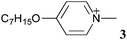
High Δε nematic liquid crystals: fluxional zwitterions of the [closo-1-CB9H10]−cluster†
Bryan
Ringstrand
and
Piotr
Kaszynski
*
Organic Materials Research Group, Department of Chemistry, Vanderbilt University, Nashville, TN 37235, USA. E-mail: piotr.kaszynski@vanderbilt.edu
First published on 24th September 2010
Abstract
A new class of nematics with high dielectric anisotropy (Δε) for display applications has been developed and characterized by thermal and dielectric methods in mixtures with 3 nematic hosts: ClEster, 6-CHBT, and ZLI-1132. The key structural element is the zwitterionic derivative of the [closo-1-CB9H10]− anion substituted at the B(10) position with either pyridinium (3) or sulfonium (4) groups. The zwitterions increase the longitudinal molecular electric dipole moment by 8 D (4) or 12 D (3) and give rise to the extrapolated Δε in the range of 22 (4a)–113 (3c). The sulfonium derivatives 4 have higher solubility in nematic hosts, lower tendency to aggregate, and lower impact on host's viscosity than pyridinium 3. This, in part, is attributed to facile epimerization at the sulfur center in 4 and existence of the trans/cis equilibrium. Experimental results are augmented with DFT calculations and analyzed using the Maier–Meier relationship.
Introduction
Polar liquid crystals are key components of mixtures for liquid crystals display (LCD) technologies.1,2 The molecular dipole moment, its magnitude and orientation, dictates the dielectric anisotropy (Δε) of the material, which directly affects the voltage characteristic of the electro-optical switching.3 Dielectric properties of the mixture are a sum of mole fraction xi of dielectric parameters of individual components (eqn (1)). Similar additivity is often observed for the nematic-isotropic transition temperature (TNI), and both of these relationships are used to formulate mixtures with desired phase range and dielectrics. Therefore, compounds that exhibit high Δε, moderate TNI, and have minimal effect on material's viscosity are of particular interest for technological applications.4 | (1) |
Compounds with a moderate molecular dipole moment and Δε in a range of 10–30 are designed using polar groups such as CN, F, CF3, and OCF3, and also dipolar rings such as pyrimidine and dioxane.1,4 Using a combination of these elements a handful of derivatives with Δε above 30 have been prepared, but typically with significantly compromised stability of the nematic phase.4 To develop materials with higher dielectric anisotropy, we have focused on zwitterionic derivatives A and B of the [closo-1-CB9H10]−cluster (I, Fig. 1) as structural elements of liquid crystals. Recently, we demonstrated first such derivatives of type A, compounds 1 and 2 (Fig. 2), as high Δε additives to a nematic host.5 The compounds were effective although poorly soluble in nematic hosts due to high melting point and tendency to aggregate. The second class of compounds, derivatives of type B in which the onium fragment is attached to the B(10) position, are much more promising. Our preliminary results6 demonstrated that pyridinium derivatives 3a and 3b exhibit nematic phases with TNI > 100 °C, high Δε, and good solubility in some nematics, but still unsatisfactory in 6-CHBT and ZLI mixtures, which are good models of LCD materials. A record high Δε of about 113 was extrapolated for 3c from dilute solutions in a nematic host, however, this compound has low solubility.6
![The structures of the [closo-1-CB9H10]− anion (I) and its polar derivatives A and B. Each vertex represents a BH fragment, the sphere is a carbon atom, and Q+ stands for an onium group such as an ammonium, sulfonium, or pyridinium.](/image/article/2011/JM/c0jm02075c/c0jm02075c-f1.gif) | ||
| Fig. 1 The structures of the [closo-1-CB9H10]− anion (I) and its polar derivatives A and B. Each vertex represents a BH fragment, the sphere is a carbon atom, and Q+ stands for an onium group such as an ammonium, sulfonium, or pyridinium. | ||
 | ||
| Fig. 2 Structures of 1–4. | ||
In order to increase solubility of the polar additives, we focused on sulfonium derivatives 4 (Fig. 2). Here we report a new class of highly polar mesogens exhibiting fluxional behavior that have positive effect on solubility of the additive and viscosity of the nematic mixture. The dielectric results for sulfonium 4 are contrasted with those for pyridinium 3 and analyzed using the Maier–Meier relationship with the aid of DFT calculations.
