Fabrication of electrode–electrolyte interfaces in all-solid-state rechargeable lithium batteries by using a supercooled liquid state of the glassy electrolytes
Hirokazu
Kitaura
a,
Akitoshi
Hayashi
a,
Takamasa
Ohtomo
b,
Shigenori
Hama
b and
Masahiro
Tatsumisago
*a
aDepartment of Applied Chemistry, Graduate School of Engineering, Osaka Prefecture University, 1-1 Gakuen-cho, Naka-ku, Sakai, Osaka 599-8531, Japan. E-mail: tatsu@chem.osakafu-u.ac.jp; Fax: +81-72-2549331; Tel: +81-72-2549331
bToyota Motor Corporation, Higashifuji Technical Center, Battery Research Division, Susono, Shizuoka 410-1193, Japan
First published on 27th September 2010
Abstract
The softening behavior of a 80Li2S·20P2S5 (mol%) glass electrolyte was investigated and a favorable electrode–electrolyte interface was fabricated by sticking the supercooled liquid state of the 80Li2S·20P2S5 electrolyte on active material particles. A dense pellet of the glass electrolyte without an obvious grain boundary or any voids was prepared by softening the 80Li2S·20P2S5 glass by means of a hot press. The electrical conductivity of the pellet was 8.8 × 10−4 S cm−1 at room temperature. Sticking the solid electrolyte on the Li4Ti5O12 active material particles increased the contact area at the electrode–electrolyte interface and the utilization of the active material was increased in the all-solid-state cells. However, LiCoO2 reacted with the solid electrolyte during the hot press and the electrochemical performance of the cells using hot-pressed LiCoO2 with the glass electrolyte degraded. LiNbO3 coating suppressed the reaction of LiCoO2 with the solid electrolyte. The all-solid-state full-cell Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2 prepared by hot press showed a larger reversible capacity of 120 mAh g−1 at 0.064 mA cm−2 compared with the full-cell prepared by cold press. The softening of the 80Li2S·20P2S5 glass electrolyte is an effective way for increasing the contact area between the active materials and solid electrolyte.
Introduction
In order to reduce emissions of CO2, high-performance lithium ion batteries have received a lot of attention as new large-scale power storage systems for eco-cars such as electric vehicles (EV) and hybrid electric vehicles (HEV), and the application of natural energy by solar and wind power. However, safety issues in lithium ion batteries become more serious with the increase in size. All-solid-state lithium secondary batteries using inorganic solid electrolytes would be the ultimate batteries to solve the safety issues. There are several important factors to construct all-solid-state batteries with high power and high energy density. Development of solid electrolytes with high lithium ion conductivities is one of the most important tasks. Li2S–P2S5 solid electrolytes have been developed and were found to show high lithium ion conductivities over 10−4 S cm−1.1,2 The 80Li2S·20P2S5 (mol%) glass showed a relatively high lithium ion conductivity of 1.7 × 10−4 S cm−1 at ambient temperature in the Li2S–P2S5 glasses.2 In addition, after the glass was heated over the crystallization temperature, lithium ion conductivity was enhanced to 7.2 × 10−4 S cm−1 because of the precipitation of a lithium ion highly conductive crystal.3The prepared glass-ceramic electrolytes were applied to the all-solid-state cells and the cells showed long cycle performance at a low current density of 0.064 mA cm−2.4,5 However, the reversible capacity and rate capability of all-solid-state cells were lower than the cells using liquid electrolytes. Active material particles which electrically connect with electrolytes are only available as lithium storage materials. In order to improve the electrochemical performance of the all-solid-state cells, an electrochemically favourable electrode–electrolyte interface has to be fabricated. Two major causes, which adversely affect electrochemical reaction at the electrode–electrolyte interface, are considered.
One is the formation of high resistance layers. Ohta et al. revealed that high resistance layers formed at the interface between the LiCoO2 positive electrode and a sulfide-based solid electrolyte after the initial charge process, by using impedance spectroscopy.6,7 In addition, they enhanced the rate capability of all-solid-state cells using LiCoO2 by insertion of oxide thin-film into the electrode–electrolyte interface to suppress the formation of resistance layers.6,7 We also investigated the interface between various positive electrode materials such as LiCoO2, LiNiO2, LiMn2O4 and LiNi1/3Co1/3Mn1/3O2, and Li2S–P2S5 solid electrolytes.8–10 The interfacial resistance was observed in all the cells and the coating of positive electrode materials was effective in decreasing the interfacial resistance.
