In search of the Golden Fleece: unraveling principles of morphogenesis by studying the integrative biology of skin appendages†‡
Michael W.
Hughes
a,
Ping
Wu
a,
Ting-Xin
Jiang
a,
Sung-Jan
Lin
abcd,
Chen-Yuan
Dong
bef,
Ang
Li
a,
Fon-Jou
Hsieh
bg,
Randall B.
Widelitz
a and
Cheng Ming
Chuong
*ab
aDepartment of Pathology, School of Medicine, University of Southern California, HMR 315B, 2011 Zonal Ave., Los Angeles, CA 90033. E-mail: cmchuong@usc.edu; Fax: +1 323 442 3049; Tel: +1 323 442 1296
bResearch Center for Developmental Biology and Regenerative Medicine, National Taiwan University, Taipei, Taiwan
cInstitute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan
dDepartment of Dermatology, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan
eDepartment of Physics, National Taiwan University, Taipei, Taiwan
fCenter for Quantum Science and Engineering, National Taiwan University, Taipei, Taiwan
gDepartment of Obstetrics and Gynecology, National Taiwan University Hospital and College of Medicine, Taipei, Taiwan
First published on 24th March 2011
Abstract
The mythological story of the Golden Fleece symbolizes the magical regenerative power of skin appendages. Similar to the adventurous pursuit of the Golden Fleece by the multi-talented Argonauts, today we also need an integrated multi-disciplined approach to understand the cellular and molecular processes during development, regeneration and evolution of skin appendages. To this end, we have explored several aspects of skin appendage biology that contribute to the Turing activator/inhibitor model in feather pattern formation, the topo-biological arrangement of stem cells in organ shape determination, the macro-environmental regulation of stem cells in regenerative hair waves, and potential novel molecular pathways in the morphological evolution of feathers. Here we show our current integrative biology efforts to unravel the complex cellular behavior in patterning stem cells and the control of regional specificity in skin appendages. We use feather/scale tissue recombination to demonstrate the timing control of competence and inducibility. Feathers from different body regions are used to study skin regional specificity. Bioinformatic analyses of transcriptome microarrays show the potential involvement of candidate molecular pathways. We further show Hox genes exhibit some region specific expression patterns. To visualize real time events, we applied time-lapse movies, confocal microscopy and multiphoton microscopy to analyze the morphogenesis of cultured embryonic chicken skin explants. These modern imaging technologies reveal unexpectedly complex cellular flow and organization of extracellular matrix molecules in three dimensions. While these approaches are in preliminary stages, this perspective highlights the challenges we face and new integrative tools we will use. Future work will follow these leads to develop a systems biology view and understanding in the morphogenetic principles that govern the development and regeneration of ectodermal organs.
Insight, innovation, integrationInsightful: We pursue a system biology understanding of ectodermal organs from the molecular level, cellular level, organ, organism, and evolutionary level. We have found common ground between diverse ectodermal organs. We have developed new concepts in topobiology, periodic patterning, and macro-environmental regulation of stem cells. Innovative: We use novel imaging techniques to study the skin, and apply bioinformatics to study recombination explants. Integration: We have taken a multidisciplinary approach to obtain a system biology understanding of the morphogenetic principles governing the development, regeneration and evolution of ectodermal organs. The disciplines include stem cell biology, development, evolution, mathematical modeling, dermatology, paleontology, etc. |
Introduction
In Greek mythology, Jason launches a journey to pursue the Golden Fleece. The Golden Fleece symbolizes rejuvenation power. In the story, nine headed dragons regenerate their heads instantly when they are severed from the body of the beast. Dragon teeth give rise to warriors when placed in contact with the right soil. In these early days of human civilization, the human mind must have been awed by the robust regenerative power of some ectodermal organ appendages. They could not understand how regeneration occurs and used myths to tell adventurous stories based in part on their observations. In the story, Jason assembled a multi-disciplined, multi-talented team of Argonauts that managed to overcome overwhelming odds to successfully obtain the Golden Fleece. Today, to search for the fundamental principles of morphogenesis and the regenerative power of skin appendages, we will still need to take a multi-disciplined, integrative biological approach.A concept animal with different types of (non-neural) ectodermal organs is shown in Fig. 1A. There are hairs, feathers, teeth, horns, nails, salivary glands, sweat glands, mammary glands, etc. During development, different ectodermal organs share the same developmental origin but become different types of ectodermal organs through interactions between the epithelia and mesenchyme (Fig. 1B).1 Since the integument forms the interface between the body and its external environment, these ectodermal organs have to endure frequent wear and tear and therefore evolved robust healing and regenerative powers. Different modes of regeneration are utilized by different ectodermal organs. Skin epidermis undergoes continuous renewal. Mammary glands undergo involution and growth phases. Hair and feathers are unique in that they undergo cyclic regeneration of the major portion of each organ under physiological conditions. During shedding or molting, the ‘older’ hairs or feathers are shed. Upon initiation of regeneration, the dermal papilla interacts with stem cells and new hairs or feathers (mini-organs) are re-made. Some interesting insights can be gained by comparing apparently different skin appendages. For example, feathers and mammary glands appear to be unrelated organs. Yet a comparison showed that both are ectodermal organs, regulated by sex hormones and each plays a key role in the evolution of the Aves and Mammalia classes, respectively.2 Trans-differentiation of ectodermal organ phenotypes also has been found by tilting the balance of molecular pathways. For example, when BMP activity is reduced in the epithelial–mesenchymal interface in K14 noggin mice, sweat glands and meibomian glands are converted into hairs3 and nipples are also converted into hair-bearing epidermis.4
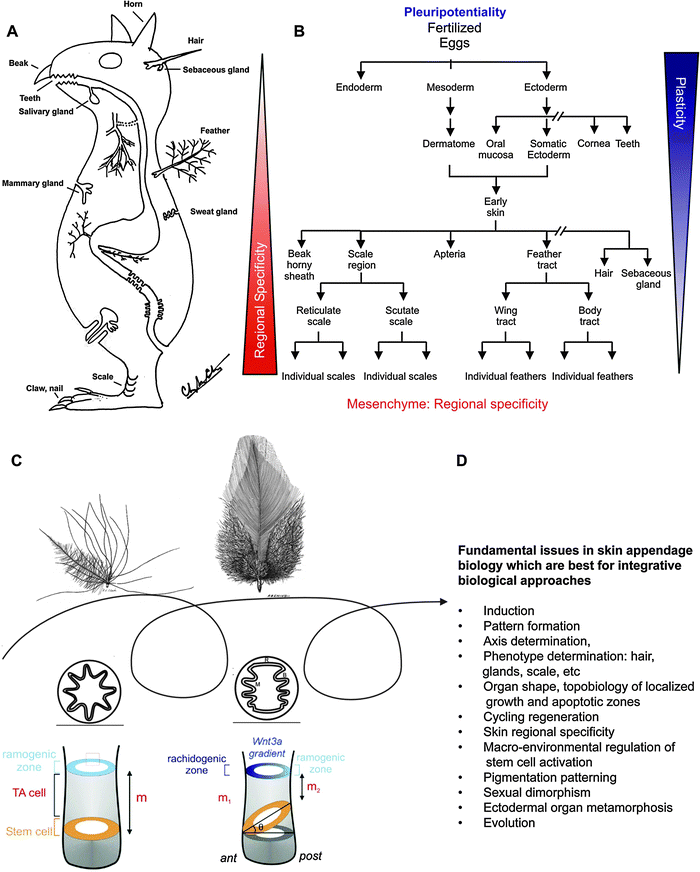 | ||
| Fig. 1 Basic concepts in ectodermal organ morphogenesis . (A) Concept animal with different forms of ectodermal skin appendages. Endodermal organs are also shown. Modified from Chuong edit, 1998.1 (B) Chart showing the progression of ectodermal development into many different types of ectodermal organs. It also shows that the plasticity in ectoderm derived epithelial cells (i.e., multi-potentiality) gradually decreases, while the complexity of mesenchyme increases. (C) Feather follicles undergo cyclic molting and regeneration. The follicles can change phenotypes between cycles. In subsequent generations of feathers, a symmetric downy feather and a contour feather emerge from the same feather follicle. Radial and bilateral feather symmetry can be determined by the topobiologcial arrangement of stem cells. Modified from Yue et al., 200622 Chuong et al., 2000.102 (D) Fundamental issue in biology that can be addressed by skin appendage model and integrative biology approach. | ||
Skin appendages are ideal models to help answer many fundamental biological issues. These are listed in Fig. 1D. Skin appendages are excellent experimental models because they are at the body surface, accessible to experimentation, and easy to observe. They develop relatively late in embryonic development, and undergo physiological regeneration even in adult life. Because there are many skin appendages on one organism, alterations are less likely to be lethal, allowing more opportunities for perturbation. Grasping this opportunity, we use skin appendages as a Rosetta stone to understand the principles of morphogenesis. We have employed a multi-disciplinary approach using feather and hair models. This integrative biology approach has been fruitful and we have gained new understanding with significance beyond skin appendage biology. The following are some examples of what we have done.
