Rapid fractionation of Al in human serum by the use of HiTrap desalting size exclusion column with ICP-MS detection†
Simona
Murko
,
Janez
Ščančar
and
Radmila
Milačič
*
Department of Environmental Sciences, Jožef Stefan Institute, Jamova 39, 1000, Ljubljana, Slovenia. E-mail: radmila.milacic@ijs.si; Fax: + 386 1 2519 385; Tel: + 386 1 477 3560
First published on 29th September 2010
Abstract
To understand the pathways of Al transport in human body it is necessary to identify and quantify Al species present in human serum. It has been demonstrated that about 90% of Al in human serum corresponds to high molecular mass species (HMM-Al) and that Al is bound exclusively to transferrin (Tf). The remaining Al exists in low molecular mass species (LMM-Al) bound predominantly to Al-citrate. In studies of the efficiencies of the chelation therapies and to perform kinetic studies of Al binding to Tf, rapid and reliable analytical procedures are required. Therefore, a new analytical procedure for the efficient, reliable and fast separation of proteins from LMM compounds in serum was developed by the use of a HiTrap desalting size exclusion column. Unspiked and spiked human serum that was left for six hours to reach approximately thermodynamic equilibrium were studied. The separation of HMM-Al from LMM-Al species was accomplished with tris-HCl buffer (pH 7.4) in 10 min. Al elution profile was followed by ICP-MS detection. In the first 5 min HMM-Al compounds were eluted, followed by the elution of LMM-Al species from 5 to 10 min. Results demonstrated that about 94% of Al was present in the HMM (protein) fraction. To prove the reliability of the fractionation procedure developed, the protein peak and LMM separated species were collected in 5 mL fractions, followed by the previously developed speciation procedures. Speciation of the protein peak by CIM® DEAE-ICP-MS verified that about 98% of Al was bound to Tf. In the LMM serum fraction Al-citrate was separated on the FPLC column coupled to ICP-MS. Speciation data confirmed the reliability of the developed fast fractionation procedure. It represents a promising tool for investigations of kinetics of Al binding to Tf and may also be applied in studies of the efficiencies of the chelation therapies in patients overloaded with Al.
Introduction
Aluminium (Al) overload in human body is related to many clinical disorders, especially in renal patients.1,2 Its accumulation in brain has also been associated to the neurodegenerative process in Alzheimer's disease.3,4 By improvement of the quality of water used for dialysis, Al overload in patients with chronic renal failure has been greatly reduced.5 However, the absorption and accumulation of Al, particularly via consumption of Al-based drugs,6 still remains the problem in nephrology. To understand Al transport through the human body it is important to identify and quantify Al species present in serum. The earliest approaches in speciation of Al were oriented to separate low molecular mass Al (LMM-Al) from high molecular mass Al (HMM-Al) species to determine their proportion in human serum. For this purpose ultrafiltration and microultrafiltration (cut off 30000 Da) were first applied.9–11Fractionation of Al was also investigated by size-exclusion chromatography (SEC).12–15 The results of ultrafiltration and SEC procedures in general suggested that about 90% of Al was bound to HMM proteins. However, problems with an excess of spiked Al, most probably as a result of contamination from the ultrafiltration membranes and column supports were reported.12 Due to contamination problems and relatively long separation times (0.5 to 1 h) these simple fractionation procedures were not reliable and were time consuming. For identification and quantification of Al species in the HMM and LMM serum fractions, more selective analytical procedures were needed. So, in the last two decades researchers intensively investigated Al speciation by the use of different chromatographic procedures in combination with element specific detection, mainly electrothermal atomic absorption spectrometry (ETAAS) and inductively coupled plasma mass spectrometry (ICP-MS).16–18Al-compounds eluted under the chromatographic peaks were characterised not only on the basis of the retention times, but were identified also by SDS page19–21 or mass spectrometry techniques.22–24 Results of numerous investigations confirmed that about 90% of Al is bound to HMM compounds and the remaining Al to LMM species. In HMM fraction Al is bound exclusively to serum protein transferrin (Tf),19–21,24–28 while in the LMM fraction Al species correspond predominantly to Al-citrate and in a lesser extent to Al-phosphate and ternary complex of Al with citrate and phosphate.21,22 By lowering of blanks arising from extraneous contamination, reagents and chromatographic supports, it was possible to identify25,27 and quantify24Al species at physiological concentration levels. Despite increasing knowledge of the presence of Al species in human serum, there is still a need for sensitive, reliable and rapid fractionation procedures for studies of the efficiencies of the chelation therapies in renal8–11,29 and Alzheimer's disease patients.30 Fast and reliable fractionation procedures are also essential in investigations of the kinetics of Al binding to serum Tf and importantly contribute to data basis for computational studies of Al distribution and its fate in human body. Such computer modeling investigations are being intensively performed31,32 and need approval by complementary analytical procedures.Therefore, the aim of our work was to develop sensitive analytical procedure for the efficient, reliable and fast separation of proteins from LMM compounds in human serum. For this purpose HiTrap desalting size exclusion column was coupled to ICP-MS. Isocratic elution with tris-hydrochloric acid buffer (pH 7.4) was applied for 10 min to separate HMM-Al from LMM-Al species. In the first 5 min HMM-Al compounds were eluted, followed by the elution of LMM-Al species from 5 to 10 min. The reliability of the fractionation procedure was checked by the previously developed analytical procedures for speciation of Al that were applied to the separated protein peak and fraction containing LMM-Al species.