The other is the small contact area between active materials and solid electrolytes. In the case of using solid electrolytes, it is considered that the contact area between active materials and solid electrolytes is smaller than the contact area between active materials and liquid electrolytes because solid electrolytes are not wettable and infiltrative like liquids. A novel approach to form a large contact area between active materials and solid electrolytes is required. We focus our attention on a characteristic of glasses. In general, glass transforms into supercooled liquid and softens at around glass transition temperatures (Tg).11 The use of a viscous flow of supercooled liquid onto solid-state materials at around Tg is effective in forming a liquid–solid interface, and the interface would give close solid–solid contact by cooling down to room temperature. We propose a new concept for fabricating the electrode–electrolyte interface with a large contact area in the all-solid-state batteries. By use of the transformation from glass to supercooled liquid in the system Li2S–P2S5, the solid–liquid interface can be formed at the interface between solid-state active materials and liquid-state sulfide electrolytes.
In this paper, we present the fabrication of all-solid-state batteries by use of a softening process of the 80Li2S·20P2S5 glass electrolyte. At the beginning, the softening behavior of the 80Li2S·20P2S5 glass was investigated. Then the supercooled liquid of the solid electrolyte was kept in close contact with active material particles by hot pressing. The reactivity of the electrolyte in a supercooled liquid state with active materials such as the LiCoO2 positive electrode and Li4Ti5O12 negative electrode were investigated by using electron microscope observations and electrochemical measurements. The all-solid-state battery was fabricated and the electrochemical performance was evaluated.
Experimental
The 80Li2S·20P2S5 (mol%) glass was prepared by a mechanochemical reaction process.2 Reagent-grade chemicals of Li2S and P2S5 were used as starting materials. The mixture of starting materials was put in a zirconia pot together with zirconia balls in an Ar filled glove box, and then the mechanochemical treatment was carried out at 500 rpm for 20 h by using a high energy planetary ball mill (Pulverisette 7, Fritsch). The volume of the pots was 45 ml and the number of balls with the diameter of 5 mm was 160.In order to investigate the softening behavior, the obtained glass with or without active materials was pressed at around glass transition temperature. Lithium cobalt oxide (LiCoO2: Toda Kogyo Co.) and lithium titanate (Li4Ti5O12: Titan Kogyo Co.) were used as positive and negative electrode active materials. It was reported that the LiNbO3 buffer layer is effective in decreasing the interfacial resistance between LiCoO2 positive electrode and thio-LISICON electrolyte and developing the rate performance of all-solid-state cells.7 The LiNbO3-coted LiCoO2 was thus used in this study and the thickness of the LiNbO3 layer was about 7 nm.12 These active materials were mixed with the glass in an agate mortar. The glass powder and the mixed powder were pressed at 180–240 °C for 2–4 h under a pressure of 360 MPa. All processes were performed in an Ar-filled glove box. X-Ray diffraction (XRD) measurements were performed using a diffractometer (M18XHF22-SRA, Bruker). The microstructure of the samples was examined by using a scanning electron microscope (SEM, JSM-5300, JEOL). The interface between an active material particle and the solid electrolyte was analyzed using a transmission electron microscope (TEM, JEM2100, JEOL). Focused ion beam (FIB) milling was done for sample-preparation for cross-sectional TEM observation. Elemental point analysis for the cross-section of the composite electrode layer was carried out using energy dispersive X-ray spectroscopy (EDX, JED-2300T, JEOL). Electrical conductivities were measured for the pellets sandwiched between the stainless-steel disks by the AC impedance measurement using an impedance analyzer (Solartron 1287 coupled with Solartron 1260).
