Research highlights
Šeila
Selimović
ab,
Omar Z.
Fisher
c and
Ali
Khademhosseini
*abde
aCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, USA. E-mail: alik@rics.bwh.harvard.edu
bHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
cDavid H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
dWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, USA
eWorld Premier International – Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan
First published on 17th November 2011
Deconstructing dendritic chemotaxis
Adaptive immunity depends on the body's ability to recognize pathogens quickly and signal a targeted immune response. This mechanism, while highly effective, can be hijacked by certain pathogens, such as HIV1 and metastatic cancer cells.2 One key process in adaptive immunity begins with dendritic cells (DC), which traffic pathogens to lymph nodes via chemotaxis. The general understanding of this pathway is limited by poor in vitro models of the complex cytokine gradients established in native tissue. To address this issue, Swartz and co-workers were able to investigate the effects of varying chemokine gradients on three-dimensional (3D) DC migration using a microfluidic platform.In biological tissues, these gradients occur in 3D space and in the presence of interstitial flow. These conditions are difficult to establish using common 2D models. Haessler et al.,3 however, succeeded in generating stable chemokine gradients by incorporating three parallel microfluidic channels into an agarose matrix (Fig. 1). The two outer channels contained chemokines at fixed concentrations, and the middle channel was filled with a hydrogel–cell mixture. The cell-laden hydrogel contained 1.5 mg ml−1 type I collagen and 10% Matrigel, an optimal combination for the migration of DCs. Proteoglycans were added to the matrix to ensure binding of ligands to cells. Before cells were loaded into the device, the gradients were established and reached a steady state in 2 h in a 3D space and were stable and independent of convection. Thus, cells were exposed to stable gradients throughout the experiment.
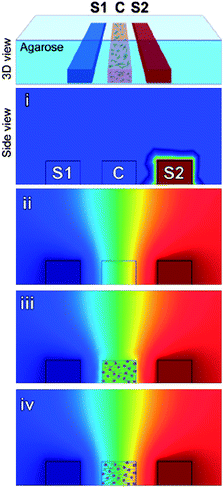 | ||
| Fig. 1 Schematic of a microfluidic device for studying chemotaxis. Cells (C), and chemokines (S1 and S2) are introduced in separate channels. Red and blue correspond to theoretical maximum and minimum concentrations, respectively. Chemokines are added to S1 (i) to establish a concentration gradient (ii), then cells are added (iii). Within minutes a concentration gradient is established across cells (iv). Figure reprinted with permission from Haessler et al.3 | ||
This system was used to identify differences in DC migration in the presence of two competing lymphoid homing chemokines that displayed similar properties in 2D. In particular, it was found that DCs moved faster in response to chemokine CCL21 when its concentration gradient increased, but for the other chemokine, CCL19, the speed remained constant after a threshold value, independent of the concentration gradient. Thus, it was quantitatively shown that dendritic cells had a stronger chemotactic response towards CCL21, driven by receptor binding kinetics.
The device described here allows researchers to study cell chemotaxis in 3D environments in more detail and with greater precision than previously possible. The chemotactic cell behavior can now be quantified with high precision, as the chemokine gradients are known exactly at any time and point in 3D. This information could facilitate greater understanding of chemotactic processes and may ultimately lead to novel immunotherapies against various diseases.
Complex shape-controlled particles
Colloidal particles of controllable shapes have unique characteristics, such as strong light scattering, and tend to differ in rheological properties from standard spherical particles.4 They are particularly interesting for studies of crystal structures and molecular interactions, and in terms of applied research, as drug delivery vehicles. Qin and colleagues have recently introduced a simple flow-focusing experiment to generate polymeric particles of a variety of complex shapes.In their paper, Zhang et al.5 describe a PDMS device utilizing two nozzles for double emulsion generation. Here, the inner phase was mineral oil, the middle phase was poly(ethylene glycol) diacrylate (PEGDA), a photosensitive gel precursor, and the outer phase was fluorinated oil FC-40. The flow rates of the three liquids were chosen to yield spherical inner droplets and plug-shaped outer droplets inside the output channel. Because of a difference in LaPlace pressure across the plugs, the inner droplet experienced a lower velocity compared to the tip of the plug and was thus observed in its rear. The emulsion was then exposed to UV light through the PDMS. The PEGDA precursor was thoroughly crosslinked, except for a thin layer on the plug surface. The crosslinking reaction was inhibited here due to the presence of oxygen, which easily permeates PDMS. This liquid layer of PEGDA enabled the removal of the inner mineral oil core during the subsequent washing step, which also eliminated the outer FC-40 phase. As a result, the crosslinked particles adopted crescent shapes. When several spherical droplets were embedded in the PEGDA plug, the particles had bipod and other complex shapes (Fig. 2). The exact dimensions of the particles were controlled by altering the flow rate ratios of the three phases.