Periodic patterning is a fundamental process in biological development.5 Since the mammalian coat and bird plumages are composed of a population of mini-organs, new properties emerge such as pattern formation (the arrangement, size and number of single skin appendages). We have made progress in applying the Turing reaction-diffusion model to study pattern formation of skin appendage primordia.6 This model has been widely applied to understanding the acquisition of self-organizing, repeated patterns in biological systems. In the Turing model, activators promote while inhibitors block the formation of an organ. Activators and inhibitors are released from the same source. Activators favor the synthesis and release of both activators and inhibitors. Inhibitors suppress the synthesis and release of activators. The model predicts that activators have a locally high effective concentration near their source of release while inhibitors diffuse further and have a higher effective concentration at a distance from the source.7,8Cells then migrate toward regions where the effective activator concentration is higher than the effective inhibitor concentration, thus enabling the formation of periodic patterns in biological systems.9
We studied how stem cells are patterned during feather induction.10 Since the timing of induction in hair and feather placodes occurs relatively late, it allows us to study the pattern determination process from the initial homogenous state. Using tissue reconstitution of feather buds from dissociated cells, we have a system in which the patterning process starts from undetermined epidermal and dermal cells.11 We and others showed that growth factors (FGF/BMP/Wnt) and their inhibitors (i.e., Sprouty/Noggin/DKK) fulfill the definition of activators/inhibitors in feathers and hairs, respectively.6,12,13Growth factors are secreted peptides that bind to specific receptors. This in turn signals through independent mechanisms to elicit changes in cell behavior. For example, FGFs are a family of growth factors which bind to FGF receptors. FGF receptors contain a tyrosine kinase domain. Binding of the ligand to the receptor can establish a phosphorylation cascade to affect several aspects of cell behavior (proliferation, differentiation, etc. in different cellular contexts.14 BMPs are another family of growth factors which bind to BMP receptors. They were initially identified in bone but have been found since in all types of tissues. BMP receptors have serine/threonine kinase domains. Upon binding of BMP ligands to their receptors, SMAD proteins (1, 5, 8 and the co-SMAD 4) are phosphorylated. SMAD 4 then translocates into the nucleus and activates downstream transcription.15 Wnts are a family of growth factors which bind to Frizzled receptors. This can lead to a stabilization of -catenin within the cell. β-catenin is then free to move into the nucleus and in conjunction with Lefs/Tcfs promote downstream transcription.16
To fully understand how the homogeneous stem cells are patterned into bud and interbud regions, and how changes in the activator/inhibitor ratios can alter the pattern configuration from spots to stripes, in collaboration with Dr Philip Maini's group, we devised a computer simulation model that nicely recapitulates this process.9 These suggest that during periodic patterning, the epidermis initially forms a homogenous feather field in which every cell is equally competent to form feather buds. Mesenchymal cells migrate and sort themselves following principles including the Turing reaction-diffusion mechanism. The chemical patterns thus are consolidated into dermal condensation patterns. In response to the dermal signals, the β-catenin positive, homogenous stem cells in the feather field (basal states) respond to form placodes (state A) with certain sizes, shapes, numbers and inter-bud spacing (state B).17
In the spirit of a multi-disciplined approach, we collaborated with robotics engineers who needed to develop algorithms for the team behavior of swarming robots. We treated each robot in a robot team as a stem cell, and developed a “digital hormone” model. This permitted robot teams to self-organize into certain configurations depending on environmental obstacles that they may encounter.18 These studies developed leads toward the regenerative patterning of swarming robots.19
We next explored possible mechanisms of how individual organs are shaped. The feather model is ideal here because feathers in the adult bird show distinct morphologies to serve different functions in different body regions. These include thermo-regulation (downy in the trunk), communication (contour and tail feathers), and flight (wing feathers) (Fig. 1C and 8B). We found the shape is based on the topological configuration of feather stem cells. Feather stem cells are configured as a ring at the bottom of the follicle.10 Interestingly, this ring is horizontally positioned in radially symmetric feathers, but tilted toward the anterior (rachis) end of bilaterally symmetric feathers. We hypothesize this topological difference leads to the break of symmetry as stem cells progress from transient amplifying cells to differentiated cells (Fig. 1C).20 We then found that there is an anterior-posterior Wnt 3a gradient in the bilaterally symmetric feathers, but not the radially symmetric feathers. By modulating the activity ratio of morphogenesis related molecules (BMP, Wnt 3a, etc.) at different times during the growth phase, different feather morphologies can be shaped along the proximal distal axis of the feather shaft.21,22 Thus feather morphogenesis is determined by micro-environmental (within the follicle) regulation of the stem cell topology. We further explored this interaction through the chimeric recombination of dermal papillae transplanted between wing/body feather follicles. Interestingly, the chimeric feathers show that their new phenotypes are dictated by the origin of the dermal papilla.22
Another new property that emerges from a population of organs is the coordination of timing in regeneration. It has been known that a single hair follicle goes through regenerative hair cycling continuously during adult life,23 but whether the thousands of hair follicles on one individual cycle randomly, simultaneously, or in coordination is not known. In mice, we observed hair regeneration propagates in waves. Boundaries form because there are refractory regions where the wave cannot pass. We show that intra-follicular Wnt signaling goes up and down, in synchrony with hair cycling. Yet, extrinsic to hair follicles, there is another cyclic molecular change; the oscillation of dermal Bmp signaling, which is asynchronous with hair cycling.24 The interactions of these two rhythms lead to the recognition of refractory and competent phases in telogen, and autonomous and propagating phases in anagen. Boundaries form when propagating anagen waves reach follicles which are in refractory telogen.25 Further, we found hair waves are reset during pregnancy, implying a systemic level of regulation by macro-environmental factors.24 The unexpected link with Bmp2 expression in subcutaneous adipocytes has implications for systems biology and Evo-Devo. Thus, there is a macro-environmental regulation of hair stem cell activities by factors elicited from the surrounding dermis, neighboring follicles, systemic hormones, and external environments (Fig. 8C).25 The macro-environmental factors serve as a bridge between stem cells and the real external environment.