Results
Synthesis
Colorless esters 4a and 4b were prepared from sulfonium acid 5 in yields >85% (Scheme 1). The acid was first converted to the corresponding acid chloride using (COCl)2 in the presence of a catalytic amount of DMF, and then reacted with an appropriate phenol in the presence of Et3N. Acid 5 was obtained by alkylative cyclization of inner salt 6 with dibromide77 under hydrolytic conditions8 (Scheme 1). For comparison purposes, ester 8 (Fig. 3), lacking the pentyl substituent at the thiacyclohexane ring, was obtained from the appropriate carboxylic acid as previously described for its methoxy analogue.8 The preparation of pyridinium esters 3 is described elsewhere.6 | ||
| Scheme 1 Preparation of compound 4 | ||
 | ||
| Fig. 3 The structure of 8. | ||
Fluxional behavior of 4
Analysis of 1H NMR spectra of sulfonium derivatives 4a, 4b and also the carboxylic acid 5 revealed a coexistence of two isomeric forms, the trans and the cis, in a ratio of about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4). Temperature-depended 1H NMR studies yielded difference in enthalpy, ΔH = 0.83 ± 0.04 kcal mol−1, and entropy, ΔS = 0.2 ± 0.1 cal mol−1 K−1, between 4a-ciscis and 4a-transtrans.9NMR experiments also demonstrated that the barrier to epimerization at the sulfur center is low and the equilibrium is established quickly within less than 10 min above ambient temperature.
1 (Fig. 4). Temperature-depended 1H NMR studies yielded difference in enthalpy, ΔH = 0.83 ± 0.04 kcal mol−1, and entropy, ΔS = 0.2 ± 0.1 cal mol−1 K−1, between 4a-ciscis and 4a-transtrans.9NMR experiments also demonstrated that the barrier to epimerization at the sulfur center is low and the equilibrium is established quickly within less than 10 min above ambient temperature.
 | ||
| Fig. 4 Interconversion of the trans and cis isomers of 4a. Two major conformers are shown. | ||
Computational analysis at the B3LYP/6-31G(d,p) level of theory confirmed these results, and the energy difference between the trans and cis forms was calculated at ΔH = 1.28 kcal mol−1 (ΔS = −3.8 cal mol−1 K−1) for both derivatives 4a and 4b in gas phase. For a better understanding of the fluxional character of mesogens 2 and 4, models 9 and 10 were analyzed using the DFT and MP2 methods.
Results shown in Table 1 demonstrate that the epimerization process is easier for the B(10) isomer 10 than for the C(1) isomer 9 by 7 kcal mol−1. The enthalpy of activation ΔH‡ calculated for the former is about 24 kcal mol−1, which is consistent with a relatively fast epimerization observed for 4 even at ambient temperature. In contrast, epimerization of the C(1) isomer 9 requires elevated temperatures, presumably above 100 °C. In addition, 9 has a shorter cage–S bond by nearly 0.1 Å, which affects the stability of the axial epimer and, consequently, the position of the axial/equatorial equilibrium. Thus, for the B-isomer with the longer cage–S bond (dS–B = 1.854 Å) the ΔG value is small and the concentration of the axial conformer is high (K298 = 2). In contrast, the steric energy for the C(1) isomers is higher by 1.8 kcal mol−1 which corresponds to a concentration of about 2% of the axial form at the equilibrium. These results are consistent with a single isomer of 2 observed by NMR spectroscopy.5
| Compound | ΔH‡/kcal mol−1 | ΔG298/kcal mol−1 | K 298 | d S–X b/Å | µ c/D |
|---|---|---|---|---|---|
| a MP2/6-31G(d,p) level calculations with B3LYP/6-31G(d,p) thermodynamic corrections. b Interatomic distance between the S and the cage's C or B atoms in the equatorial epimer. c Dipole moment of the equatorial epimer. | |||||
| 9, X = C, Y = B | 31.2 | 2.2 | 42 | 1.750 | 14.4 |
| 10, X = B, Y = C | 24.2 | 0.4 | 2 | 1.854 | 8.75 |
Thermal properties
Polarized optical microscopy (POM) and differential scanning calorimetry (DSC) demonstrated that only one sulfonium derivative, ester 4b, exhibits a monotropic nematic phase with a clearing temperature of 98 °C (Table 2). A supercooled sample of ester 4a crystallized at 60 °C, 38 K below melting, without showing a mesophase. In contrast all three pyridine derivatives 3a–3c exhibit a mesophase, although only one, the butoxy 3b has an enantiotropic nematic phase.No mesophase was observed in 8 even in a sample supercooled by 20 K. A comparison of 4b and 8 demonstrated that the pentyl chain on the thiacyclohexane ring lowers the melting point by 38 K.