 | ||
| Fig. 2 Complex-shape PEGDA particles formed from double emulsions with two or more inner droplets. Images of the double emulsions (top row), bright field images of the crosslinked particles (middle row) and corresponding fluorescence photographs (bottom row). Scale: 100 μm. Reprinted with permission from Zhang et al.5 | ||
The utility of the particles was demonstrated when magnetic nanobeads were encapsulated in the gel. The particles were shown to align with a static external magnetic field and to rotate in response to a rotating magnetic field. This indicates the ability to functionalize the particles and offers future applications such as particle sorting and even magnetofaction.
This emulsion based method of forming shape-controlled particles stands out because of its application of seemingly undesirable experimental occurrences. For example, perfectly spherical particles are usually the goal of flow-focusing droplet fabrication, while here any aberration of this goal is preferred. Also, the permeability of PDMS to oxygen inhibits certain chemical reactions, as shown here, but the researchers utilized this effect to eliminate the inner lipid core of the double emulsion. By manipulating these experimental disadvantages into solutions, the researchers succeeded in fabricating highly complex, functionalized particles that can be useful for rheological studies and as functional probes in biological systems, for example for drug delivery.
Click-ing proteins in place
Microscale cell culture often requires excellent control over the cellular microenvironment, including surface chemistry and topography.6 While there are several chemical techniques for functionalizing substrates with proteins that affect cell behavior, Maynard and co-workers have recently introduced a method relying on Click reactions. These reactions are used to join small molecules together; they are modular, stable, and high in yield.The Click reactions developed by Broyer et al.7 utilize PEG with aminooxy and alkyne functional groups for conjugation with other proteins. Initially, functionalized PEG with 8 arms for higher surface density was spin coated on a silicon wafer and patterned into 1 μm triangles and squares via electron beam lithography. This processing step also crosslinked PEG to the silicon surface. The patterning parameters were adjusted separately for the two functionalized PEG materials. Then, myoglobin and ubiquitin were functionalized to yield α-ketoamide and azide groups, respectively. Last, the functionalized myoglobin was allowed to react with the aminooxy-PEG, and azide-carrying ubiquitin with the alkyne PEG, catalyzed with copper. To verify the presence of the two proteins on selected patches of the silicon substrate, fluorescence images were recorded showing myoglobin in green and ubiquitin in red.
Beside this parallel application of the two Click reactions, the researchers showed that patterning of interdigitated features with different proteins was also possible. For example, they created alternating square arrays of triangles and squares and showed that, due to the highly precise e-beam lithography, a feature separation of 1 μm and possibly even less could be achieved. The background noise was much lower than the signal (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 and more), indicating low non-specific adsorption of the proteins on the silicon wafer. Further, the two different PEG materials only adsorbed one type of protein, such that purely myoglobin-coated and purely ubiquitin-coated features could be achieved.
33 and more), indicating low non-specific adsorption of the proteins on the silicon wafer. Further, the two different PEG materials only adsorbed one type of protein, such that purely myoglobin-coated and purely ubiquitin-coated features could be achieved.
Last, it was also shown that an orthogonal application of the Click chemistry could yield even more complex features. By first patterning large squares with aminooxy-PEG and ubiquitin, and then partially covering them with smaller features of alkyne-PEG and myoglobin, complex topographies and surface functionalization was achieved (Fig. 3).
 | ||
| Fig. 3 Protein patterns achieved via orthogonal Click reactions. Aminooxy-functionalized PEG was crosslinked into large squares, then alkyne-PEG was coated on top of the squares in the form of 4 × 4 square arrays: (a) layout of the functionalized surface, (b) image displaying both myoglobin (green) and ubiquitin (red) on the substrate, (c) and (d) separate fluorescence images of the two proteins. Scale: 5 μm. Reprinted with permission from the Royal Society of Chemistry from Broyer et al.7 | ||
The advantage of using Click reactions for protein patterning is their stability, high yield and modular nature. This paper demonstrates these characteristics in localized patterning of two proteins on a single substrate. As both reactions are modified to bind only one functionalized protein, high accuracy in the patterning process can be achieved. The application of e-beam lithography further adds high precision in spatial patterning of the substrate. Both methods also allow for generation of topographically and chemically complex surfaces. Thus, several important applications of this patterning process are envisioned: single-cell studies, mono- and co-culture of cells, and microrheology studies on proteins and DNA.