Experimental
Instrumentation
To separate proteins from LMM species size exclusion HiTrap desalting Sephadex G-25 Superfine column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) of the following column characteristics: dimensions 16 × 25 mm, matrix cross-linked dextran, pH stability 2–13, bead size 15–70 μm, exclusion limit Mr 5000, was applied. A weak anion-exchange CIM® (Convective Interaction Media) DEAE monolithic disk (Bia Separations, Ljubljana, Slovenia) with matrix supports made of highly porous poly(glycidyl methacrylate-co-ethylene dimethacrylate) bearing weak anion exchange diethylamino (DEAE) functional group (dimensions 12 × 3 mm, pH stability 2–14) was used for speciation of HMM-Al species. For speciation of LMM-Al compounds a strong anion-exchange FPLC column of Mono Q HR 5/5 (Pharmacia, Uppsala, Sweden), column characteristics: dimensions 5 × 50 mm, matrix polystyrene/divenyl benzene, pH stability 2–12, particle size 10 μm, was applied.HPLC separations were carried out using a high performance liquid chromatography pump Series 1100 from Agilent (Tokyo, Japan) equipped with a sample injection valve Rheodyne, Model 7725i (Cotati, California, USA), fitted with a 1 mL injection loop for HiTrap desalting column. A 0.1 and 0.5 mL injection loops were used for CIM®DEAE disk and FPLC column, respectively. A UV-vis detector (Agilent 1100 Series Diode Array and Multiple Wavelength Detector, DAD/MWD) was used on-line with HPLC equipment for absorption measurements at 278 nm. The HPLC system was biocompatible. Pressures were within the range of the FPLC pumps, preventing denaturation of proteins.
Element-specific detection of Al after chromatographic separation as well as the total concentration of Al in serum was performed using ICP-MS model 7500ce from Agilent Technologies (Agilent, Tokyo, Japan). The outlet of the chromatographic support was directly connected to the Babington nebuliser and a Scott-type spray chamber of ICP-MS instrument. A nickel sampler and skimmer with 1.0 and 0.4 mm cone orifices, respectively, were used. Treatment of data was performed with the Agilent ChemStation software. Data processing was based on the peak area. ICP-MS operating conditions for determination of Al are listed in Table 1.
| Parameter | Value |
|---|---|
| RF power (W) | 1500 |
| Sampling depth (mm) | 8.0 |
| Outer gas flow rate (L min−1) | 15.0 |
| Carrier gas flow-rate (L min−1) | 0.80 |
| Make-up gas flow-rate (L min−1) | 0.11 |
| He gas flow-rate (mL min−1) | 3.0 |
| m/z monitored | 27 |
| Integration time (s) | 3.0 |
| Total acquisition time (s) | 600–900 |
| QP bias (V) | −15 |
| Oct bias (V) | −18 |
| Extract 1 (V) | −0.3 |
| Extract 2 (V) | −150 |
A WTW (Weilheim, Germany) 330 pH meter was employed to determine the pH.
Reagents and materials
Merck (Darmstadt, Germany) suprapur acids and Milli-Q water (Direct-Q 5 Ultrapure water system, Millipore Watertown, MA, USA) were used for the preparation of samples and standard solutions. All other reagents were of analytical reagent grade. A stock standard solution of Al (1000 ± 0.002 mg L−1 in 5% HNO3) used for calibration in ICP-MS determinations was obtained from Merck. A stock Al3+ solution (100 μg Al mL−1) used for the spiking of serum sample and standard proteins was prepared in a 100 mL calibration flask by dissolving 0.1388 g of Al(NO3)3· 9H2O (Riedel-de Haën, Hannover, Germany) in water. A stock Al-citrate solution (100 μg Al mL−1) was made by mixing citric acid (Merck) and aluminium nitrate-9-hydrate in 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 citric acid to Al molar ratio.22 Fresh working standard solutions were prepared daily by dilution of stock solutions with water. In the HiTrap desalting column procedure buffer A consisted of 0.05 mol L−1 tris-HCl buffer (Merck) that was adjusted to 7.4 with an appropriate amount of 1 mol L−1 of hydrochloric acid. Buffer B contained 1 mol L−1 of ammonium chloride (Merck) and was prepared by dissolving 53.49 g NH4Cl in 1 L of buffer A. In the CIM®DEAE disk procedure buffer A (pH 7.4) consisted of 0.05 mol L−1 tris-HCl buffer + 0.03 mol L−1NaHCO3 (Merck). Buffer B contained 1 mol L−1 of ammonium chloride dissolved in buffer A. In the FPLC procedure 4 mol L−1ammonium nitrate eluent was prepared by dissolving 320.16 g of HN4NO3 in 1 L water. Standard proteins (Sigma-Aldrich, Steinheim, Germany) of known molecular masses: albumin (66000 Da), Imunoglobulin G (IgG) (150000 Da) and transferrin (Tf) (77000 Da) (0.5–25 g L−1) were used for separation of protein peak on the HiTrap desalting column and for calibration of the CIM DEAE disks. Chelex 100 (Na+ form, 100–200 mesh) chelating ion-exchange resin (Sigma) and a silica based LiChrosorb RP-18 HPLC column (150 × 4.6 mm i.d.) were used for purification of the eluents.22 For the accuracy check the certified serum sample, SeronormTM Trace Elements of Serum L-1, obtained from Sero AS (Billingstad, Norway) was used.