Binary mixtures
Pyridinium and sulfonium derivatives 3 and 4, and also 11, a carborane analogue of 3c,6 were investigated as low concentration additives to three nematic hosts: ClEster, 6-CHBT, and ZLI-1132 (Fig. 5). The first host with a small negative Δε was found to be a good solvent for zwitterions of type A (Fig. 1).5 The last two hosts have significant positive Δε (+7.6 for 6-CHBT and +11.5 for ZLI-1132) and are models for industrial LCD materials. Results for binary mixtures are shown in Table 3. | ||
| Fig. 5 Structures of nematic hosts and compound 11. | ||
| Host | [TNI]/°C | ε ‖ | ε ⊥ | Δε | S app b | g b | |
|---|---|---|---|---|---|---|---|
| a For details of data analysis see ESI1. b Error ≤ 0.01. | |||||||
| 3a | ClEster | 103 ± 1 | 54.8 ± 0.3 | 12.8 ± 0.1 | 42.0 ± 0.3 | 0.62 | 0.32 |
| 3c | ClEster | 95.5 ± 1 | 136.2 ± 0.3 | 22.8 ± 0.3 | 113.4 ± 0.6 | 0.66 | 0.35 |
| 4a | ClEster | 89 ± 2 | 35.0 ± 0.2 | 9.7 ± 0.2 | 25.3 ± 0.2 | 0.59 | 0.46 |
| 6-CHBT | 112 ± 2 | 41.3 ± 0.2 | 11.8 ± 0.2 | 29.5 ± 0.3 | 0.57 | 0.56 | |
| ZLI-1132 | 36 | 30.7 | 8.6 | 22.1 | — | — | |
| 4b | 6-CHBT | 133 ± 2 | 47.7 ± 0.1 | 12.2 ± 0.1 | 35.5 ± 0.1 | 0.63 | 0.72 |
| 11 | ClEster | 98 ± 1 | 21.0 ± 0.1 | 5.8 ± 0.1 | 15.2 ± 0.1 | 0.59 | 0.35 |
Three esters, 3a, 4a, and 3c, were tested in ClEster host. While the first two compounds showed good solubility to at least 15 mol%, the nitrile 3c gave stable solution only at or below 3 mol%, and 5.6 mol% solution was inhomogenous after 4 h at rt. Despite good solubility in ClEster, the pyridinium ester 3a did not form homogenous solutions with 6-CHBT at a concentration even as low as 2.5 mol%. In contrast, both sulfonium esters 4a and 4b were soluble in ClEster and 6-CHBT hosts at concentrations up to about 11 mol%, which was the highest concentration tested. In addition, sulfonium 4a formed a stable 2.8 mol% solution in ZLI-1132.