These studies demonstrate that many biological issues in skin appendages are achieved by modulating epithelial stem cell activity with different hierarchical levels of environmental control. This process is modulated from the adjacent dermal papilla, surrounding dermis, and/or systemic physiological conditions. In this regard, Dr Bissell's pioneering work has served as an inspiration. Twenty five years ago, using RSV tumor virus as a tool, Dr Bissell probed the nature of interactions between viral oncogenic activity and the environment. She demonstrated that the embryonic environment restricts the sarcoma forming ability of src.26 Later she further showed that this ability is due to cell types; only endothelial cells could become neoplastic.27
Others have also demonstrated that growth factors, extracellular matrix and proteolytic enzymes present within the stroma are critical for tumorigenesis. Dr Mintz preimmunized female host mice against the syngeneic male melanotic skin grafts. Subsequent transplants of male skin grafts were delayed in forming tumors. These cells could develop tumors faster when subsequently transplanted to control, non-immunized hosts.28 They interpret the delay as being due to the destruction of the donor male stroma and replacement by the host. The increased growth rate recovered melanotic tumor cells were transplanted was attributed to selection of a faster growing population. Dr Werb has added major contributions on the role of tumor-stromal interactions in mammary gland development and breast cancer with a particular focus on the extracellular matrix and metalloproteinases. For example, her group found that estradiol can induce amphiregulin,29 which is shed from the epithelium by the action of metalloproteinase and then communicates with the underlying stroma via the FGF Receptor.30 Dr L. Coussens has examined the role of the immune response and inflammation on tumor formation. The basic concept is that chronic inflammation can promote tumor formation; however, some immune responses may promote while others may fight tumorigenesis. Cytokines, chemokines and mediators of immune response produced within the tumor environment mediate these responses.31,32
The role of mesenchymal–epithelial interactions in prostate development and disease has been shown by the Cunha group. Androgen signaling through the androgen receptor is essential for prostate development. Recombining epithelium and mesenchyme from wildtype mice and mice with testicular feminization he demonstrated that androgen binds its receptor in the mesenchyme.33 His group later showed that embryonic rat mesenchyme could induce human prostate development from adult bladder epithelium.34,35 Furthermore, they showed that androgens plus estrogens could cause an immortalized human prostate cell line to form metastatic tumors when transplanted to a male nude mouse kidney capsule.36
All together, these studies focus more on tumor–stroma interactions and oncogenesis (dys-regulated new growth), these profound observations serve as a reference for us who study the epithelial–mesenchymal interactions in regulated new growth during the development, maintenance and regeneration of organs.37 More recently, Dr Bissell's work further demonstrates that normal mammary gland growth and breast cancer are under similar micro- and macro-environmental regulation.38,39 Conceptually, this is gratifying as we have treated the mammary gland as one of the skin appendages (Fig. 1)1 and now we have come to appreciate similar principles by focusing on different ectodermal organs (mammary glands versus feathers) and different processes (tumorigenesis versus normal development).
Here we present a perspective on how the field has grown to where it is today. To continue the trend of an integrative biology approach, we search for the molecular basis that defines different ectodermal organ phenotypes, feather versus scales, and different types of feathers. We also are eager to visualize the real time processes that occur in the initial phase of periodic patterning of dermal condensations and epithelial placodes. We wish to describe our view of how current methodologies can be applied to skin biology for major advances in future research. We show examples of how these novel methodologies have redefined the classical phenomena at a higher level of resolution. While these data represent work in progress, they reveal unexpected complexity of processes regulating organ morphogenesis and the exciting new potential unraveled by integrative biological approaches.40
Results
Timing of commitment in epithelial–mesenchymal interactions during feather/scale formation
Epithelial appendages are the product of epithelial–mesenchymal interactions. Tissue recombination experiments showed that in general, the dermis determines the phenotype of the epithelial appendage.41,42 Here we sought to evaluate these abilities by coupling classical tissue recombination experiments with microarray. Chicken dorsal skin epithelium interacts with its underlying mesenchyme to form feathers beginning at E7 (H&H stage 31), while metatarsal scale epithelium interacts with its mesenchyme to form scales beginning at E9 (H&H stage 35) which stabilize around E12 (H&H stage 38). To do this, we designed a set of experiments using E7 dorsal skin (normally feather region), E9, E11 (H&H stage 37) and E12 metatarsal skin (normally scale region). We separated the epithelium and mesenchyme and recombined them to cover interacting components at different ages (Fig. 2). Different epithelia are positioned in different rows, and different dermis in different columns.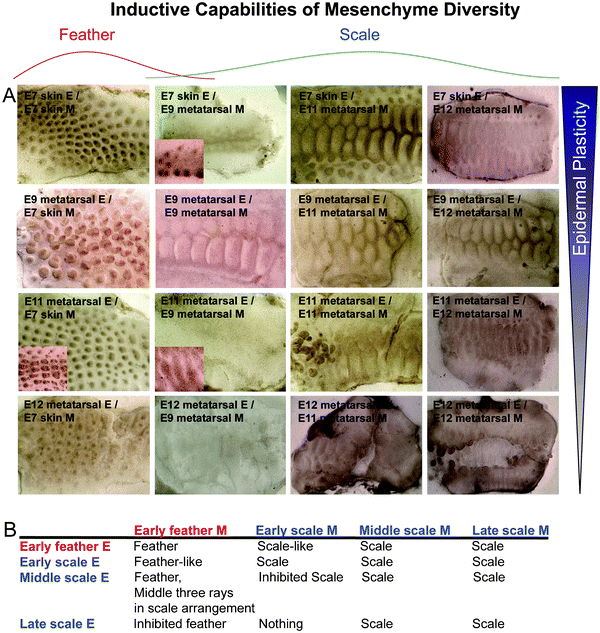 | ||
| Fig. 2 Timing of commitment in feather/scale tissue recombination experiments. (A) Results of chimeric explants. (B) Summary of results. Different rows represent different sources of epidermis: E7 dorsal skin epidermis (normally feather forming), E9, E11 and E12 metatarsal skin epidermis (normally scale forming). Different columns represent different sources of mesenchyme: E7 dorsal skin mesenchyme (normally feather inducing), E9, E11 and E12 metatarsal skin mesenchyme (normally scale inducing). We can observe the gradual restriction of epidermal plasticity and the beginning of dermal complexity, echoing what we see in Fig. 1B. Red, feather derived tissue; blue, scale derived tissue. | ||
When E7 feather epithelium is combined with E7 feather mesenchyme, feathers form. However, when this epithelium is recombined with different stage scale mesenchyme (E9–E12), scales form to varying degrees. E11 scale mesenchyme had the highest capacity to induce scale formation from E7 epithelium, while both E9 and E12 scale mesenchyme are weaker in their inducing ability. These findings suggest that the inducing ability of the mesenchyme is transient (Fig. 2, 1st row). Also, as animal develops, different inductive mesenchymal signals arise, forming diverse types of ectodermal organs.
In a reciprocal experimental design, when E7 feather dermis is recombined with different stage scale epithelium (Fig. 2, 1st column), feathers are produced with E9 scale epidermis, but this ability declines with advancing age of the scale epidermis. With E12, the chimeric feather/scale appendages are arranged in a scale pattern. With E11 scale epidermis, feather buds form, but are in scale pattern in the 3 rows around the midline which matures faster than the flanking regions. We also examined the inducing ability of scale dermis (E9–E12) to induce scale epithelium (E9–E12). It shows similar trends. Thus the competence of epidermis, i.e., the multi-potential ability of the epidermal progenitors, to respond to the inducing signal and become specific types of skin appendages is also transient, higher in the earlier stages of the epidermis. This ability is gradually restricted with developmental timing. We wondered what molecules might regulate competence vs. non-competence in developing tissues. We also wondered what molecules might underlie regional specificity, in other words, why some regions develop in to feathers and others into scales.