1 citric acid to Al molar ratio.22 Fresh working standard solutions were prepared daily by dilution of stock solutions with water. In the HiTrap desalting column procedure buffer A consisted of 0.05 mol L−1 tris-HCl buffer (Merck) that was adjusted to 7.4 with an appropriate amount of 1 mol L−1 of hydrochloric acid. Buffer B contained 1 mol L−1 of ammonium chloride (Merck) and was prepared by dissolving 53.49 g NH4Cl in 1 L of buffer A. In the CIM®DEAE disk procedure buffer A (pH 7.4) consisted of 0.05 mol L−1 tris-HCl buffer + 0.03 mol L−1NaHCO3 (Merck). Buffer B contained 1 mol L−1 of ammonium chloride dissolved in buffer A. In the FPLC procedure 4 mol L−1ammonium nitrate eluent was prepared by dissolving 320.16 g of HN4NO3 in 1 L water. Standard proteins (Sigma-Aldrich, Steinheim, Germany) of known molecular masses: albumin (66000 Da), Imunoglobulin G (IgG) (150000 Da) and transferrin (Tf) (77000 Da) (0.5–25 g L−1) were used for separation of protein peak on the HiTrap desalting column and for calibration of the CIM DEAE disks. Chelex 100 (Na+ form, 100–200 mesh) chelating ion-exchange resin (Sigma) and a silica based LiChrosorb RP-18 HPLC column (150 × 4.6 mm i.d.) were used for purification of the eluents.22 For the accuracy check the certified serum sample, SeronormTM Trace Elements of Serum L-1, obtained from Sero AS (Billingstad, Norway) was used.
Recommended cleaning procedures for the laboratory ware, tubes, eluents and ICP-MS system
To avoid contamination by extraneous Al, polyethylene or Teflon laboratory ware and tubes were used. Teflon bottles were also used for rinsing solutions in the ICP-MS system. Before use, all laboratory ware and tubes for chromatographic separations and ICP determinations were treated with 10% nitric acid for 48 h, rinsed well with MilliQ water and dried at room temperature. In order to reduce memory effects from ICP-MS system five rinses after each analysis of serum sample (the first rinse with 5% nitric acid and the following four with water) were applied. All eluents used in the chromatographic separations were efficiently cleaned by the batch chelating ion-exchange (Chelex 100, Na+ form) procedure, followed by further purification through the silica based reversed-phase HPLC column.22,23Recommended cleaning procedures for chromatographic supports
The following cleaning procedures were applied after 10 consecutive sample separations:Sample preparation
Venous blood (venous puncture) from transplanted renal patients was taken during clinical examination after informed consent was obtained. It was collected into Al-free Becton-Dickinson vacutainers without additives. Sample was centrifuged for 10 min at 855 g. Serum aliquots were transferred into 1 mL polyethylene tubes with polyethylene pipette and stored in a freezer at −20 °C. Prior to analysis samples were equilibrated to room temperature.Standard proteins (25 g L−1 of albumin, 5 g L−1 of IgG and 2.5 g L−1 of Tf) used for the optimization of the analytical procedures for the separation of serum proteins were dissolved in buffer A (pH 7.4). A certified serum sample was reconstituted following the producer's instructions.
In order to study the speciation of HMM-Al and LMM-Al compounds, spiked and unspiked serum samples were used. The spiking of the serum was performed with 100 μL of Al3+ solution (Al-nitrate salt) added to 3 mL of serum, so that the final concentrations of Al in serum were 150 to 300 ng mL−1. Spiked serum was left at room temperature for 6 h to reach approximately thermodynamic equilibrium.22,23 Speciation of Al was then performed following the Recommended analytical procedures.
Recommended analytical procedures
Sample preparation, chromatographic separations and determination of Al by ICP-MS were carried out under clean-room conditions (class 10000).If not stated otherwise, all the analysis were done in two replicates.