All-solid-state half-cells were assembled to investigate the effects of solid–solid interface prepared by hot press. The mixture of LiCoO2 (non-coated or LiNbO3-coated) and 80Li2S·20P2S5 glass (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 weight ratio) or Li4Ti5O12 and 80Li2S·20P2S5 glass (50
30 weight ratio) or Li4Ti5O12 and 80Li2S·20P2S5 glass (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 weight ratio) was pressed at 210 °C for 4 h under 360 MPa. The pellet was ground in an agate mortar and the obtained composite electrode powder was used as the working electrode. The glass-ceramic electrolyte was used for the separation between the working and counter electrodes. The composite electrode (10 mg) and the glass-ceramic electrolyte powder (80 mg) were set in a polycarbonate tube and were then pressed under 360 MPa. Then In foil (thickness, 100 μm) was put on the solid electrolyte layer as a counter and a reference electrode; a pressure of 240 MPa was applied to the three-layered pellet by cold press and was then relieved. Finally, two-electrode cells sandwiched with two stainless steel rods as current collectors were obtained. For comparison, the mixture of active materials and the 80Li2S·20P2S5 glass-ceramic electrolyte was used as a working electrode.
50 weight ratio) was pressed at 210 °C for 4 h under 360 MPa. The pellet was ground in an agate mortar and the obtained composite electrode powder was used as the working electrode. The glass-ceramic electrolyte was used for the separation between the working and counter electrodes. The composite electrode (10 mg) and the glass-ceramic electrolyte powder (80 mg) were set in a polycarbonate tube and were then pressed under 360 MPa. Then In foil (thickness, 100 μm) was put on the solid electrolyte layer as a counter and a reference electrode; a pressure of 240 MPa was applied to the three-layered pellet by cold press and was then relieved. Finally, two-electrode cells sandwiched with two stainless steel rods as current collectors were obtained. For comparison, the mixture of active materials and the 80Li2S·20P2S5 glass-ceramic electrolyte was used as a working electrode.
All-solid-state full-cells were assembled by using the softening process. The positive electrode was composed of LiNbO3-coated LiCoO2 (38.5 wt.%), the 80Li2S·20P2S5 glass (57.7 wt.%), and vapor grown carbon fiber (VGCF) (3.8 wt.%) powders. The negative electrode was composed of Li4Ti5O12 (28.3 wt.%), the solid electrolyte (66.0 wt.%), and VGCF (5.7 wt.%) powders. The three-layered pellet (negative electrode/80Li2S·20P2S5 glass/positive electrode) sandwiched between stainless steel disks was prepared by pressing at 210 °C for 4 h under 360 MPa. For comparison, the all-solid-state battery using 80Li2S·20P2S5 glass-ceramic electrolyte was assembled by cold press. Electrochemical tests were conducted at 25 °C in an Ar atmosphere using charge-discharge measuring devices (BTS-2004, Nagano Co.).
Results and discussion