In search of molecular pathways involved in tissue competence and regional specificity
To search for molecules involved in competence we chose to perform expression studies on competent E7 and non-competent at E9 feather forming skin. We previously had shown that differences in wing or body feathers were based on differences within the dermal papilla, while epidermal cells in the proximal follicle represent stem cells that can be modulated into different types of feathers.22 To examine differences in regional specificity we compared the above results with results from competent E9 and non-competent E11 metatarsal scale forming regions as well as the proximal follicle epidermis and dermal papilla in adult wing, body and tail feather follicles. These time points were chosen based on the results from experiments described in Fig. 2.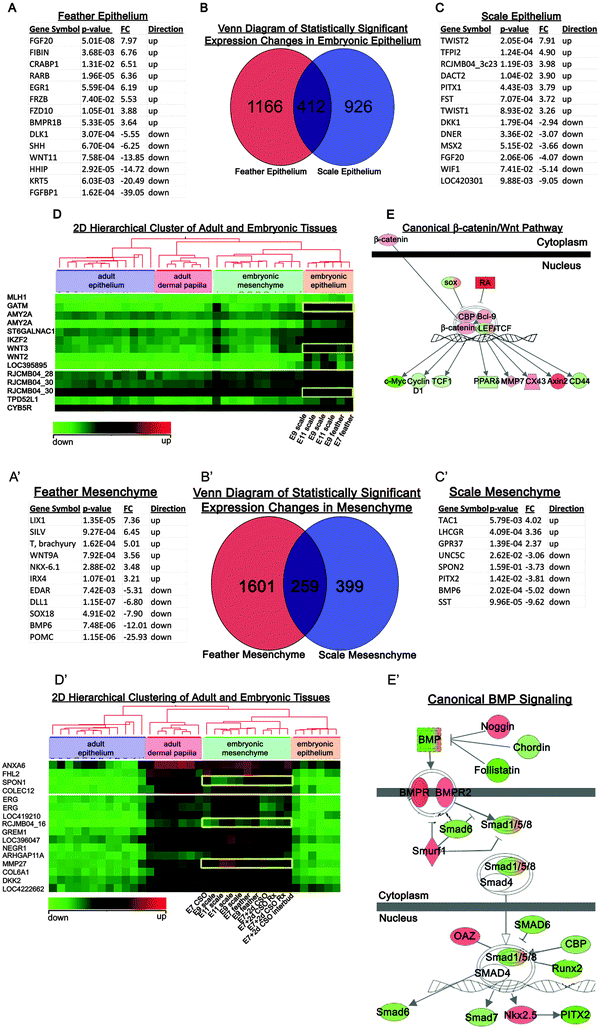 | ||
| Fig. 3 Differential gene expression analysis of embryonic chicken feather/scale regions. (A–E) Differential gene expression analysis of embryonic epithelium. The gene expression of E7 feather was compared to E9 feather forming epithelium, and E9 scale was compared to E11 scale forming epithelium. (B) Venn diagram separates the genes that contribute to scale forming, feather forming, or non-specific forming epithelium. A representative list of genes that contribute to the formation of scale (A) or feather epithelium (C) are listed. (D) Two dimensional hierarchical cluster exhibiting differential gene expression for embryonic epithelium. (E) Example showing genes involved in epithelium formation were enriched in the canonical β-catenin/Wnt pathway. Red is up-regulated and green is down-regulated gene expression. (A′–E′) Differential gene expression analysis of embryonic mesenchyme. The gene expression of E7 feather was compared to E9 feather forming mesenchyme, and E9 scale was compared to E11 scale forming mesenchyme. (B′) Venn diagram separates the genes that contribute to scale forming, feather forming, or non-specific forming mesenhcyme. Representative lists of genes contributing to the formation of feather (A′) or scale mesenchyme (C′). (D′) Two dimensional hierarchical cluster exhibiting differential gene expression for embryonic mesenchyme. (E′) Genes involved in mesenchyme formation were enriched in the canonical BMP pathway. Red is up-regulated and green is down-regulated gene expression. | ||
The mesenchymal tissues of E7 and E9 feather forming, as well as E9 and E11 scale forming were also compared (Fig. 3A′–E′). According to the ANOVA performed, WNT9A, NKX-6.1 and IRX4 were up-regulated while EDAR, SOX18 and BMP6 were down-regulated in the feather forming mesenchyme (Fig. 3A′). The scale forming mesenchyme exhibited an up-regulation of TAC1 and GPR37 while UNC5C, PITX2 and BMP6 were down-regulated (Fig. 3C′). A Venn diagram demarcates gene lists that may be playing a role in feather or scale mesenchyme inducing ability. Two dimensional hierarchical clustering analysis suggested that SPON1 and MMP27 play a role in modulating mesenchyme inducing ability (Fig. 3D′). Entering the common gene list into IPA generated canonical pathways and networks that demonstrated how mesenchymal inducing capability might be regulated (Fig. 3E′). In this case BMP pathway activity appears to play an important role.
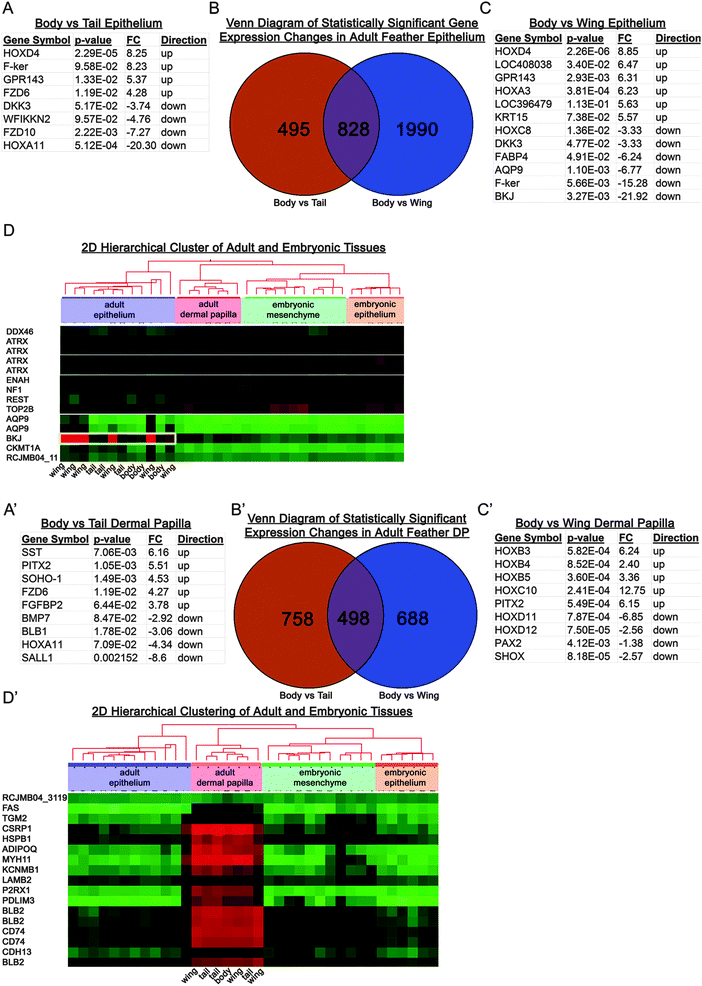 | ||
| Fig. 4 Differential gene expression analysis of feather follicles from different body regions of adult chickens. (A–D) Differential gene expression analysis of adult chicken epithelium. The gene expression of body feather epithelium was compared to wing feather or tail feather epithelium. Representative gene lists that contribute to the formation of tail epithelim (A) and wing epithelium (C) are listed. (B) Venn diagram separates the genes that contribute to form wing feather or tail feather epithelium. (D) Two dimensional hierarchical cluster exhibits up-regulation of genes in the wing epithelium and their respective down-regulation in other tissues and ages. Red is up-regulated and green is down-regulated gene expression. (A′–D′) Differential gene expression analysis of adult chicken feather mesenchyme. The gene expression of body feather dermal papilla was compared to wing feather or tail feather dermal papilla. Representative gene lists that contribute to the formation of tail dermal papillae (A′) and wing dermal papilla (C′) are tabulated. (B′) Venn diagram separates the genes that contribute to form wing feather or tail feather dermal papillae. (D′) Two dimensional hierarchical cluster exhibits up-regulation of genes in the dermal papillae and their respective down-regulation in other tissues and ages. Red is up-regulated and green is down-regulated gene expression. | ||
We next looked at the feather dermal papillae (Fig. 4A′–D′) because data from recombination experiments and the literature demonstrate that the mesenchyme determines what skin organ develops (Fig. 2).41 Using the body feather as the control or non-specialized feather, we compared body to wing feather dermal papillae gene expression patterns (Fig. 4C). ANOVA identified numerous changes in gene expression. Up-regulating Hoxb3, Hoxb4 and PITX2, while simultaneously down-regulating of Hoxd11, Hoxd12 and PAX2 are associated with dermal papillae in the body region (Fig. 4A). Two dimensional hierarchical clustering identified genes that were commonly up-regulated in the adult dermal papilla (Fig. 4D).
