steinbacher, J. Mol. Biol., 1994, 240, 188–192 CrossRef CAS.
- A. Worn and A. Pluckthun, Biochemistry, 1998, 37, 13120–13127 CrossRef CAS.
- S. J. Demarest, G. Chen, B. E. Kimmel, D. Gustafson, J. Wu, J. Salbato, J. Poland, M. Elia, X. Tan, K. Wong, J. Short and G. Hansen, Protein Eng., Des. Sel., 2006, 19, 325–336 CrossRef CAS.
- L. Jermutus, A. Honegger, F. Schwesinger, J. Hanes and A. Pluckthun, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 75–80 CrossRef CAS.
- L. Jespers, O. Schon, K. Famm and G. Winter, Nat. Biotechnol., 2004, 22, 1161–1165 CrossRef CAS.
- S. Jung, A. Honegger and A. Pluckthun, J. Mol. Biol., 1999, 294, 163–180 CrossRef CAS.
- K. Proba, A. Worn, A. Honegger and A. Pluckthun, J. Mol. Biol., 1998, 275, 245–253 CrossRef CAS.
- L. Borras, T. Gunde, J. Tietz, U. Bauer, V. Hulmann-Cottier, J. P. Grimshaw and D. M. Urech, J. Biol. Chem., 2010, 285, 9054–9066 CrossRef CAS.
- A. Honegger, A. D. Malebranche, D. Rothlisberger and A. Pluckthun, Protein Eng., Des. Sel., 2009, 22, 121–134 CAS.
- M. Kugler, C. Stein, M. Schwenkert, D. Saul, L. Vockentanz, T. Huber, S. K. Wetzel, O. Scholz, A. Pluckthun, A. Honegger and G. H. Fey, Protein Eng., Des. Sel., 2009, 22, 135–147.
- S. K. Holmgren, L. E. Bretscher, K. M. Taylor and R. T. Raines, Chem. Biol., 1999, 6, 63–70 CrossRef CAS.
- S. K. Holmgren, K. M. Taylor, L. E. Bretscher and R. T. Raines, Nature, 1998, 392, 666–667 CrossRef CAS.
- T. Steiner, P. Hess, J. H. Bae, B. Wiltschi, L. Moroder and N. Budisa, PLoS One, 2008, 3, e1680 CrossRef.
- N. Budisa, Engineering the Genetic Code: Expanding the Amino Acid Repertoire for the Design of Novel Proteins, Wiley-VCH, Weinheim, 1st edn, 2006 Search PubMed.
- N. Voloshchuk and J. K. Montclare, Mol. BioSyst., 2010, 6, 65–80 RSC.
- N. Budisa, W. Wenger and B. Wiltschi, Mol. BioSyst., 2010, 6, 1630–1639 RSC.
- B. Wiltschi and N. Budisa, Appl. Microbiol. Biotechnol., 2007, 74, 739–753 CrossRef CAS.
- E. A. Kabat, T. T. Wu, H. Perry, K. Gottesman and C. Foeller, Sequences of Proteins of Immunological Interest, NIH Publication, Bethesda, MD, 5th edn, 1991, pp. 91–3242 Search PubMed.
- S. Edwardraja, R. Neelamegam, V. Ramadoss, S. Venkatesan and S. G. Lee, Biotechnol. Bioeng., 2010, 106, 367–375 CAS.
- G. Maulik, A. Shrikhande, T. Kijima, P. C. Ma, P. T. Morrison and R. Salgia, Cytokine Growth Factor Rev., 2002, 13, 41–59 CrossRef CAS.
- N. Ayyadurai, R. Neelamegam, S. Nagasundarapandian, S. Edwardraja, H. S. Park, S. J. Lee, T. H. Yoo, H. Yoon and S.-G. Lee, Biotechnol. Bioprocess Eng., 2009, 14, 257–265 Search PubMed.
- C. Renner, S. Alefelder, J. H. Bae, N. Budisa, R. Huber and L. Moroder, Angew. Chem., Int. Ed., 2001, 40, 923–925 CrossRef CAS.
- C. Dugave and L. Demange, Chem. Rev., 2003, 103, 2475–2532 CrossRef CAS.
- W. J. Wedemeyer, E. Welker and H. A. Scheraga, Biochemistry, 2002, 41, 14637–14644 CrossRef CAS.
- E. J. Milner-White, L. H. Bell and P. H. Maccallum, J. Mol. Biol., 1992, 228, 725–734 CrossRef.
- D. Pal and P. Chakrabarti, J. Mol. Biol., 1999, 294, 271–288 CrossRef CAS.
- M. Jager and A. Pluckthun, FEBS Lett., 1997, 418, 106–110 CrossRef CAS.
- N. Budisa, B. Steipe, P. Demange, C. Eckerskorn, J. Kellermann and R. Huber, Eur. J. Biochem., 1995, 230, 788–796 CAS.
- E. Selvakumar, N. Rameshkumar, S. G. Lee, S. J. Lee and H. S. Park, ChemBioChem, 2010, 11, 498–501 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Frequency profile, sequence information, total cell expression profile, purification, LC-ESI-MS/MS, model structure. See DOI: 10.1039/c0mb00154f |
| This journal is © The Royal Society of Chemistry 2011 |