The 80Li2S·20P2S5 sample showed the glass transition temperature at 200 °C and crystallization temperature at 240 °C. Fig. 1(a) shows the XRD pattern of the 80Li2S·20P2S5 sample. Although the diffraction peaks due to Li2S crystal are slightly observed, the 80Li2S·20P2S5 sample was almost amorphous. The 80Li2S·20P2S5 sample was a glassy material, which was consistent with previous reports.2 The photograph of the 80Li2S·20P2S5 glass pressed at room temperature is shown in Fig. 2(a). The yellowish pellet was obtained by pressing at room temperature. The glassy powder was pressed at 180–240 °C for 2 h under 360 MPa. Fig. 2(b)–(d) show photographs of the pellets prepared by pressing the 80Li2S·20P2S5 glass at (b) 180 °C, (c) 210 °C and (d) 240 °C for 2 h. The color of the pellets turned brownish with increasing temperature. In addition, the largely translucent pellet was obtained by pressing at 210 °C for 2 h. After pressing at 240 °C, a fragile pellet was obtained and its color was opaque black. Fig. 1(b)–(d) shows XRD patterns of the pellet prepared by pressing the 80Li2S·20P2S5 glass at (b) 180 °C, (c) 210 °C and (d) 240 °C for 2 h. The similar XRD patterns to the 80Li2S·20P2S5 glass (a) were observed in the pellet pressed at the temperature up to 210 °C. On the other hand, the peaks attributable to the thio-LISICON II analog (Li3.25P0.95S4) phase appeared in the pellet pressed at 240 °C.3 It was considered that the translucence of the pellet pressed at around glass transition temperature was ascribed to an increase in transmittance by the disappearance of the grain boundary. In contrast, the precipitated crystal would form a grain boundary. Hence, the opaque pellet was obtained after pressing at around the crystallization temperature of 240 °C.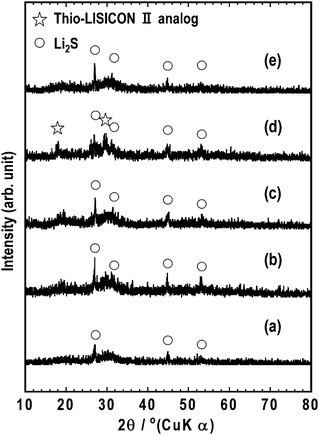 | ||
| Fig. 1 XRD patterns of (a) the 80Li2S·20P2S5 sample and its pellets pressed at (b) 180 °C, (c) 210 °C and (d) 240 °C for 2 h, and (e) 210 °C for 4 h. | ||
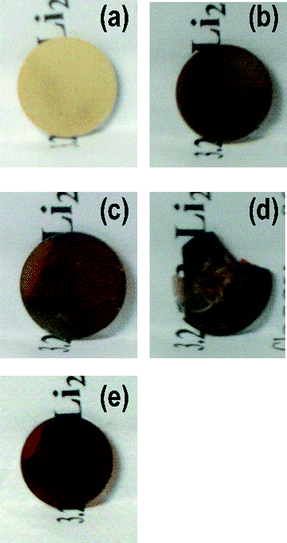 | ||
| Fig. 2 Photographs of the pellets of the 80Li2S·20P2S5 sample pressed at (a) room temperature, (b) 180 °C, (c) 210 °C and (d) 240 °C for 2 h, and (e) 210 °C for 4 h. | ||
To prepare an entirely translucent pellet, the period of hot-pressing was optimized. Finally, a translucent pellet was obtained after pressing at 210 °C for 4 h under 360 MPa and the crystallization of the 80Li2S·20P2S5 glass was not observed, as shown in Fig. 1(e) and Fig. 2(e). The microstructure of the pellets was characterized by SEM. Fig. 3 shows the SEM images of a cross-section of the pellet pressed at (a) room temperature and (b) 210 °C. The pellet pressed at room temperature had a grain boundary. On the other hand, a smooth cross-section was observed in the pellet pressed at 210 °C. Softening adhesion among 80Li2S·20P2S5 glass particles occurred by pressing at around the glass transition temperature and obvious grain boundary and voids were not observed. Fig. 4 shows the impedance spectra of the pellets pressed at room temperature (□) and 210 °C (○). The electrolyte pellets were sandwiched between two stainless-steel disks as ion-blocking electrodes. The impedance spectra of both the pellets gave one straight line, which is a typical profile for ion conductors. The resistance decreased and electrical conductivity increased by pressing at 210 °C. The pellet pressed at 210 °C showed the higher conductivity of 8.8 × 10−4 S cm−1 compared to the conductivity of 3.7 × 10−4 S cm−1 in the pellet pressed at room temperature. The electrical conductivity was increased by decreasing the grain boundary and voids. A DC polarization test was performed for the pellet pressed at 210 °C and the electronic conductivity of the pellet was estimated to be 10−7 S cm−1; this conductivity was almost four orders of magnitude lower than the total conductivity determined by AC impedance method. It was confirmed that ionic conduction is dominant in the electrolyte pellet pressed at 210 °C.