Thermal analysis of the solutions revealed that the TNI of a mixture increases approximately linearly with increasing concentration of the additive in ClEster and 6-CHBT (Fig. 6) with the least linear behavior of 3c. Interestingly, the [TNI] values (virtual TNI) extrapolated for the two sulfonium esters 4a and 4b are significantly higher than those measured for the pure compounds, by 36 K for the latter and at least 40 K for the former in 6-CHBT. This indicates that the nematic phase in binary mixtures of 4 in 6-CHBT and ClEster is markedly expanded. Results for 4a also demonstrate a strong dependence of the [TNI] on the host with a difference of about 50 K observed between ClEster and ZLI-1132 (Table 3). In contrast to sulfonium esters, the [TNI] values obtained for the pyridinium esters 3 are within the expectation and moderately lower than those measured for pure compounds.
 | ||
| Fig. 6 A plot of peak temperature of the N–I transition for binary mixtures pyridinium 3a in ClEster (circles) and sulfonium 4b in 6-CHBT (diamonds). | ||
Analysis of solution dielectric data shows that the permittivity of the mixtures increases approximately linearly with the increasing mole fraction of the additive in accordance with eqn (1) (Fig. 7). This indicates that none or little aggregation of the polar molecules takes place in solution at this concentration, which is in sharp contrast with compounds of type A.5 The extrapolated dielectric parameters for pure additives 3 and 4 are impressive (Table 3), especially those obtained for the cyano derivative 3c (ε‖ = 136 and Δε = 113), which are the highest ever recorded for a nematic material.4 Comparison with nitrile 11, a non-zwitterionic analogue of 3c, shows that replacement of the C–C fragment in 11 with the isosteric polar fragment N+–B− in 3c results in an increase of Δε by nearly 100! Dielectric anisotropy, Δε, extrapolated for the sulfonium ester 4a is lower than that for pyridinium analogue 3a by about 17 in ClEster (Table 3).
 | ||
| Fig. 7 Dielectric parameters as a function of the mole fraction of 4a in 6-CHBT. The last data point deviates from linearity. | ||
Further analysis of dielectric data revealed that the pyridinium and sulfonium additives increase elastic constant K11 of the ClEster host by about 50–100% (Fig. 8a). In contrast the K11 value for solutions of sulfonium 4a and 4b in 6-CHBT remains approximately constant at about 14 pN.
 | ||
| Fig. 8 Elastic constant K11 (a) and viscosity γ (b) of binary mixtures of pyridinium 3a (diamond) and 3c (square), sulfonium 4a (triangle up), sulfonium 4b (triangle down), and carborane 11 (circle) in ClEster and 6-CHBT. Lines are guide for the eye. | ||
Similar differences between the two hosts were also observed for the viscosity of the solutions. In ClEster host viscosity of the binary mixture appears to increase with increasing Δε of the additive (Fig. 8b). A comparison of solutions of sulfonium 4a in ClEster with those in 6-CHBT demonstrates that the additive increases viscosity much less in the latter. Assuming linear increase of γ with concentration the extrapolated viscosity is 0.58 P s for 4a and 0.31 P s for 4b. For comparison, extrapolation from 3 data points gives a value of 10.8 P s for 3c and 1.25 P s for the non-zwitterionic 11 in ClEster.
Unfortunately, direct comparison of pyridinium and sulfonium in 6-CHBT is not possible.