We then used qPCR to validate a subset of 5 genes that showed significant differences in expression levels as determined by microarray (Table 1). Our qPCR study confirmed that these genes were expressed to significantly different levels as a function of competence vs. determination or of regional specificity.
| Comparison | Gene | Regulation |
|---|---|---|
| Several genes shown to be up or down regulated by microarrays were chosen and qPCR was performed with the samples indicated. | ||
| E7 Epithelium vs. E9 Epithelium | RARb | Upregulated |
| E9 Scale Epithelium vs. E11 Scale Epithelium | Tac1 | Upregulated |
| Body Feather Epithelium vs. Tail Feather Epithelium | HoxD4 | Upregulated |
| Body Feather Epithelium vs. Wing Feather Epithelium | BKJ | Down regulated |
| Body Feather Epithelium vs. Tail Feather Epithelium | Pitx2 | Upregulated |
Next we compared gene lists derived from our microarray study on dermal papilla with those from mouse hair dermal papilla (Table 2).44 We next compared a gene list derived from wing growth feather collar epithelium (the site of feather precursor cells)10 with the gene list from hair bulge enriched genes (Table 3).45 We found many genes in common, highlighting their fundamental importance in these two skin appendages that evolved convergently. These molecules will be the target for future investigations.
| Growing Wing Feather Dermal Papilla | Hair Dermal Papilla Genes |
|---|---|
| Information of hair dermal papilla are from Rendl et al., 2005. | |
| Frizzled 2 | Frzd Related Protein, Secreted Frzd Related Protein 2 |
| Hox D4 | Hox C8 |
| FGFR Activation Protein 1 | FRGR1 |
| Melanocortin Receptor 5 | FGFR Activation Protein 1 |
| Solute Carrier 16 | Solute Carrier 16 |
| Insulin-like Growth Factor Binding Protein 1 | Insulin-like Growth Factor Binding Protein 3 |
| Potassium Voltage-gated Channel Shaker | Potassium Voltage-gated Channel Shaker |
| Related Family, Member 2 | Related Family, Member 2 |
| Growing Wing Feather Collar Epithelium | K15 Positive Hair Bulge Cell Enriched Genes |
|---|---|
| Information for K15 positive hair bulge cells are from Morris et al., 2004. | |
| G protein coupled receptor family C, Group 6, member A | G protein-coupled receptor 49 |
| Potassium channel subfamily K, member 2 | Potassium channel subfamily K, member 2 |
| FGF2, FGF7, FGF10 | FGF1 |
| Frizzled Homolog 4 | Frizzled Homolog 2 |
| Frizzled Related Protein | Secreted Frizzled Related Protein 1 |
| TNF, member 13b | TNF Receptor 11b |
| Col1A2, Col11A1, ColA52 | Col5 alpha2 |
| Annexin A6 | Annexin A6 |
| Tenascin C | Tenascin C |
| CD34 | CD34 |
| Solute Carrier, Family 4, 7, 18 | Solute Carrier, Family 29 |
We performed whole mount in situ hybridization using two Hox genes in every cluster to examine the expression in H&H stage 26 (E5) and 29 (E6.5) chicken embryos (Fig. 5). Sagittal sections and some cross sections were collected from the whole mount in situ hybridization samples. The feather placodes started to form in dorsal tract at E6.5. Our purpose is to examine the role of Hox genes in regional specificity leading to pteric versus apteric regions, short feather versus long feather regions, why feathers grow on the wing versus scale growth on the leg, etc. The results show some interesting regional differences. Overall, the body skin did not strictly follow the co-linear expression patterns with lower numbers of Hox genes in the anterior trunk or proximal limbs. This result is similar to that of Reid and Gaunt49 which only studied sagittal sections. We observed some hox genes at E6.5, such as Hoxa13 (Fig. 5B) and Hoxc8 (Fig. 5E) expressed in the feather placode at the region which did not have expression at E5, suggested that these genes may take part in future feather morphogenesis. Some hox, such as Hoxb8, is absent at the future apteric region (Fig. 5D, red arrow) suggesting it may play some role in regional specification. Currently, the data is limited at this stage and more work on hox gene expression patterns and functional studies are required to investigate their role in regional specification.
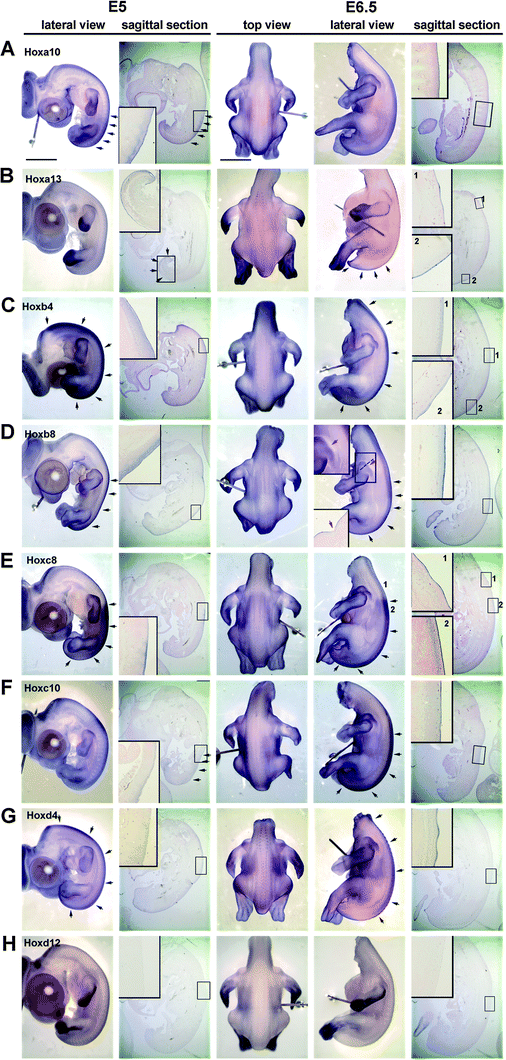 | ||
| Fig. 5 Expression of Hox genes in chicken embryonic skin at E5 (stage 26) and E6.5 (stage 29) as determined by wholemount in situ hybridization. (A) Hoxa10 is expressed in the epithelium and mesenchyme at E5 but only in the epithelium at E6.5. (B) Hoxa13, is expressed in the distal limb bud and tail region epithelium and mesenchyme at E5. However, feather placodes in the upper dorsal tract (insert 1) start to express Hoxa13 at E6.5 only in the epithelium. (C) Hoxb4 and (D) Hox b8 are expressed in the epithelium and dermis at E5 and E6.5. However, Hoxb4 expression extends more proximally than Hoxb8. At E6.5, there is a Hoxb8 negative region between the scapular and dorsal feather tract that extends posteriorly until the boundary between the femoral and dorsal tract (red arrow). The dashed line shows the plane of section shown in the inset. This non-Hoxb8 area may be related to the apteric regionof the chicken skin. (E) Hoxc8 expression is the same at E5 and E6.5. However, in region 1, Hoxc8 is expressed in the epidermis of feather placodes. This region did not have strong staining under the skin (compare to region 2). (F) Hoxc10 did not show a clear staining pattern in skin as compared to Hoxc8, but it can be found in the skin from the sagittal section at both E5 and E6.5. (G) Hoxd4 is weakly expressed in all dorsal epidermis at E5. Staining is stronger in the epithelium of bud and interbud regions at E6.5. (H) Hoxd12 is expressed in the distal and ventral limb bud but not in the skin at both E5 and E6.5. Black arrows indicate the intense expression region in the sample. Scale bar: 2 mm. | ||
Visualizing cellular events during feather morphogenesis
The exciting aspect of using an integrative biology approach to study the morphogenesis of skin appendages is that it offers an opportunity to understand the clearly visible skin appendage phenotype from the level of molecules, cells, tissue interactions, all the way to organ shape. Heterotypic recombination defines the tissue interaction question, illustrating that organ phenotype is established by the epidermis and dermis together. Microarray analyses help us identify critical molecular signaling pathways involved in this process. Our current understanding of these processes is depicted in Fig. 6A. In order to understand the cellular events that lead to feather morphogenesis at a deeper level, we need to visualize cell movements in real time and observe specific interactions among cells during the process of skin morphogenesis. Here we apply several types of modern imaging technologies for this purpose.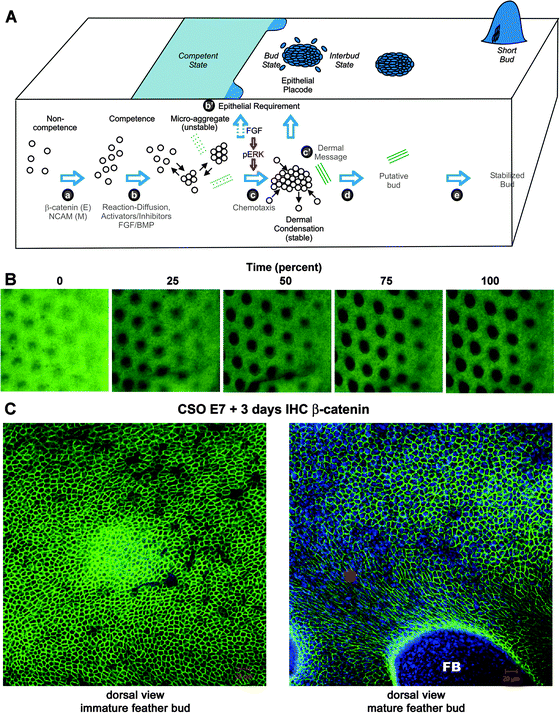 | ||