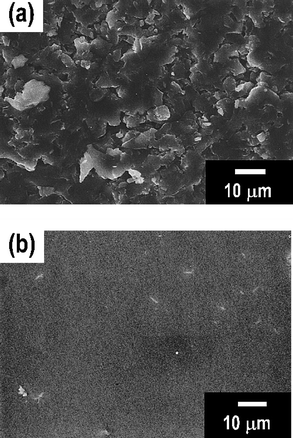 | ||
| Fig. 3 SEM images of a cross-section of the 80Li2S·20P2S5 pellets pressed at (a) room temperature and (b) 210 °C. | ||
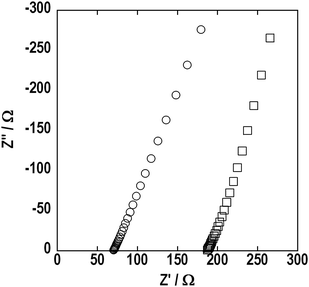 | ||
| Fig. 4 Impedance spectra of the 80Li2S·20P2S5 pellets pressed at room temperature (□) and 210 °C (○). | ||
To improve the electrode–electrolyte interface in the all-solid-state batteries, the softening adhesion between active materials and the 80Li2S·20P2S5 glass was examined. Fig. 5 shows the SEM images of the cross-section of the pellet composed of LiCoO2 and the 80Li2S·20P2S5 glass after pressing at (a) room temperature and (b) 210 °C. The pellet pressed at room temperature showed an obvious grain boundary between LiCoO2 and 80Li2S·20P2S5 glass particles. On the other hand, the grain boundary between LiCoO2 and the 80Li2S·20P2S5 glass particles was ambiguous in the pellet pressed at 210 °C. The 80Li2S·20P2S5 glass stuck to LiCoO2 particles and this would increase the contact area at the electrode–electrolyte interface.
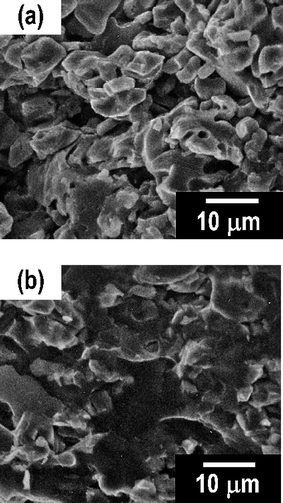 | ||
| Fig. 5 SEM images of a cross-section of the pellets composed of LiCoO2 and 80Li2S·20P2S5 glass after pressing at (a) room temperature and (b) 210 °C. | ||
The effects of the hot-pressing on the electrochemical performance of all-solid-state cells were evaluated. Fig. 6 shows the first charge-discharge curves of the all-solid-state half-cells at 0.064 mA cm−2: (a) In/80Li2S·20P2S5/LiCoO2 and (b) In/80Li2S·20P2S5/Li4Ti5O12. The charge-discharge curves of the all-solid-state cells using the composite electrode pressed at 210 °C is denoted by the solid line. The dashed line indicates the charge-discharge curves of the all-solid-state cells using the composite electrode pressed at room temperature (a conventional procedure). In the voltage profiles of the all-solid-state cells, the vertical axis on the left side denotes the potential versus the Li-In electrode as a counter electrode; the vertical axis on the right side denotes the potential versusLi electrode, which was calculated based on the potential difference between Li-In and Li.13 The reversible capacity of about 100 mAh g−1 was obtained for the cells using LiCoO2 assembled in the previous procedure. Using LiCoO2 composite electrode pressed at 210 °C, the cell exhibited a small capacity of about 40 mAh g−1. On the contrary, the cell using the Li4Ti5O12 composite electrode pressed at 210 °C showed a capacity of about 80 mAh g−1 despite the absence of conductive additives, and the capacity was larger than the capacity of about 40 mAh g−1 for the cell assembled in the previous procedure.