Bio-breadboard
Cell adhesion and detachment are important factors in many physiological processes as well as diseases.8 Thus, understanding the underlying mechanisms of cell–substrate interactions is paramount to increasing control over cell behavior. To gain insight into the adhesion and detachment processes, Mofrad and colleagues have developed a microscale platform capable of selective activation that encourages cell adhesion or removal.The so-called biological breadboard (BBB), described by Yoon et al.9 consists of an array of gold electrodes on a Pyrex substrate coated with PEG. The electrodes were patterned on the Pyrex substrate via e-beam evaporation and using an organic solvent. The electrodes were 10 μm to 500 μm wide. All electrodes were subsequently coated with an arginine-glycine-aspartic acid peptide terminated thiol (RTT). This increased the electrodes' affinity to cell attachment. When no potential was applied to these electrodes, cells preferentially adhered to the electrodes and did not adhere to the bare Pyrex/PEG substrate. When a negative potential, however, was applied to the electrodes, the RTT detached due to a breaking of bonds between RTT and gold. This lowered the affinity of the gold electrodes to cell adhesion. Thus, by a simple activation of selected electrodes with negative potential (−0.90 to −1.65 V) before and after seeding, the platform could control cell adhesion and detachment, respectively.
The authors conducted a variety of characterization experiments on the electrodes, including contact angle measurements, X-ray photoelectron spectroscopy and potentiodynamic electrochemical characterization of the detachment of the gold–thiol self-assembled monolayer. All experiments showed that the RTT-functionalized gold layer was indeed preferred to untreated gold and to PEG for cell attachment and that the resistive properties of the gold electrodes changed depending on the presence of the RTT layer. Further, it was shown that cell seeding could be localized by selectively actuating certain electrodes. NIH 3T3 fibroblasts did not adhere to negatively charged (bare gold) electrodes, because they lacked the RTT layer, but they did attach to RTT-coated electrodes, where no potential was applied. Upon activating those electrodes, as well, the fibroblasts detached. Hence, it was demonstrated that the BBB allowed for spatiotemporal control over both cell attachment and removal. For example, the time needed for 95% of cells to separate from the substrate or the CD-time was shown to be on the order of 30–50 s and inversely proportional to the cell confluency, at an activation potential of −1.5 V. Furthermore, the ratio of the CD-time and the activation potential in the case of fully confluent cells reached 100% within a few seconds at −1.8 V, but at lower applied voltages (∼−0.3 V) the ratio reached 50% only after 30 s and 100% after one minute. This confirmed that cell detachment depended strongly on the activation potential.
Low cell viability is a concern when using such high potentials. However, live/dead assays showed that at −1.5 V all cells remained viable upon detachment from the substrate, and at −1.8 V 80% of the cells were viable. Thus, the application of the BBB for cell detachment studies does not carry a large risk of false positives, that is dead cells that could naturally detach from the substrate rather than due to the activation potential.
The BBB has several advantages that make it attractive for cell adhesion and detachment experiments. The platform is simple to use, reusable, and allows for multiple studies on both subcellular and cellular scales. Its ability to control cell adhesion temporally and spatially makes the platform compatible with physiological cell studies, as well as signaling and motility studies.
References
- D. McDonald, et al., Recruitment of HIV and its receptors to dendritic cell-T cell junctions, Science, 2003, 300(5623), 1295–1297 CrossRef CAS.
- J. D. Shields, et al., Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling, Cancer Cell, 2007, 11(6), 526–538 CrossRef CAS.
- U. Haessler, et al., Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(14), 5614–5619 CrossRef CAS.
- J. A. Champion, Y. K. Katare and S. Mitragotri, Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers, J. Controlled Release, 2007, 121(1–2), 3–9 CrossRef CAS.
- Q. Zhang, B. Lin and J. Qin, Synthesis of shape-controlled particles based on synergistic effect of geometry confinement, double emulsion template, and polymerization quenching, Microfluid. Nanofluid., 2011, 1–7, DOI:10.1007/s10404-011-0846-x.
- D. Falconnet, et al., Surface engineering approaches to micropattern surfaces for cell-based assays, Biomaterials, 2006, 27(16), 3044–3063 CrossRef CAS.
- R. M. Broyer, et al., Dual Click reactions to micropattern proteins, Soft Matter, 2011, 7, 9972–9977 RSC.
- A. J. Ridley, et al., Cell Migration: Integrating Signals from Front to Back, Science, 2003, 302(5651), 1704–1709 CrossRef CAS.
- S.-H. Yoon, et al., A biological breadboard platform for cell adhesion and detachment studies, Lab Chip, 2011, 11, 3555–3562 RSC.
| This journal is © The Royal Society of Chemistry 2011 |