Analysis of dielectric data
Dielectric parameters extrapolated for pure additives were analyzed using the Maier–Meier relationship,10,11 which connects molecular and phase parameters. Using experimental ε‖ and Δε values and DFT-calculated parameters µ, α, and β (Table 4), eqn (2) and (3) permitted the calculation of the apparent order parameter Sapp and the Kirkwood factor g (eqn (4)) shown in Table 3. The field parameters F and h in eqn (2) and (3) were calculated using the extrapolated dielectric parameters for each additive and electronic polarizability α of the host. Full protocol for the calculations is given in the ESI†.12 | (2) |
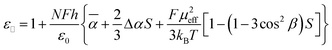 | (3) |
| µeff2 = gµ2 | (4) |
| µ ‖/D | µ ⊥/D | µ/D | β b/° | Δα/Å3 | α avrg/Å3 | |
|---|---|---|---|---|---|---|
| a Vacuum dipole moments and polarizabilities obtained at the B3LYP/6-31G(d,p) level of theory. Polarizability units were converted from a.u. to Å3 using the factor 0.1482. b Angle between the net dipole vector µ and µ‖. For details see the ESI1. c Calculated for an average molecule at the equilibrium ([cis] = 21.5 mol%). | ||||||
| 3a | 13.99 | 3.31 | 14.38 | 13 | 51.81 | 61.06 |
| 3c | 20.23 | 1.79 | 20.31 | 5 | 52.23 | 55.01 |
| 4a-transtrans | 9.62 | 2.31 | 9.89 | 13 | 41.87 | 57.26 |
| 4a-ciscis | 8.49 | 3.02 | 9.02 | 20 | 35.89 | 56.73 |
| 4a c | 9.03 | 2.50 | 9.64 | 15 | 40.16 | 57.06 |
| 4b-transtrans | 8.89 | 2.62 | 9.27 | 16 | 42.73 | 56.34 |
| 4b-ciscis | 7.69 | 3.45 | 8.43 | 24 | 36.66 | 55.86 |
| 4b c | 8.63 | 2.80 | 9.07 | 18 | 41.50 | 56.17 |
| 11 | 8.10 | 0.51 | 8.11 | 4 | 51.00 | 54.35 |
The calculated Sapp values are about 0.6 (Table 3), which indicates that all additives have reasonably high local order parameter and are well aligned with the nematic director of the host. A detailed comparison shows that the sulfonium derivatives 4 have slightly lower Sapp (less aligned) than pyridinium 3 and carborane 11, which is presumably related to the epimerization at the S-center and less anisometric average molecular shape.
The Kirkwood parameter g is moderate and about 0.35 for pyridinium 3, while for the sulfonium 4 is markedly greater reaching a value of 0.72 for 4b in 6-CHBT. This indicates less aggregation of the polar molecules in the liquid phase, which is consistent with higher solubility of the sulfonium 4 than pyridinium 3.
Discussion and conclusions
Sulfonium esters 4 are more soluble, have lower local order parameter Sapp, and higher Kirkwood parameter g than the pyridinium derivatives 3. The size of the molecular dipole moment alone cannot satisfactorily explain these observed differences. For instance, carborane 11 and sulfonium 4 have similar dipole moments, yet the g value for the former is similar to that of highly polar pyridinium 3c. However, the observed differences in behavior of 3 and 4 are consistent with the fluxional behavior of the sulfonium and fast interconversion of the trans and cis epimers in the latter in the nematic phase.Fast interconversion of each molecule of 4 between two forms, linear (4-transtrans) and bent (4-ciscis, Fig. 4), results in an average molecule shape that is less anisometric than that for the rigid pyridinium 3. In consequence, the more linear pyridinium exhibits better alignment with the nematic director of the host (higher Sapp) than the shape-shifting sulfonium (Table 3). Also, fast epimerization at the S-center decreases the tendency of polar molecules to aggregate, which results in higher solubility and greater Kirkwood factor g for sulfonium than the pyridinium analogues. This fluxional shape is also a likely factor contributing to lower viscosity observed for nematic solutions of sulfonium 4 than pyridinium 3. Such an effect of sterically demanding additives, including carborane derivatives,13 on phase viscosity has been observed before. Thermal and dielectric results also indicate that the additive's effect on phase properties is host-dependent, and sulfonium 4 appears to be particularly compatible with 6-CHBT: the additive expands the nematic phase and has high g factor.
In summary, sulfonium derivatives 4 represent a new concept in designing polar additives. They combine the polar zwitterionic fragment that gives rise to a large positive Δε, and shape-shifting ability, which results in high solubility, high effective electric dipole moment µeff, and relatively low contribution to rotational viscosity γ. Further modification of polar properties and consequently Δε of sulfonium derivatives of type B can be accomplished by proper substitution of the phenol part of the ester 4 as it was demonstrated for the pyridinium derivatives 3a and 3c.
