Using the Man9(GlcNAc)2–DC-SIGN pairing to probe specificity in photochemical immobilization†
Suzanne J.
Dilly
a,
Andrew J.
Clark
a,
Daniel A.
Mitchell
*b,
Andrew
Marsh
*a and
Paul C.
Taylor
*a
aDepartment of Chemistry, University of Warwick, Coventry CV4 7AL, UK. E-mail: a.marsh@warwick.ac.uk; p.c.taylor@warwick.ac.uk; Fax: +44 (0)24 7652 4112; Tel: +44 (0)24 7652 4565 Tel: +44 (0)24 7652 4375
bClinical Sciences Research Institute, Warwick Medical School, University of Warwick, Coventry CV2 2DX, UK. E-mail: d.mitchell@warwick.ac.uk; Tel: +44 (0)24 7696 8596
First published on 9th November 2010
Abstract
We demonstrate the expected preference of an immobilised oligosaccharide Man9(GlcNAc)2 upon a 96-well photochemical array, for its known receptor, the cell-surface lectin Dendritic Cell-Specific ICAM3 Grabbing Nonintegrin (DC-SIGN) when compared to immobilised competing monosaccharides.
Oligosaccharide surface display is used widely in medicinal chemistry discovery programmes,1,2 glycomic approaches to understanding function,3 and associated biophysical measurements. Display methods often presume that a particular regiochemical point of attachment will lead to a desired bioactive partnership, however this may not always be the case,4–6 potentially resulting in sub-optimal activity. In particular, a limited range of surface coupling chemistries are often utilized,7,8 which, compounded by the low availability of saccharide to be immobilized leads to real synthetic and biological challenges. Surface-mediated photoactivation strategies9,10 offer the possibility of more diverse immobilization, enabling greater exploration of ligand–receptor space than might be expected through a single functional group approach. The design of high-throughput assays for lectins has also been noted to be complicated by their tendency to bind weakly to monovalent carbohydrate ligands, hence methods for mimicking multivalency such as that found in the Man9GlcNAc2/DC-SIGN pairing.11,12 We here demonstrate that an array of photoactivatable chemistries 1–5, Fig. 1, in a standard 96-well format (Magic Tag®)13 allows rapid immobilisation of both mono- and oligosaccharides in a fashion that retains their anticipated biological activity. Recent work has shown the applicability of related surface-mediated photoactivation strategies14 towards similar goals.15,16
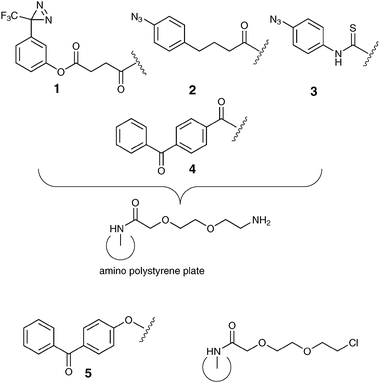 | ||
| Fig. 1 Magic Tag® photochemistries 1–5 on ethylene glycol linker derivatised polystyrene 96-well plates. | ||
DC-SIGN is a human lectin primarily expressed on the surface of dendritic cells, and is known to interact with host glycoproteins of the immune system such as ICAM-3via binding to selected glycans.17 In addition, it also binds to carbohydrate structures on the surfaces of pathogens including HIV-1 and Mycobacterium tuberculosis.18,19
Glycan array and radioligand competition assays have shown that soluble recombinant DC-SIGN binds selectively to branched N-linked high mannose oligosaccharides, notably Man9GlcNAc2, 7 (Fig. 2, a component of the gp120 envelope glycoprotein of HIV-1) and also to fucosylated oligosaccharides such as Lewis-X and Blood Groups A and B.20,21
In competition assays, monosaccharides such as mannose and fucose interact with DC-SIGN with KI values between 0.6 and 1.0 mM, but in the case of Man9GlcNAc2, the KI value is significantly lower at 16 μM, indicating higher affinity.21 Crystallographic studies have shown that this enhanced affinity for selected oligomeric glycan structures is supported by extended binding surfaces of the lectin domain, allowing for multiple sites of protein–oligosaccharide contact.22 However, our understanding of the extent of DC-SIGN ligand identity and mode of binding remains incomplete.
We have demonstrated that Magic Tag®, a chemical genomics tool developed in our laboratories13,23 can be applied to reveal potentially interesting interactions between small biologically active molecules and polypeptides from a phage displayed library representing a proteome. Of particular relevance is our earlier study in which we immobilised abscisic acid.13 Extension of our easy-to-use array of photochemistries including a diazirine141, two aryl azides242, 3 and two benzophenones244, 5 to explore binding events between saccharides and lectins is an appealing concept, but we had not previously validated Magic Tag® for this purpose. Herein, we demonstrate that the known specificities of DC-SIGN in solution are maintained towards three immobilized monosaccharides.