Results and discussion
Determination of total Al concentrations in serum samples
The total Al concentrations in serum samples were determined by ICP-MS under the optimal operating conditions given in Table 1. Before analysis serum samples were diluted (1 + 4) with water. In the separated HMM and LMM serum fractions after HiTrap desalting SEC procedure, samples were already diluted five times during the chromatographic separation step. The concentration of Al in serum samples (mean of three parallel analysis) was determined by the standard addition method. The accuracy of the determination of total Al was checked by the analysis of reference serum sample (SeronormTM Trace Elements of Serum L-1). The determined Al concentration (7.1 ± 0.6 ng mL−1) agreed well with the certified value (7.6 ± 0.7 ng mL−1), confirming the accuracy of the analytical procedure applied.Optimization of the analytical procedure for the separations of serum proteins from LMM compounds by the use of HiTrap desalting SEC with UV-ICP-MS detection
To separate serum proteins from LMM species HiTrap desalting SEC column was used. 1 mL of a synthetic solution of standard proteins (25 g L−1 of albumin, 5 g L−1 of IgG and 2.5 g L−1 of Tf) prepared in buffer A (50 mmol L−1 tris-hydrochloric acid, pH 7.4) was injected onto the column. The chromatographic run was carried out at a flow rate of 1 mL min−1. Isocratic elution using buffer A was applied for 10 min. The separation of proteins was followed on-line by UV detection at 278 nm, while Al elution profile was detected by ICP-MS. The UV chromatogram of a synthetic solution of standard proteins albumin, IgG and Tf separated on the HiTrap desalting SEC column is presented in Fig. 1A. It is evident that proteins were eluted in a single peak from 1.5 to 4 min. The same chromatographic conditions were used for the separation of undiluted human serum. The UV chromatogram is presented in Fig. 1B. As it can be seen from Fig. 1Bproteins were separated from 1.5 min to 5 min, while LMM species present in serum were eluted from 5 to 10 min. Since synthetic solution of standard proteins (albumin, IgG and Tf) and undiluted serum contained traces of Al (total concentration 3.3 ± 0.3 ng Al mL−1 and 2.6 ± 0.2 ng Al mL−1, respectively), it was possible also to follow the Al elution profiles by ICP-MS (Fig. 1C). It can be seen from Fig. 1C that the majority of Al in serum was eluted at the elution time corresponding to HMM-Al compounds. In order to prove that LMM compounds present in serum did not co-elute with serum proteins it was necessary to examine the behaviour of Al-citrate on the HiTrap desalting SEC column. For this purpose a synthetic solution of Al-citrate (10 ng mL−1Al) was prepared in buffer A and separation of Al species performed following the Recommended analytical procedure. Data are presented in Fig. 1C. From the Al elution profile for Al-citrate (Fig. 1C) it can be seen that this LMM-Al species was eluted from 5 to 8 min. Data from Fig. 1 (A-C) confirmed that the developed HiTrap desalting SEC procedure efficiently and rapidly separates proteins from LMM compounds in serum.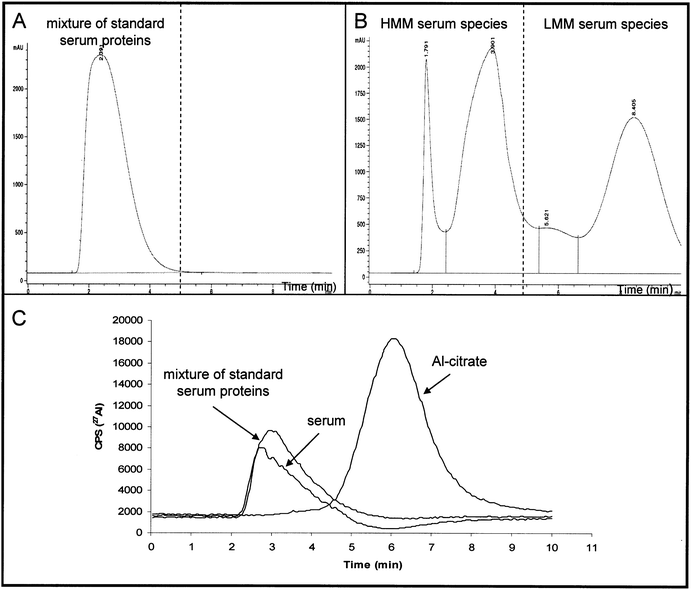 | ||
| Fig. 1 Separation of the mixture of standard serum proteins, undiluted human serum and synthetic solution of Al-citrate (10 ng mL−1Al) on a HiTrap desalting SEC column followed by UV (278 nm) and ICP-MS detection: (A) UV chromatogram of the mixture of standard serum proteins (25 g L−1 of albumin, 5 g L−1 of IgG and 2.5 g L−1 of Tf), (B) UV chromatogram of undiluted human serum, (C) Al elution profiles for the separation of the mixture of standard serum proteins (3.3 ng mL−1Al), undiluted human serum (2.6 ng Al mL−1) and synthetic solution of Al-citrate (10 ng mL−1Al). | ||
Reliability of the HiTrap desalting SEC fast fractionation procedure