| Fig. 6 Video microscopy imaging of developing chicken skin explant cultures. (A) Schematic drawing on our current concept of periodic patterning process (from Lin et al.9). This concept is based on current biochemical and functional perturbation data. However, the detailed cellular processes and tissue interactions remain to be elucidated. The microarray data earlier showed differences in molecular expression. The following imaging data give us a glimpse on the complexity of the cellular behavior and redefine this classical phenomenon. Green dotted and solid lines represent the development of extracellular matrices. (B) Time lapse videomicroscopy. Movie is in the ESI.‡ E9 skin explants were photographed every 15 min for 17 h to track the process of early feather morphogenesis. Representative images are taken from the movie. Each black spot represent a bud, which is about 10 cells long in diameter. The dorsal skin midline lies to the left of the images, and buds to the right side of the panel are in earlier stage than those in the left. Scale bar = 500 μm. (C) E7 chicken skin organs were cultured for 3 days. Immunohistochemistry with antibodies against β-catenin was used. (A) Cells within the immature feather bud exhibit a rather homogenous hexagonal cell shape. (B) In the more mature bud region (*), the cell shape changes as the feather bud (FB) forms and elongates. Size bars: 20 microns. Beta-catenin stains Green, DAPI stains blue. | ||
For example, cells can be visualized without staining by the autofluorescent NAD(P)H in the cytoplasm. In skin, keratin and elastic fibers also have unique fluorescent signatures that can help to identify specific cells and extracellular matrix networks.56 Using the ultrashort femtosecond laser, a non-linear polarization effect of second harmonic generation (SHG) can also be effectively achieved for imaging.51,58,60 The interaction of incident light with biological structures of non-centrosymmetry, including collagen and myosin, can produce photons of exactly half the wavelength of the incident photons. Since SHG is a direct polarization effect without absorption of incident photons, there is no heat generated in the process and the photodamage to the targets of interest is minimized. Under the same incident laser, the autofluorescence wavelength is longer than the SHG. Hence, signals from autofluorescence and SHG can be easily spectrally separated for imaging. For example, autofluorescent elastic fibers and SHG-generating collagen fibers can be imaged at the same time by use of multiphoton microscopy.55 Furthermore, since SHG is structurally sensitive, it can be used to analyze the structural transition and denaturation of collagen fibers under various physiological and pathological conditions.56,58,59,61,62
We have employed multiphoton microscopy to analyze the dynamic cell rearrangements. In Fig. 7A and B, the embryonic skin specimen is labeled with the Hoechst nuclear dye and cultured as an explant. Serial en face mulitphoton images are taken from the surface down to the bottom and a three-dimensional image can be reconstructed from the serial images. To differentiate cells at different depths and to facilitate single cell tracing, cells at depth from the epidermal surface (blue) to the bottom of the dermis (red) are graded by pseudocolor. Reconstituted three-dimensional images that were taken at various time points during explant culture allowed us to analyze the dynamics of cell rearrangements during feather morphogenesis. In Fig. 7B, we can see the trend of dermal cell movement toward the right and also toward the bottom of the dermis from time zero to 70 min. For easier analysis of three-dimensional cell movement, we can project the image to either X-Y, X-Z or Y-Z planes and calculate the cell movement vector on each plane. For example, the X-Y projection image of the depth-graded pseudocolor image allows us to trace single cell movement on the X-Y plane (Fig. 7A, right panel).
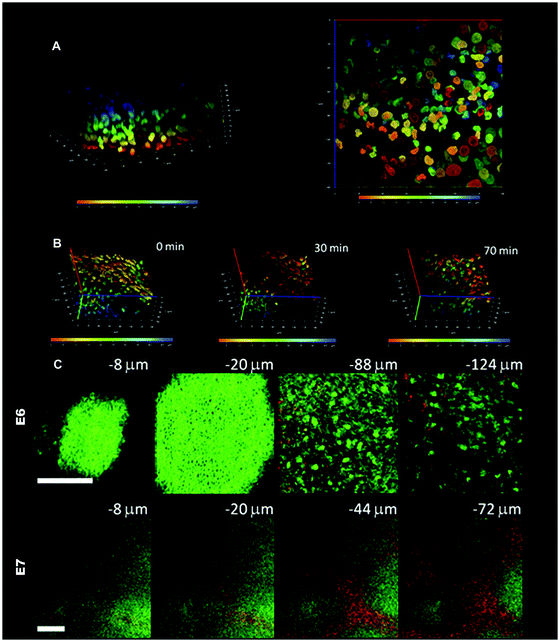 | ||
| Fig. 7 Multiphoton microscope imaging of developing chicken skin explant cultures. (A) The embryonic E6 skin is labeled with Hoechst 33342 dye and cultured as an explant. Serial images from the surface to the bottom are reconstituted into a three-dimensional image. The nuclei at different depths from the surface are graded with pseudocolor from blue (epithelial surface) to red (50 μm from surface) to facilitate single cell tracing. The left picture shows the three-dimensional distributions of cells and the right panel shows a bottom view of the nuclei in an en face projection to X-Y plane. The X or Y axis is 140 μm and the Z axis is 50 μm. (B) Time-lapse multiphoton tracing of cell rearrangement. To highlight the mesenchymal cell movement, the dermal side is on the top and epidermal side at the bottom. The depth-graded pseudocolor helps to delineate individual cells and facilitate single cell tracing. There is a trend of movement toward the right hand side of the figure and to the dermal side. The X or Y axis is 140 μm and the Z axis is 50 μm. (C) Multiphoton auto-fluorescence and second harmonic generation (SHG) images of unstained developing feather bud. The upper panel shows the images of E6 skin at different depths before dermal condensation formation. In E6, the dermal cells have an even cell distribution and there is scanty SHG signals from collagen (−88 and −124 μm). The epithelial cells can also be visualized with an autofluorescent cytoplasm and a nuclear halo (−8 and −20 μm). The lower panel shows the images of E7 skin at different depths when dermal condensates appear. In the lower power view of E7 skin, single cells cannot be delineated. The dermal condensates have higher autofluorescence due to the higher cell density and interbud area is rich in SHG signals from the collagen. The low power image clearly demonstrates the preferential cell and collagen distribution in the developing skin. Autofluorescence is green and second harmonic generation is red; bars: 100 μm. | ||
Another strength of multiphoton microscopy is the ability to acquire autofluorescence and SHG signals simultaneously.51,52,59 Since the cells are rich in autofluorescent cytoplasmic NAD(P)H and collagen fibers are an effective SHG generator, we can visualize dynamic three-dimensional changes of cell and collagen organization within the developing tissue. The nature and organization of the extracellular matrix is another macro-environmental factor that can influence cell behavior. In Fig. 7C, we use unlabeled embryonic chicken skin for multiphoton imaging. We found that the autofluorescence signals (green color) from the cytoplasm allow us to visualize both the epithelial and mesenchymal cells. From the epithelial surface down to 20 μm in depth, the epithelial cells have autofluorescent cytoplasm and the nuclei appear as halos since they lack NAD(P)H. Further down into the dermis, the dermal cells as well as the collagen networks can be seen. On E6, before dermal condensations appear there is no preferential distribution of collagen (Fig. 7C, upper panel). Dot-like short collagen fibers are scattered in the dermis. On E7 when dermal condensates start to build up, there is preferential distribution of collagen in the interbud region and cell density is higher in the dermal condensate (Fig. 7C, lower panel). The collagen fibers are longer and interwoven into connecting networks at this stage.