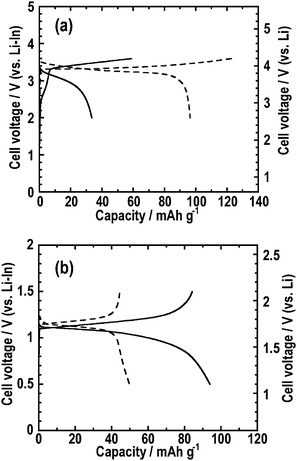 | ||
| Fig. 6 First charge-discharge curves at 0.064 mA cm−2 of the all-solid-state half-cells (a) In/80Li2S·20P2S5/LiCoO2 and (b) In/80Li2S·20P2S5/Li4Ti5O12. The charge-discharge curves of the all-solid-state cells using the composite electrode pressed at 210 °C is denoted by the solid line. The dashed line indicates the charge-discharge curves of the all-solid-state cells using the composite electrode pressed at room temperature (a conventional procedure). | ||
We investigated the structural change of LiCoO2 and Li4Ti5O12 by pressing with the 80Li2S·20P2S5 glass at high temperature. DTA-TG analysis of the mixture of LiCoO2 (or Li4Ti5O12) and the 80Li2S·20P2S5 glass and XRD measurements of the pellet pressed at 210 °C were carried out. However, noticeable thermal changes and structural changes were not observed. More detailed investigation was conducted by the TEM-EDX measurements. The cross-section of the composite LiCoO2 and Li4Ti5O12 electrodes was observed by scanning transmission electron microscopy (STEM). Fig. 7 shows the high-angle annular dark-field (HAADF)-STEM images of the interface between the LiCoO2 or Li4Ti5O12 active material and the solid electrolyte in the composite electrodes pressed at room temperature ((a) and (b)), and pressed at 210 °C ((c) and (d)). In contrast to the interface formed by pressing at room temperature in Fig. 7(a) and (b), there was an interfacial layer between LiCoO2 and the 80Li2S·20P2S5 glass after pressing at 210 °C, as shown in Fig. 7(c). On the other hand, an obvious interfacial layer was not observed at the interface between Li4Ti5O12 and the 80Li2S·20P2S5 glass after pressing at 210 °C.
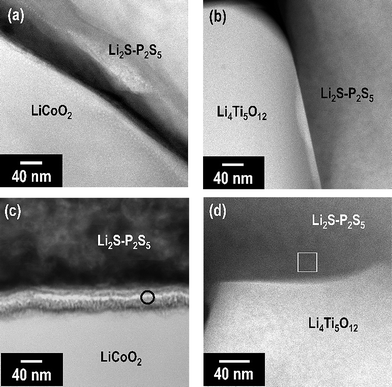 | ||
| Fig. 7 HAADF-STEM images of the interface between the LiCoO2 or Li4Ti5O12 active material and the solid electrolyte in the composite electrodes pressed at room temperature ((a) and (b)), and pressed at 210 °C ((c) and (d)). | ||
The elemental point analyses using EDX spectroscopy were carried out on the solid electrolyte and interfacial layer. Fig. 8(a) and (c) show the EDX-point analysis data at the positions marked by the circle and square in Fig. 7(c) and (d), respectively. Fig. 8(b) also shows the EDX-point analysis data at the solid electrolyte 300 nm from the interface in Fig. 7(c). At all the positions Si and Ga elements were detected. The elements of Si and Ga are derived from the sample stage of the TEM measurements and a residue by Ga ion beam for FIB milling, respectively. The O element, which would be ascribed to the impurity of starting materials of the solid electrolyte and contamination during sample preparation and transfer process for TEM observations, was also detected. On the solid electrolyte, S and P elements derived from the 80Li2S·20P2S5 electrolyte were observed as shown in Fig. 8(b) and (c). S and P elements were detected even at the interfacial layer in Fig. 7(c). At the same time, the Co element existed in the interfacial layer between LiCoO2 and the 80Li2S·20P2S5 glass. It indicates that the elements of LiCoO2 and the 80Li2S·20P2S5 glass mutually diffused by pressing at 210 °C. In addition, the Co diffusion was observed at a distance of further than 300 nm as shown in Fig. 8(b). The Co diffusion suggests the degradation of LiCoO2 and it is a reason why the all-solid-state cell using LiCoO2 electrode pressed at 210 °C showed the smaller capacity as shown in Fig. 6(a). By contrast, there was no Ti element on the solid electrolyte at the vicinity of the Li4Ti5O12 particle. The larger capacity in the all-solid-state cell using Li4Ti5O12 electrode pressed at 210 °C was caused by the increase of contact area between Li4Ti5O12 and the 80Li2S·20P2S5 solid electrolyte.