Results
Photochemical immobilization on surface tethered chemistries 1–5 together with Corning® photochemistry Universal-BIND™ of Man9GlcNAc2 (Fig. 2), the known ligand for DC-SIGN revealed that diazirine 1 gave the strongest response when challenged with biotin-conjugated DC-SIGN and subsequently exposed to fluorescein isothiocyanate labelled anti-biotin (Fig. 3). The same pattern of relative fluorescence was also seen when D-mannose, 6 (the ligand used in solid-phase to purify soluble recombinant DC-SIGN from bacterial cell extracts) was similarly immobilized and again exposed to DC-SIGN and streptavidin conjugates.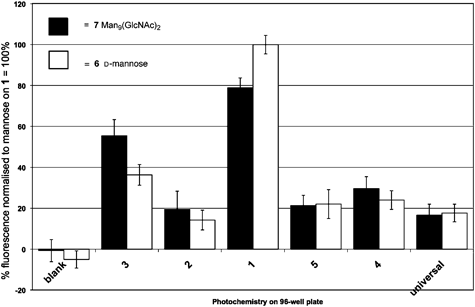 | ||
| Fig. 3 Response of derivatised surfaces 1–5, blank reference and Corning® Universal-BIND™ to biotin-conjugated DC-SIGN probed with fluorescein isothiocyanate labelled anti-biotin. | ||
In order to further explore the specificity of these novel readily prepared glyco-surfaces, two further monosaccharides, D-fucose and D-glucose (Fig. 4) were immobilized using the same array of photochemistries (Fig. 5). In turn, these surfaces gave lower fluorescent responses than D-mannose or the natural ligand, reflecting the known affinities of these monosaccharides (L-fucose 6.7 ± 0.5 mM, D-mannose KI = 13.1 ± 0.4 mM and D-glucose, KI = 23 ± 1 mM respectively).21 Note the difference in expected vs. our observed response for fucose may arise because we used D-fucose herein rather than L-fucose used in the earlier solution assay, both of which occur in nature.
 | ||
| Fig. 5 Comparison of D-glucose (front), D-fucose (middle) and D-mannose (back) immobilized on the photochemical array 1–5 and Corning® Universal-BIND™. | ||
Overall, the results demonstrate that Magic Tag® has significant potential in the lectin field. The successful rapid immobilization of a complex natural ligand for DC-SIGN Man9(GlcNAc)2 that subsequently gave a positive response in our assay is very pleasing.
A second important observation is that the specific nature of the interaction between Man9GlcNAc2 and DC-SIGN (KI = 16 μM)21 appears to have been maintained. Both D-glucose and D-fucose, which are known to have lower binding constants (vide supra) with respect to DC-SIGN, gave the expected weak responses in our assay. On the other hand, D-mannose, which is known to bind to DC-SIGN, gave a significant response, paralleling that of the ligand Man9(GlcNAc)2 (Fig. 3).
The data contain some interesting subtleties. Firstly, diazirine 1 shows greatest response to DC-SIGN when the known ligands Man9GlcNAc2, D-mannose and D-fucose are immobilized. 3-Trifluoromethyl-3H-phenyl diazirine has previously been found to be a good photocrosslinker for DNA, with markedly different reactivity from both aryl azides and benzophenones. Our observations, taken in conjunction with work to delineate surface vs. solution chemoselectivities25 indicate the diazirine moiety to be especially useful in saccharide C–H or O–H bond insertions. Secondly, the responses to Man9GlcNAc2 and to mannose are closer than might have been expected from literature binding competition data.21 This observation, while at first surprising, is in fact consistent with literature observations of surface density and avidity effects on immobilisation of saccharide ligands.12 In our case, it may be that when the monosaccharide mannose is immobilized, clusters of the sugar result that mimic to some extent the natural ligand, Man9GlcNAc2, or that receptor oligomerization may be arising at the glycosylated surface. Indeed, the interaction between DC-SIGN and mannan, a natural oligomer of mannose, has been observed by 1D saturation transfer difference NMR26 and surface-mediated DC-SIGN oligomerization itself has been postulated to play a key role in pathogen infectivity.27
Conclusions
The experiments described in this communication suggest that Magic Tag® photoimmobilisation is an efficient method for the capture of saccharides in order to probe their interactions with lectins. Molecular recognition of the immobilised sugars by DC-SIGN reproduced the known binding profile of this lectin, though mannose gave a surprisingly strong response, probably due to surface-mediated avidity effects. The use of a range of chemical functionalities in parallel enables rapid evaluation of conditions for photocapture and recognition of synthetically challenging small molecule ligands.Acknowledgements
We thank Professor Richard Napier and Dr Andrew J. Thompson (Warwick HRI) for helpful discussions, BBSRC & EPSRC ExGen programme (SJD, ref. 88/EGM17690) and BBSRC Follow-on Fund (SJD, ref. BB/C524338/1) for funding. DAM is a Research Councils UK Academic Fellow.Notes and references
- C. Y. Wu, P. H. Liang and C. H. Wong, Org. Biomol. Chem., 2009, 7, 2247–2254 RSC.