In order to verify that the fractionation on the HiTrap desalting SEC column does not make any influence on Al species present in human serum, further speciation of the protein peak and the LMM serum fractions was performed by the previously developed CIM®DEAE disk28 and FPLC22,23 analytical procedures. For this purpose human serum was spiked with 300 ng mL−1Al, so that the total Al concentration in serum was 305 ± 6 ng mL−1, and left to equilibrate for 6 h at room temperature.22 1 mL of sample was injected onto the HiTrap desalting SEC column and separation performed following the Recommended analytical procedure. The protein peak was collected from 0 to 5 min, while the fraction of LMM compounds was collected from 5 to 10 min (HMM and LMM fractions were diluted five times). Analysis of the total concentration of Al in the protein peak and the LMM serum fraction determined by ICP-MS demonstrated that 284 ± 6 ng Al mL−1 (93%) of the total Al in spiked serum corresponded to HMM-Al species and 22.6 ± 0.8 ng Al mL−1 (7.4%) to LMM-Al compounds.Speciation of the serum protein peak by the CIM®DEAE procedure
Speciation of the HMM-Al species was examined by applying a 0.1 mL aliquot of the protein peak onto the CIM®DEAE disk. Concentration of Al in a five time diluted protein peak fraction was 57 ± 1 ng mL−1. The chromatographic procedure was performed as described under the Recommended analytical procedure. The elution profiles of separated proteins followed by UV and Al by ICP-MS detection are presented in Fig. 2A and Fig. 2B, respectively. It may be seen From Fig. 2A and Fig. 2B that Al from the protein peak fraction was eluted under the Tf peak. For quantification of the amount of Al bound to Tf, calibration curve was prepared by spiking of Tf, dissolved in 0.05 mol L−1 tris-HCl buffer + 0.03 mol L−1NaHCO3, pH 7.4, with known amounts of Al. The Al-Tf standard solutions were separated on the CIM®DEAE disk under the same chromatographic conditions as a protein peak sample. Al concentration that corresponded to Al-Tf was calculated on the peak area basis and was found to be 280 ± 6 ng Al mL−1. This data confirmed that Al in the HMM serum fraction was quantitatively eluted as Al-Tf species. The Al binding ligand Tf was identified in our previous studies not only on the basis of the retention volume, but also by the SDS-PAGE21 and acquity ultra performance liquid chromatography-electrospray ionization mass spectrometry (UPLC-ESI-MS).24 Therefore, the above described observations are in agreement with our previous observations21,24,28 and observations of other researchers.19,20,25–28 In addition, experimental data obtained by CIM®DEAE - ICP-MS procedure (Fig. 2) proved that the separation of serum on the HiTrap desalting SEC column did not influence the speciation of HMM-Al species.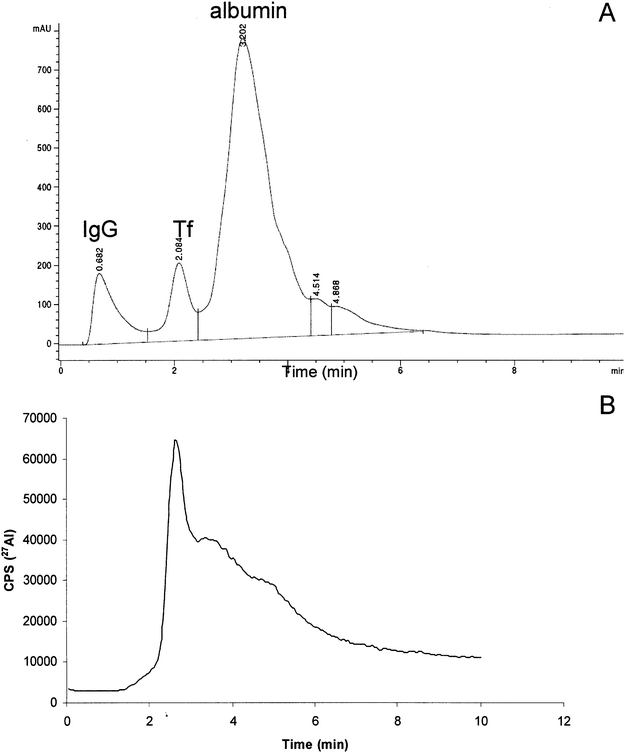 | ||
| Fig. 2 Separation of spiked human serum (300 ng mL−1 of Al) on anion-exchange CIM®DEAE disk followed by UV (278 nm) and ICP-MS detection. 1 mL of spiked serum was first injected onto the HiTrap desalting SEC column. A protein peak was collected from 0 to 5 min (5 mL). Concentration of Al in a five times diluted protein peak was 57 ± 1 ng mL−1. 0.1 mL aliquot of the protein peak was then injected onto the CIM®DEAE disk: (A) UV chromatogram and (B) Al elution profile. | ||
Speciation of the LMM serum fraction by the FPLC procedure
In the LMM serum fraction eluted from HiTrap desalting SEC column the speciation of LMM-Al compounds was performed by the use anion-exchange FPLC. Concentration of total Al in a five time diluted LMM serum fraction was 4.5 ± 0.1 ng mL−1. A 0.5 mL aliquot of the LMM fraction was injected onto the column and chromatographic separation was accomplished following the Recommended analytical procedure. The elution profile of Al obtained by ICP-MS is presented in Fig. 3. In the same Fig. elution profile of a synthetic solution of Al-citrate (10 ng mL−1Al, pH 7.4) is overlayed. It can be seen from Fig. 3 that LMM-Al species in serum were almost completely eluted at the elution time corresponding to Al-citrate. The small LMM-Al peak from serum fraction can also be observed around 2 min, corresponding presumably to Al-phosphate.22,23 The identification of Al binding ligands (citrate and phosphate) was performed in our previous investigations not only on the basis of the retention volume, but also by the by electrospray (ES)-MS-MS analysis.22,23 Quantification of Al-citrate in the LMM serum fraction was made on the basis of the calibration curve (peak area) composed of a synthetic solutions of Al-citrate (pH 7.4). The Al-citrate standard solutions were separated on the FPLC column under the same chromatographic conditions as a LMM serum fraction. Al concentration in the LMM serum fraction that corresponded to Al-citrate was calculated on the peak area basis and was found to be 4.3 ± 0.1 ng Al mL−1. Experimental results obtained by FPLC-ICP-MS procedure (Fig. 3) proved that the separation of serum on the HiTrap desalting SEC column did not influence the speciation of LMM-Al species.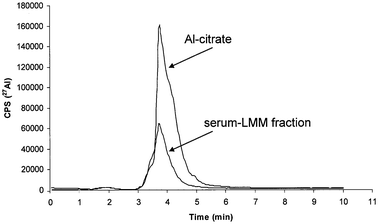 | ||