Discussion
Just as Jason and his Argonauts possessed different indispensible skills in their pursuit of the Golden Fleece, today's research science also requires a multi-disciplinary approach that is a hallmark of integrative biology. We have applied this approach to understand the intricate cellular and molecular interactions that lead to specific skin appendage formation. In this study we integrate data from many sources to paint a picture of feather morphogenesis based upon tissue interactions, and molecular profiling that may be behind the tissue interactions.Microarray transcriptome analyses help identify candidate molecules, but we are still in search of the molecular basis of competency and regional specificity in feather development/regeneration
Microarray analyses allow investigators to examine the complete transcriptome from a small number of cells at certain cellular states. By analyzing transcriptomes from different cellular states, one may gain unbiased clues as to which genes and pathways may be involved either as the cause or the consequence cellular state changes. These cellular fates can change during normal development; tissues can obtain different fates in different body regions (Fig. 1B), and normal fates can be trans-differentiated when different mesenchyme are imposed or molecular pathway activities are tilted. For example, rabbit cornea can be forced to form hairs63 and chicken oral mucosa can be induced to form tooth like appendages.64 It also has been possible to achieve this by modulating molecular pathway activities. Classical experiments demonstrated that retinoic acid can convert hair follicles into glands65 in the mouse, and feather buds to form on chicken scales.66 This scale – feather transforming ability is also observed in response to over-expression of delta,67 a BMP dominant negative receptor68 and β-catenin.69 In mice, by enhancing noggin expression and reducing BMP 2, 4, 7 activities at the epithelial-mesenchymal junction, we were able to convert sweat glands and sebaceous glands into hairs.3 In these mice, nipples are also converted into hair forming epithelia.4 Here we use two examples for microarray analyses: the first one is the determination of feather and scale fate, the second one is the determination of wing and body feathers.Tissue recombination experiments demonstrated embryonic chicken skin epithelial competency is restricted to a specific time during feather bud morphogenesis (Fig. 3). E9 feather forming epithelium and E11 scale forming epithelium responded poorly to inducing signals arising from the underlying mesenchyme. In order to discover which genes and their relevant pathways were modulating this competency, we integrated microarray analysis with tissue recombination experiments. The result was a comparison of transcriptomes between competent and non-competent tissues, E7 vs. E9 feather forming and E9 vs. E11 scale forming epithelia (Fig. 3). We observed the classical genes involved in tissue development such at Fgf, Wnt pathway members (Dkk, Frz) and SHH. These were all differentially expressed during modulation of epithelial tissue competency. We also found some interesting putative players in the role of tissue competency. Msx2 was found to be significantly suppressed during scale epithelial tissue competency (Fig. 3A). Further, Msx2 was found to be enriched in a network with Msx1, Bmp3 and Dkk (data not shown). Msx2 has been shown to be a downstream effector of the Bmp pathway70 and Msx2 has been shown to work alongside Msx1 during tissue development.71 The surprising interaction suggested by the network is Msx2 and Dkk (data not shown). Msx2 is known to activate the Wnt pathway during bone anabolsim,72 and Msx2 is activated by the Wnt pathway during stem cell neural crest induction.73 Our data suggests that Msx2 is also involved in epithelial tissue competency and may be working in concert with the Wnt pathway. There are many other candidate pathways. However, the analyses of these data also suffer from a lack of annotation of chicken genes even though efforts to re-annotate continue.74 When more annotation data become available, we will revisit our database and deduce additional relevant pathways.
The mechanism of region specific gene expression has tantalized scientists for decades. The adult chicken provides a fantastic scientific model to explore this question. In the young chick, all feathers appear to be of the same downy type. Yet different morphologies of feathers can emerge from the same feather follicle in the adult (Fig. 8B). To study the molecular mechanism of this process, we first want to know the transcriptome difference among feathers from different body regions. The flight feathers develop on the wings, the tail feathers develop on the tail, and contour feathers develop on the body. We applied microarray analysis to micro-dissected adult chicken feather tissues (Fig. 4A–C, A′–C′). Two dimensional hierarchical clustering yielded some genes specific to the dermal papilla (Fig. 4D and D′). They also yield some difference in Hox genes.
In the vertebrae and in the limb Hox genes were found to be expressed in a collinear pattern.75,76 This led to the concept that Hox codes specify skeletal identities.77,78 Based on this finding and our own observations in the skin,78 we have proposed the “Skin Hox code hypothesis”,79 proposing that combinatorial Hox expression might be involved in determining skin specification (i.e., apteric or pteric, anterior or posterior, medial or lateral, scale or feather). In the developing skin and in the dermal papilla from wing and body feathers, we found some distinct Hox expression patterns and some that are region specific (Fig. 5). Hoxb8 was not expressed at the apetric region at E6.5 suggesting that Hoxb8 may take part in the determining the pteric and apteric regions of the skin. As more research goes on, we can test our prediction that more hox genes are involved in this developmental process.
Dhouailly's group examined the expression of Hoxc8, d9, d11 and d13 in the dermis of developing limb and found some region specific expression patterns.48 These are in general consistent with our finding. In cultured fibroblasts derived from different parts of the human body, microarray analysis shows specific HOX expression related to their topographic origin.80,81 Combined, these studies show there is region specific Hox expression in the skin. Complexity is added when we consider skin as being composed of epithelium and mesenchyme, and that it is a two dimensional plane, in contrast to the one dimensional spine or limb axis. Some Hox genes may also be involved in different functions such as growth control82–84 or other molecules may have to work together to establish skin regional specificity. This complex issue will require further investigation.
Imaging technology revealed complex cellular flow and matrix organization in the developing skin explants
Our molecular expression studies suggest that there are temporal and spatial differences which may guide cells toward specific locations and to specific cell fates. To trace cell movements we have turned to modern imaging technologies. The developing chicken skin explants are unique because the patterning events take place in flat skin composed of a single layer of epidermis and dermis which is about 10 cell layers thick. This unique system offers a wonderful opportunity to explore cell migration during organ (feather) morphogenesis.We previously had addressed this issue using replication defective spleen necrosis virus to deliver beta-galactosidase to the developing skin and feather buds.85 Based on still images we found that cells seemed to migrate from the midline across the dorsal feather tract. As feather buds formed they contained a mixture of labeled and unlabeled cells suggesting that feathers are derived from a multiple cell lineage. We also used DiI labeling to track the motility of cells in different regions within the feather bud and interbud. With DiI labeling, we examined the role of the p-ERK signaling pathway in cellular chemotaxis.9 Suppression of p-ERK signaling with U0126 led to a more rapid dispersion of dermal cells and a loss of feather bud boundaries, leading to feather bud fusion.
Here, we continued along this line of study using several imaging modalities to learn more about the roles of cell motility and changes of cell shapes during feather morphogenesis. If one were to try to imagine the rules of a football game from watching still images taken at different time points during a game, the task would be very difficult. However, by watching the players in action, it becomes more manageable. Similarly, understanding the dynamic process of tissue morphogenesis from fixed still images is difficult. By capturing the skin appendage morphogenetic events as a movie, it makes the whole process easier to comprehend. We used time-lapse video visible light microscopy to demonstrate the cell movements that are necessary to form the early feather buds. This enables us to begin to see patterns that may be crucial to proper organ formation. Do cells enter the forming buds from all directions or from specific directions? Once in the buds do they stay or can they leave? Do they stay at the base of the bud or do they migrate toward the distal tip? These questions will take some time to answer. Since feathers are made of epithelium and mesenchyme, the formation of epithelial placodes and dermal condensations are in different, albeit related, tissue layers. Along these lines we developed techniques to trace individual cell movements within the epidermis or dermis alone. This technical advance will help us view the relative mobility of cells located at different locations in the morphogenetic explants.