- Y. Liu, A. S. Palma and T. Feizi, Biol. Chem., 2009, 390, 647–656 CrossRef CAS.
- N. Laurent, J. Voglmeir and S. L. Flitsch, Chem. Commun., 2008, 4400–4412 RSC.
- S. Peddibhotla, Y. J. Dang, J. O. Liu and D. Romo, J. Am. Chem. Soc., 2007, 129, 12222–12231 CrossRef.
- J. J. Tate, J. Persinger and B. Bartholomew, Nucleic Acids Res., 1998, 26, 1421–1426 CrossRef CAS.
- M. Wiegand and T. K. Lindhorst, Eur. J. Org. Chem., 2006, 4841–4851 CrossRef CAS.
- E. Clo, O. Blixt and K. J. Jensen, Eur. J. Org. Chem., 2010, 540–554 CAS.
- M. Kohn, J. Pept. Sci., 2009, 15, 393–397 CrossRef.
- G. Carroll, D. Wang, N. Turro and J. Koberstein, Glycoconjugate J., 2008, 25, 5–10 CrossRef.
- D. Wang, G. T. Carroll, N. J. Turro, J. T. Koberstein, P. Kováč, R. Saksena, R. Adamo, L. A. Herzenberg and L. Steinman, Proteomics, 2007, 7, 180–184 CrossRef CAS.
- Y. M. Chabre and R. Roy, Curr. Top. Med. Chem., 2008, 8, 1237–1285 CrossRef CAS.
- N. Jayaraman, Chem. Soc. Rev., 2009, 38, 3463–3483 RSC.
- S. J. Dilly, M. J. Bell, A. J. Clark, A. Marsh, R. M. Napier, M. J. Sergeant, A. J. Thompson and P. C. Taylor, Chem. Commun., 2007, 2808–2810 RSC.
- N. Kanoh, S. Kumashiro, S. Simizu, Y. Kondoh, S. Hatakeyama, H. Tashiro and H. Osada, Angew. Chem., Int. Ed., 2003, 42, 5584–5587 CrossRef CAS.
- L. H. Liu, H. Dietsch, P. Schurtenberger and M. D. Yan, Bioconjugate Chem., 2009, 20, 1349–1355 CrossRef CAS.
- O. Norberg, L. Q. Deng, M. D. Yan and O. Ramstrom, Bioconjugate Chem., 2009, 20, 2364–2370 CrossRef CAS.
- T. B. H. Geijtenbeek, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk and C. G. Figdor, Cell (Cambridge, Mass.), 2000, 100, 575–585 CrossRef CAS.
- T. B. H. Geijtenbeek, D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, J. Middel, I. Cornelissen, H. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor and Y. van Kooyk, Cell (Cambridge, Mass.), 2000, 100, 587–597 CrossRef CAS.
- A. Tanne, B. Ma, F. Boudou, L. Tailleux, H. Botella, E. Badell, F. Levillain, M. E. Taylor, K. Drickamer, J. Nigou, K. M. Dobos, G. Puzo, D. Vestweber, M. K. Wild, M. Marcinko, P. Sobieszczuk, L. Stewart, D. Lebus, B. Gicquel and O. Neyrolles, J. Exp. Med., 2009, 206, 2205–2220 CrossRef CAS.
- Y. Guo, H. Feinberg, E. Conroy, D. A. Mitchell, R. Alvarez, O. Blixt, M. E. Taylor, W. I. Weis and K. Drickamer, Nat. Struct. Mol. Biol., 2004, 11, 591–598 CrossRef CAS.
- D. A. Mitchell, A. J. Fadden and K. Drickamer, J. Biol. Chem., 2001, 276, 28939–28945 CrossRef CAS.
- H. Feinberg, D. A. Mitchell, K. Drickamer and W. I. Weis, Science, 2001, 294, 2163–2166 CrossRef.
- S. R. Ladwa, S. J. Dilly, A. J. Clark, A. Marsh and P. C. Taylor, ChemMedChem, 2008, 3, 742–744 CrossRef CAS.
- S. A. Fleming, Tetrahedron, 1995, 51, 12479–12520 CrossRef CAS.
- N. Kanoh, T. Nakamura, K. Honda, H. Yamakoshi, Y. Iwabuchi and H. Osada, Tetrahedron, 2008, 64, 5692–5698 CrossRef CAS.
- S. Mari, D. Serrano-Gomez, F. J. Canada, A. L. Corbi and J. Jimenez-Barbera, Angew. Chem., Int. Ed., 2005, 44, 296–298 CrossRef CAS.
- D. Serrano-Gomez, E. Sierra-Filardi, R. T. Martinez-Nunez, E. Caparros, R. Delgado, M. A. Munoz-Fernandez, M. A. Abad, J. Jimenez-Barbero, M. Leal and A. L. Corbi, J. Biol. Chem., 2008, 283, 3889–3903 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Immobilisation of saccharides and screening versusbiotin-conjugated DC-SIGN. See DOI: 10.1039/c0mb00118j |
| This journal is © The Royal Society of Chemistry 2011 |