| Fig. 3 Al elution profiles of the LMM-Al compounds in spiked human serum (300 ng mL−1 of Al) and a synthetic solution of Al-citrate (10 ng mL−1Al) separated on the anion-exchange FPLC column followed by ICP-MS detection. 1 mL of spiked serum was first injected onto the HiTrap desalting SEC column. A LMM serum peak was collected from 5 to 10 min (5 mL). Concentration of Al in a five times diluted LMM peak was 4.5 ng mL−1. 0.5 mL aliquot of the LMM peak was then injected onto the anion-exchange FPLC column. | ||
Performances of the HiTrap desalting SEC with ICP-MS detection
The repeatability of the fractionation procedure was tested for six consecutive separations of spiked (300 ng Al mL−1) serum sample. The RSD for Al determined in the HMM serum fraction was 2% while in the LMM serum fraction 8%. The limit of detection for the determination of Al in the HMM and LMM serum fractions calculated as 3 s of the blank sample was found to be 0.2 ng Al mL−1.Fractionation of Al species in human serum by the use of HiTrap desalting SEC with ICP-MS detection
In order to study the potential of the developed fast HiTrap desalting SEC-ICP-MS procedure, fractionation of HMM-Al and LMM-Al species was applied in several serum samples from transplanted renal patients. To find out whether spiking influence the distribution of Al species, an unspiked serum (sample A) containing 2.6 ± 0.2 ng Al mL−1 and sample A spiked with Al3+ solutions of 150 and 300 ng mL−1Al was examined. In addition, four other serum samples spiked with 300 ng mL−1Al were analysed. Samples were injected onto the HiTrap desalting SEC column and the separation of HMM from LMM compounds was performed following the Recommended analytical procedure. Total Al content and concentrations of Al in separated HMM and LMM fractions were determined by ICP-MS. The results are presented in Table 2. Data in Table 2 indicate that spiking does not influence the Al distribution in serum samples. It is evident that about 94% of Al is eluted as HMM-Al species (Al bound to proteins) and that the remaining Al corresponds to LMM-Al compounds. These findings support the previously reported literature data on fractionation of Al in serum samples by the ultrafiltration and SEC procedures.7–15 However, it should be emphasised that spiked samples may not represent the in vivo situation. The latter has recently been investigated computationally with some initial success in the group of Exley.31,32| Sample | Total Al/ng mL−1 | Al in HMM fraction/ng mL−1 | Al in LMM fraction/ng mL−1 | Al in HMM fraction (%) | Al in LMM fraction (%) |
|---|---|---|---|---|---|
| Serum A | 2.6 ± 0.2 | 2.4 ± 0.2 | 0.2 ± 0.03 | 92 ± 8 | 7.6 ± 1.1 |
| Serum A + 150 ng mL−1Al | 157 ± 6 | 149 ± 5 | 7.8 ± 0.4 | 95 ± 3 | 5.0 ± 0.2 |
| Serum A + 300 ng mL−1Al | 305 ± 6 | 284 ± 6 | 22.6 ± 0.8 | 93 ± 2 | 7.4 ± 0.2 |
| Serum B + 300 ng mL−1Al | 344 ± 7 | 330 ± 6 | 14.1 ± 0.4 | 96 ± 2 | 4.1 ± 0.1 |
| Serum C + 300 ng mL−1Al | 384 ± 7 | 365 ± 7 | 19.2 ± 0.5 | 95 ± 3 | 5.0 ± 0.1 |
| Serum D + 300 ng mL−1Al | 455 ± 7 | 423 ± 7 | 32.4 ± 0.4 | 93 ± 2 | 7.1 ± 0.2 |
| Serum E + 300 ng mL−1Al | 348 ± 6 | 331 ± 6 | 17.4 ± 0.3 | 95 ± 3 | 5.0 ± 0.2 |
There are several advantages of the presently developed fast fractionation procedure. The major benefits lie in the simplicity and the speed of separation of the HMM from the LMM serum constituents. Partitioning of Al between serum proteins and the LMM-Al compounds may be quantified in both, the HMM serum fraction (first 5 min of the separation procedure), and in the LMM serum fraction (the following 5 min of the separation procedure). In comparison to ultrafiltration and SEC procedures7–15 that take 0.5 to 1 h, the separation time of the HiTrap desalting SEC procedure is much shorter. In addition, ultrafiltration procedures enable quantification of Al