Computational details
Quantum-mechanical calculations were carried out using Gaussian 09 suite of programs.14 Geometry optimizations for unconstrained conformers of 3 and 4 with the most extended molecular shapes were undertaken at the B3LYP/6-31G(d,p) level of theory using default convergence limits. Geometry and total energy for model compounds 9 and 10 were obtained using the MP2/6-31G(d,p) method. Dipole moments and exact electronic polarizabilities for 3 and 4 for analysis with the Maier–Meier relationship were obtained using the B3LYP/6-31G(d,p) method. Dipole moment components µ and polarizability tensors α were calculated in Gaussian standard orientation of each molecule (charge based), which is close to the principal moment of inertia coordinates (mass based).Experimental part
General
NMR spectra were obtained at 128.4 MHz (11B) and 400.1 MHz (1H) in CD3CN or CDCl3. 1H NMR spectra were referenced to the solvent, and 11B NMR chemical shifts were referenced to an external boric acid sample in CH3OH that was set to 18.1 ppm. Thermal analysis was performed using a TA Instruments 2920 DSC. Transition temperatures (onset) and enthalpies were obtained using small samples (1–2 mg) and a heating rate of 5 K min−1 under a flow of nitrogen gas.Preparation of esters 4 and 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reaction mixture was evaporated to dryness, and the crude product was passed through a short silica-gel plug (CH2Cl2 or CH2Cl2/hexane, 1
1). The reaction mixture was evaporated to dryness, and the crude product was passed through a short silica-gel plug (CH2Cl2 or CH2Cl2/hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The product was passed through a cotton plug, washed with hot hexane, and repeatedly recrystallized typically from combinations of cold CH3CN, cold iso-octane/toluene mixtures, or cold aqueous EtOH. Analytical data and preparation of acid 5 are given in the ESI†.
1). The product was passed through a cotton plug, washed with hot hexane, and repeatedly recrystallized typically from combinations of cold CH3CN, cold iso-octane/toluene mixtures, or cold aqueous EtOH. Analytical data and preparation of acid 5 are given in the ESI†.
Binary mixtures preparation
Solutions of esters in hosts ClEster, 6-CHBT or ZLI-1132 (∼10 mg) were prepared in an open vial with agitation using a closed-end capillary tube with moderate heating supplied by a heat gun. The binary mixtures were analyzed by polarized optical microscopy (POM) to ensure that the mixtures were homogeneous. The mixtures were then allowed to condition for 3 h at room temperature. The clearing temperature of each homogeneous mixture was determined by DSC as the peak of the transition.Electrooptical measurements
Dielectric properties of solutions of selected esters in ClEster,156-CHBT16 or ZLI-1132 were measured by a Liquid Crystal Analytical System (LCAS—Series II, LC Analytical Inc.) using GLCAS software version 0.951, which implements literature procedures for dielectric constants.17 The homogeneous binary mixtures were loaded into ITO electro-optical cells by capillary forces with moderate heating supplied by a heat gun. The cells (about 4 µm thick, electrode area of 0.581 cm2 and anti-parallel rubbed polyimide layer 2° to 3° pretilt) were obtained from LCA Inc., and their precise thickness (±0.05 mm) was measured by LCAS using the capacitance method before the cells were filled. The filled cells were heated to an isotropic phase and were cooled to ambient temperature (23 °C) before measuring the dielectric properties. Default parameters were used for measurements: triangular shaped voltage bias ranging from 0.1–15 V for 3 and 4 and 0.1–30 V for 11 at 1 kHz frequency. The threshold voltage Vth was measured at a 5% change. For each mixture, the measurement was repeated seven times for two cells. The first two measurements for each cell were discarded as conditioning measurements, and the remaining ten results were averaged to calculate the mixture's parameters. Dielectric parameters of pure hosts 6-CHBT and ZLI-1132 were obtained using the same protocol. For consistency, dielectric parameters ClEster were obtained by averaging values obtained by extrapolation from a series of measurements for each additive. The averaged extrapolated values are higher than those measured in a set of homeotropic cells by about 0.2 for ε‖ and ε⊥ and lower by 0.03 for Δε. The value for elastic constant K11 of 10 ± 0.3 pN was assumed from the measurement in homeotropic cells. The viscosity γ from these measurements was not used for comparison purposes.4-(trans-4-Hexylcyclohexyl)phenylisothiocyanate16 (6-CHBT) was vacuum distilled prior to measurements, and ZLI-1132 was used as supplied (E. Merck, Ind). Results for pure hosts at 23 °C: 6-CHBT: ε‖ = 11.65 ± 0.05, ε⊥ = 4.02 ± 0.05, Δε = 7.63 ± 0.05, K11 = 13.7 ± 0.7 pN, and γ = 111 ± 1 mP s. ZLI-1132: ε‖ = 16.12 ± 0.06, ε⊥ = 4.64 ± 0.01, Δε = 11.48 ± 0.05, and γ = 225 ± 1.5 mP s.