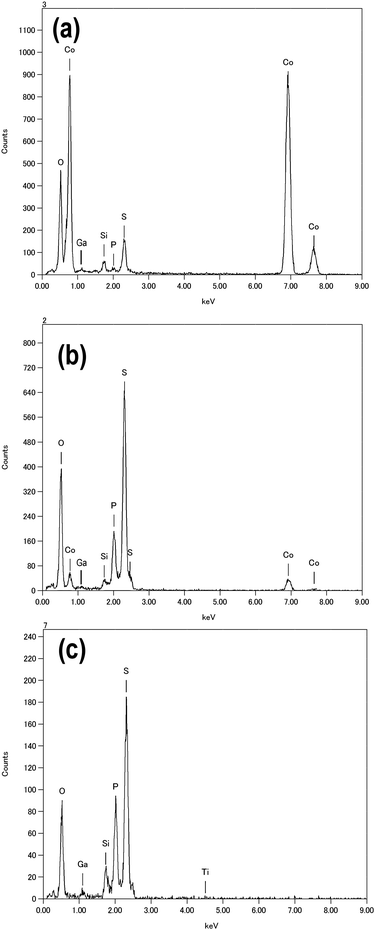 | ||
| Fig. 8 EDX-point analysis data at the following positions: (a) the position marked with a circle in Fig. 7(c), (b) the position at the solid electrolyte 300 nm from the interface in Fig. 7(c), and (c) the position marked with a square in Fig. 7(d). | ||
It was reported that the coating of LiCoO2 with LiNbO3 suppressed the formation of an interfacial resistive layer during charging in the all-solid-state cells.7 The suppression of mutual diffusion between LiCoO2 and the 80Li2S·20P2S5 solid electrolyte during charging by a coating technique was also reported. In order to suppress the mutual diffusion between LiCoO2 and the 80Li2S·20P2S5 solid electrolyte during pressing at 210 °C, the LiNbO3 thin layer was interposed at the electrode–electrolyte interface. Fig. 9 shows the charge-discharge curves of the all-solid-state cell In/80Li2S·20P2S5/LiNbO3-coated LiCoO2 at 0.064 mA cm−2. The first reversible capacity was about 100 mAh g−1. The larger capacity was obtained compared to the all-solid-state cell using the LiCoO2 electrode pressed at 210 °C. The coating suppressed the degradation of LiCoO2 during pressing at 210 °C.
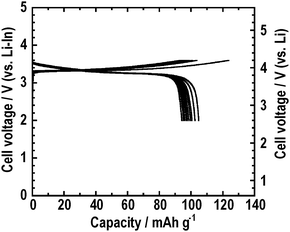 | ||
| Fig. 9 Charge-discharge curves at 0.064 mA cm−2 of the all-solid-state half-cell In/80Li2S·20P2S5/LiNbO3-coated LiCoO2. The composite electrode prepared at 210 °C was used. | ||
Finally, we assembled the all-solid-state full-cell Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2 by pressing at 210 °C. Fig. 10 shows the first charge-discharge curves of the all-solid-state full-cells Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2. The dashed and solid lines indicate the batteries pressed at room temperature and 210 °C, respectively. The all-solid-state full-cells were charged and discharged at 0.064 mA cm−2 in the voltage range between 1.4 and 2.7 V. In this voltage region, the all-solid-state full-cell pressed at room temperature exhibited the first reversible capacity of about 50 mAh g−1. On the other hand, the first reversible capacity was about 120 mAh g−1 for the all-solid-state full-cell pressed at 210 °C. Fig. 11 shows the cycle performance of the all-solid-state full-cells Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2 at 0.064 mA cm−2. The closed and open circles denote the batteries pressed at room temperature and 210 °C, respectively. The cell pressed at room temperature retained about 80% of the initial reversible capacity after 100 cycles. On the other hand, the cell pressed at 210 °C retained a capacity of 110 mAh g−1, which was larger than 90% of the initial reversible capacity, after 100 cycles. The softening of the 80Li2S·20P2S5 glass electrolyte improved the electrode–electrolyte interface in the all-solid-state cells. Sticking the solid electrolyte onto active materials increased the contact area at the electrode–electrolyte interface and the utilization of the active materials was increased.