only in the LMM serum fraction,7–11 while by the use of the conventional SEC procedures the quantification of Al is in general possible only in the protein fractions.11–15 It is of great importance that the HiTrap desalting SEC fractionation procedure does not influence the chemical speciation of the serum constituents and therefore enables further speciation procedures to be applied in the separated HMM and LMM serum fractions. The fast fractionation procedure is also reliable and sensitive enough to perform fractionation of serum samples containing Al at physiological concentrations levels. This is a great advantage in comparison to previously applied ultrafiltration and SEC procedures7–15 that made possible only analysis of serum samples with elevated Al concentrations or in spiked serum samples. Since there was enough evidence that HMM-Al was exclusively bound to Tf, the developed fast fractionation procedure is highly applicable for investigations of the kinetics of binding of Al to Tf. It represents also a promising tool for studies of the efficiencies of the chelation therapies in patients overloaded with Al. Such therapies have been already performed in dialysis patients8–11 and are presently again actual in modalities for other disorders. The latest discoveries on close association between brain metal dishomeostasis and onset or progression of Alzheimer's disease, rendered chelation therapy as a promising option also for the treatment of this neurological disease.29
Conclusions
A new analytical procedure for the fast, efficient, sensitive and reliable separation of proteins from LMM compounds in human serum was developed. HiTrap desalting SEC column coupled to ICP-MS enables separation of HMM from LMM species in serum in 10 min. By the use of isocratic elution with tris-hydrochloric acid buffer (pH 7.4), HMM-Al species were eluted in the first 5 min followed by the elution of LMM-Al compounds from 5 to 10 min. To follow the partitioning of Al in human serum, 5 mL fractions were collected and the concentration of Al determined by ICP-MS in the HMM (protein) and LMM serum fractions. Data of the presented study demonstrated that about 94% of Al in unspiked (Al concentration at physiological concentration levels) as well as in spiked serum samples was present in the protein fraction. To prove the reliability of the fractionation procedure developed, the protein peak and the LMM separated species were subjected to further speciation procedures by CIM® DEAE-ICP-MS and FPLC - ICP-MS, respectively. Results indicated that about 98% of Al in the HMM serum fraction was bound to Tf, while Al in the LMM serum fraction corresponded primarily to Al-citrate. The speciation data verified that during the fractionation on the HiTrap desalting SEC, no species transformation occurred and hence confirmed the reliability of the developed fast fractionation procedure.Due to the rapidness, sensitivity and reliability, developed analytical procedure is a promising tool for investigations of kinetics of Al binding to Tf. As the exact mechanism of Al binding to Tf is still not known, the results obtained in kinetic studies could represent an important basis for construction of an Al-Tf model at equilibrium and non-equilibrium state, at the point when equilibrium is being approached. The developed fractionation procedure is also very suitable for studies of the efficiencies of the chelation therapies in patients overloaded with Al. With slight modifications it may be applied also in investigations of the partitioning of some other metals in serum samples.
Acknowledgements
This work was supported by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia (Program group P1-0143). Authors thank Miha Benedik, M.Sc, MD from Centre for Dialysis, Department of Nephrology of University Medical Centre Ljubljana, Slovenia, for providing serum samples.References
- A. I. Arieff, Am. J. Kidney Dis., 1995, 6, 317–321 Search PubMed.
- E. Reuche, Acta Neuropathol., 1997, 94, 612–616 CrossRef.
- C. Exley, J. Alzheimer's Dis., 2007, 12, 313–315.
- D. Drago, M. Bettella, S. Bolognin, L. Cendron, J. Ščančar, R. Milačič, F. Ricchelli, A. Casini, L. Messori, G. Tognon and P. Zatta, Int. J. Biochem. Cell Biol., 2008, 40, 731–746 CrossRef CAS.