The results are collected in Tables S3 and S4 in ESI†.
Acknowledgements
Financial support for this work was received from the National Science Foundation (DMR-0907542). We thank Prof. R. Dabrowski for the gift of ClEster.References
- P. Kirsch and M. Bremer, Angew. Chem., Int. Ed., 2000, 39, 4216–4235 CrossRef CAS , and references therein.
- D. Pauluth and K. Tarumi, J. Mater. Chem., 2004, 14, 1219–1227 RSC.
- (a) L. M. Blinov, in Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess, and V. Vill, Wiley-VCH, New York, 1998, vol. 1, pp. 477–534 Search PubMed; (b) L. M. Blinov and V. G. Chigrinov, Electrooptic Effects in Liquid Crystal Materials, Springer-Verlag, New York, 1994, and references therein Search PubMed.
- S. Naemura, in Physical Properties of Liquid Crystals: Nematics, ed. D. A. Dunmur, A. Fukuda and G. R. Luckhurst, IEE, London, 2001, pp. 523–581, and references therein Search PubMed.
- B. Ringstrand, P. Kaszynski, A. Januszko and V. G. Young, Jr, J. Mater. Chem., 2009, 19, 9204–9212 RSC.
- B. Ringstrand and P. Kaszynski, J. Mater. Chem., 2010, 20 10.1039/c0jm02876b.
- J. Thomas and D. Clough, J. Pharm. Pharmacol., 1963, 15, 167–177 CAS; N. P. Volynskii and L. P. Shcherbakova, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.), 1979, 28, 1006–1009 Search PubMed.
- B. Ringstrand, P. Kaszynski, V. G. Young, Jr and Z. Janousek, Inorg. Chem., 2010, 49, 1166–1179 CrossRef CAS.
- For details see the ESI†.
- S. Urban, in Physical Properties of Liquid Crystals: Nematics, ed. D. A. Dunmur, A. Fukuda and G. R. Luckhurst, IEE, London, 2001, pp. 267–276, and references therein Search PubMed.
- A. Januszko, K. L. Glab, P. Kaszynski, K. Patel, R. A. Lewis, G. H. Mehl and M. D. Wand, J. Mater. Chem., 2006, 16, 3183–3192 RSC.
- For another approach to use the Maier–Meier relationship see: D. Demus and T. Inukai, Mol. Cryst. Liq. Cryst., 2003, 400, 39–58 Search PubMed.
- W. Piecek, K. L. Glab, A. Januszko, P. Perkowski and P. Kaszynski, J. Mater. Chem., 2009, 19, 1173–1182 RSC.
- M. J. Frisch, et al., Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- R. Dabrowski, J. Jadzyn, J. Dziaduszek, Z. Stolarz, G. Czechowski and M. Kasprzyk, Z. Naturforsch., A: Phys. Sci., 1999, 54, 448–452 CAS.
- J. W. Baran, Z. Raszewski, R. Dabrowski, J. Kedzierski and J. Rutkowska, Mol. Cryst. Liq. Cryst., 1985, 123, 237–245 CAS.
- S.-T. Wu, D. Coates and E. Bartmann, Liq. Cryst., 1991, 10, 635–646 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and characterization data for compounds 4, 5, and 8, details of thermal and dielectric data, dielectric analysis, archive of calculated equilibrium geometries for 3, 4, and 9–11. See DOI: 10.1039/c0jm02075c |
| This journal is © The Royal Society of Chemistry 2011 |