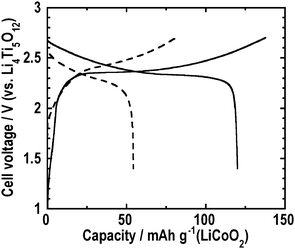 | ||
Fig. 10 First charge-discharge curves at 0.064 mA cm−2 of the all-solid-state full-cells Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2 prepraed by hot press (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and cold press ( ) and cold press (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). ). | ||
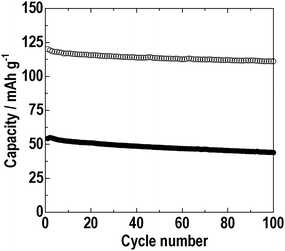 | ||
| Fig. 11 Cycle performance at 0.064 mA cm−2 of the all-solid-state full-cells Li4Ti5O12/80Li2S·20P2S5 glass/LiNbO3-coated LiCoO2 prepraed by hot press (○) and cold press (●). | ||
Conclusions
The 80Li2S·20P2S5 glassy electrolyte was softened by pressing at around the glass transition temperature. Softening adhesion among 80Li2S·20P2S5 glass particles decreased the grain boundary and voids in the pellet and increased the electrical conductivity up to 8.8 × 10−4 S cm−1. Li4Ti5O12 is stable toward supercooled liquid state of the solid electrolyte and the utilization of Li4Ti5O12 as an active material increased in the all-solid-state cell. In contrast, a reaction product by hot press at the interface between LiCoO2 and the solid electrolyte was confirmed by TEM observations and a coating layer to suppress the reaction was needed. The capacity of the all-solid-state full-cell Li4Ti5O12/80Li2S·20P2S5 solid electrolyte/LiNbO3-coated LiCoO2 prepared by hot press increased up to 120 mAh g−1 at 0.064 mA cm−2, compared with the full-cell prepared by cold press. The softening of the 80Li2S·20P2S5 glass electrolyte is an effective way for increasing the contact area between active materials and the solid electrolyte.Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors thank Dr Takada at the National Institute for Materials Science for providing the LiNbO3-coated LiCoO2 particles.References
- Z. Zhang and J. H. Kennedy, Solid State Ionics, 1990, 38, 217 CrossRef CAS
.
- A. Hayashi, S. Hama, H. Morimoto, T. Minami and M. Tatsumisago, J. Am. Ceram. Soc., 2001, 84, 477 CAS
.
- A. Hayashi, S. Hama, T. Minami and M. Tatsumisago, Electrochem. Commun., 2003, 5, 111 CrossRef CAS
.
- F. Mizuno, S. Hama, A. Hayashi, K. Tadanaga, T. Minami and M. Tatsumisago, Chem. Lett., 2002, 1244 CrossRef CAS
.
- M. Tatsumisago and A. Hayashi, J. Non-Cryst. Solids, 2008, 354, 1411 CrossRef CAS
.
- N. Ohta, K. Takada, L. Zhang, R. Ma, M. Osada and T. Sasaki, Adv. Mater., 2006, 18, 2226 CrossRef CAS
.
- N. Ohta, K. Takada, I. Sakaguchi, L. Zhang, R. Ma, K. Fukuda, M. Osada and T. Sasaki, Electrochem. Commun., 2007, 9, 1486 CrossRef CAS
.
- A. Sakuda, H. Kitaura, A. Hayashi, K. Tadanaga and M. Tatsumisago, J. Electrochem. Soc., 2009, 156, A27 CrossRef CAS
.
- H. Kitaura, A. Hayashi, K. Tadanaga and M. Tatsumisago, J. Electrochem. Soc., 2010, 157, A407 CrossRef CAS
.
- H. Kitaura, A. Hayashi, K. Tadanaga and M. Tatsumisago, Electrochim. Acta, 2010 DOI:10.1016/j.electacta.2010.017.066
.
-
R. H. Doremus, in Glass Science, John Wiley and Sons Inc., New York, 2nd edn, 1994, p. 113 Search PubMed
.
- A. Sakuda, A. Hayashi, T. Ohtomo, S. Hama and M. Tatsumisago, Electrochem. Solid-State Lett., 2010, 13, A73 CrossRef CAS
.
- K. Takada, N. Aotani, K. Iwamoto and S. Kondo, Solid State Ionics, 1996, 86–88, 877 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2011 |