- M. E. De Broe, P. C. D'Haese, M. M. Couttenye, G. F. Van Landeghem and L. V. Lamberts, Nephrol. Dial. Transplant., 1993, 1, 47–50.
- C. Exley, E. Burgess, J. P. Day, E. H. Jeffery, S. Melethil and R. A. Yokel, J. Toxicol. Environ. Health, 1996, 48, 569–584 CrossRef CAS.
- J. Pérez Parajón, E. Blanco González, J. B. Cannata and A. Sanz-Medel, Tr. Elements in Medicine, 1989, 6, 41–46 Search PubMed.
- K. Wróbel, E. Blanco González and A. Sanz-Medel, Tr. Elements in Medicine, 1993, 10, 97–103 Search PubMed.
- K. Wróbel, E. Blanco González and A. Sanz-Medel, J. Anal. At. Spectrom., 1994, 9, 281–284 RSC.
- A. Canteros, C. Díaz Corte, J. L. Fernández Martín, C. Fernández Merayo and J. Cannata, Nephrol., Dial., Transplant., 1998, 13, 1538–1542 CrossRef CAS.
- E. Blanco González, J. Pérez Parajón, J. I. García Alonso and Sanz-Medel, J. Anal. At. Spectrom., 1989, 4, 175–179 RSC.
- H. Keirsse, Smeyers-Verbekke, D. Verbeele and D. L. Massart, Anal. Chim. Acta, 1987, 196, 103–114 CrossRef CAS.
- M. Favarato, C. A. Mizzen and D. R. McLachlan, J.Chromatogr., 1992, 576, 271–285 CrossRef CAS.
- H. B. Röllin and C. M. C. A. Nogueira, Eur. J. Clin. Chem. Biochem., 1997, 35, 215–222 Search PubMed.
- J. Ščančar, R. Milačič, M. Benedik and I. Križaj, Talanta, 2003, 59, 355–364 CrossRef CAS.
- A. Sanz-Medel, A. B. Soldado Cabezuelo, R. Milačič and T. Bantan Polak, Coord. Chem. Rev., 2002, 228, 373–383 CrossRef CAS.
- R. Milačič, in Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine & Occupational Health, ed. R. Cornelis, H. Crews, J. Caruso and K. Heumann, John Wiley & Sons, Chichester, U.K., 2005, p.p. 27–39 Search PubMed.
- R. Milačič, S. Murko and J. Ščančar, J. Inorg. Biochem., 2009, 103, 1504–1513 CrossRef CAS.
- K. Wróbel, E. Blanco González, Kz. Wróbel and A. Sanz-Medel, Analyst, 1995, 120, 809–815 RSC.
- M. H. Nagaoka and T. Maitani, Biochim. Biophys. Acta, 2000, 1523, 182–188 CrossRef CAS.
- Bl. Kralj, J. Ščančar, I. Križaj, M. Benedik, P. Bukovec and R. Milačič, J. Anal. At. Spectrom., 2004, 19, 101–106 RSC.
- T. Bantan, R. Milačič, B. Mitrović and B. Pihlar, J. Anal. At. Spectrom., 1999, 14, 1743 RSC.
- T. Bantan Polak, R. Milačič, B. Mitrović and M. Benedik, J. Pharm. Biomed. Anal., 2001, 26, 189 CrossRef CAS.
- S. Murko, R. Milačič, B. Kralj and J. Ščančar, Anal. Chem., 2009, 81, 4929–4936 CrossRef CAS.
- A. Soldado Cabezuelo, M. Montes Bayón, E. Blanco González, J. I. García Alonso and A. Sanz-Medel, Analyst, 1998, 123, 865–869 RSC.
- M. H. Nagaoka and T. Maitani, Analyst, 2000, 125, 1962–1965 RSC.
- M. H. Nagaoka and T. Maitani, J. Inorg. Biochem., 2005, 99, 1887–1894 CrossRef CAS.
- S. Murko, R. Milačič and J. Ščančar, J. Inorg. Biochem., 2007, 101, 1234–1241 CrossRef CAS.
- A. Soldado Cabezuelo, E. Blanco González and A. Sanz-Medel, Analyst, 1997, 122, 573–577 RSC.
- M. L. Hedge, P. Bharathi, A. Suram, C. Venugopal, R. Jagannathan, P. Poddar, P. Srinivas, K. Sambamurti, K. J. Rao, J. Ščančar, L. Messon, L. Zecca and P. Zatta, J. Alzheimer's Dis., 2009, 17, 457–468.
- J. Beardmore, G. Rugg and C. Exley, J. Inorg. Biochem., 2007, 101, 1187–1191 CrossRef CAS.
- J. Beardmore and C. Exley, J. Inorg. Biochem., 2009, 103, 205–209 CrossRef CAS.
Footnote |
| † This article is part of a themed issue highlighting outstanding and emerging work in the area of speciation. |
| This journal is © The Royal Society of Chemistry 2011 |