Feather buds, after all, are not as flat as a single layer. To capture these events we turned to confocal and multi-photon microscopy. Confocal microscopy allows a three dimensional reconstruction of tissues and their corresponding molecular expression patterns. Feather bud morphogenesis begins with a uniform field of epithelium overlying mesenchyme at E7. At this stage the epidermal cells are multi-potential, with an equal chance to become bud or interbud epidermis).9,11 Across this stem cell field, β-catenin expression in the epithelium is uniform and is located at each cell's membrane. The result is a pentagonal or hexagonal cell shape pattern (Fig. 6C). As feather buds develop, the suprabasal layer expresses β-catenin on the lateral sides but not at the apical side of the cell. The basal layers have a more complex expression pattern with β-catenin expression at the cell membrane, in the cytoplasm, and in the nucleus (data not shown). In the patterning stage, β-catenin is localized to the membrane and thus can help us to visualize changes in cell shape. After H&H stage 31, explants are cultured for 3 days. During this growth period feather buds form and are starting to elongate. Clear distinctions can be seen between the shapes of cells in the interbud zone from those within the feather buds. The β-catenin expression pattern of the suprabasal layer near the base of the feather bud has changed from pentagonal to oblong and diamond shaped, with predominantly four sides (Fig. 6C). This change in cell shape is integral to feather morphogenesis and may be caused by tensile forces. This tension probably derives from the upward growth of the feather bud pulling on the epithelium. These forces result in tension on each of these cells, causing the cells to become elongated and narrow. Conversely, the cells in the interbud region remain pentagonal in shape due to minimal forces acting on them.
Multiphoton microscopy allows for deep imaging without damaging the tissues to be characterized. Auto-fluorescence captured by mulitphoton microscopy enables us to discern differences in cell matrix, keratins and elastic fibers without a need for molecular staining or external illumination. We have shown that a reaction-diffusion mechanism is involved in determining the initial spacing and size of a dermal condensation.9,12 It has been shown that dermal cell proliferation stops for about 24 h in the early stage of dermal condensation formation.86 Hence, active cell reorganization should happen during this process and the cell movement should be non-random. However, the events of dermal condensation have not been captured in high resolution, in terms of spatial rearrangements and time intervals. Time-lapse multiphoton images will greatly enhance our appreciation of this patterning process. Here we show two examples on how it starts to change our understanding of the system. First, dermal condensation used to be considered as centripetal migration of dermal cells toward the center of the condensation. Preliminary data here revealed that there is also a non-random Z axis movement which should be taken into consideration in constructing a model. Second, in the developing skin, birefringence has been thought to be derived from dermal collagen. Analysis of birefringence led to the suggestion that a lattice-like system of collagen tracts could have played a guidance role for the alignment and migration of mesenchymal cells during the process of dermal condensation formation.87 Our observation here showed that these collagens are unorganized at the time of patterning and get organized later when feather buds form. It appears that the collagen becomes excluded from the dense dermal condensates. Therefore, the collagen lattice is the consequence, not the cause of the dermal condensation process. It is the dermal condensations that appear first and instruct collagen matrix formation that feeds back to regulate subsequent organization of the dermis. It is interesting that at the same time fibronectin accumulates within the dermal condensations.88 What's the possible role of the dynamic rearrangement of extracellular matrix in this process? Our unpublished data (SJL) suggests that fibronectin is able to down regulate Bmp2 and Bmp4 expression in mesenchymal cells. Since Bmp proteins are inhibitors for feather bud formation, the selective down regulation of BMP by fibronectin may help to sharpen the ratio of activator/inhibitors in feather buds against the interbud area. Hence, the dynamic arrangement of extracellular matrix during feather bud formation may have a role in stabilizing the initial feather buds.
Cycling skin appendages as a model for systems biology research
In general, over the past several decades developmental biology took a reductionist approach, by taking the system apart and identifying individual components. While these analytical approaches are insightful in providing clues on what pathways are involved, we now aspire to achieve a holistic understanding of how the whole system works. Systems biology represents the trend to understand the function of a whole system by studying the interactions of its different components. This complexity can be appreciated at molecular, structural, temporal, emergence and algorithmic levels. Through these studies, cycling appendages are argued to be an ideal model for systems biological research.89With the ambitious goal of using system biology to understand complexity, we have employed an integrative biology approach that integrates different, complementary disciplines. Based on our molecular data, we have collaborated with mathematical biologists to develop computer simulation models that can describe the system behavior and also identify some molecular bases of model parameters. One model is the periodic patterning behavior in forming spots and stripes based on Turing activator/inhibitor and chemotaxis.6,9 The other model is the behavior of the regenerative hair wave.24 These approaches have been fruitful and indeed bring our understanding of the whole system to a higher level.
To completely understand a system, we need to know its origin and how it is built. Here, we want to know the Evo-Devo of ectodermal organs (Fig. 8A and C). Since an organism tends to have thousands of hairs or feathers which are individually dispensable, they may be lost or altered without lethal effects. This may provide one path for evolutionary change in hair/feather structures resulting in the acquisition of different functions. Because of the plasticity of the ectodermal organs, we can have variations in lengths and shapes. This variation may provide a small percentage of organisms with an advantage for their particular niche. Natural selection working on those organisms over time may have produced regional specificity. In the last two decades, different fossils of feathered dinosaurs and Mesozoic birds unearthed in the Jehol Biota of China90–92 have provided valuable information that inspires our thoughts on how feathers evolved.90,93,94 Indeed, feathers have come a long way in the evolution of ectodermal appendages (Fig. 8). Through novel molecular pathways and cellular processes (invagination, branching, etc.), localized growth and apoptotic zones95,96 work together to sculpt out different forms of skin appendages.
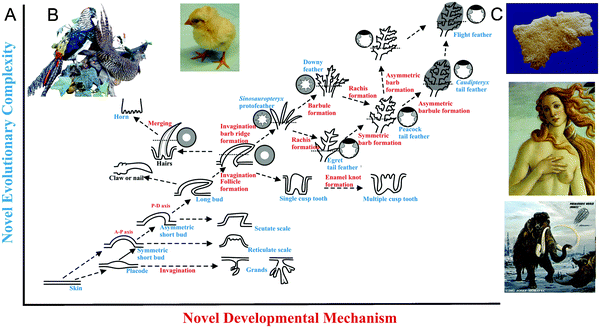 | ||
| Fig. 8 Evo-Devo of ectodermal appendages. (A) X axis represents addition of new developmental mechanisms. Y axis represents emergence of new phenotypes. Glandular structures, invaginations, protrusion, branching, etc. evolved from the flat epidermis. Complex feather forms have come a long way. Modified from Wu et al., 2004.90 (B) Young chicks show downy feathers all over the body, while an adult pheasant shows diverse feather types that are sexually dimorphic. Female pheasant does not show these spectacular feathers (not shown). (C) Mammalian ectodermal organs evolve with different emphasis under different physiological conditions as well as in evolutionary time. Top panel shows a fleece. Middle panel: scalp hairs and mammary gland. Bottom panel: long body hairs and tasks. | ||
What the Golden Fleece represents is the distilled essence of the principles of morphogenesis that allow ectodermal organs to generate and regenerate a myriad of forms. The pursuit of the Golden Fleece is fruitful as we have already been enlightened by many unexpected new things on the journey. Even if we do not find the ultimate answer, as Denis Duboule says our scientific journey represents “an intellectual adventure, a trip into ourselves”.97 While this paper represents the status of a work in progress, using an integrative biology approach, we expect many more fascinating facets of the Golden Fleece will be unraveled from different perspectives. We anticipate that this approach will enable us to gain a holistic understanding of ectodermal organ morphogenesis and regeneration in the years to come.
Materials and methods
Chicken embryos
Chicken embryos for epidermis/dermis recombination and whole mount in situ hybridization were from SPAFAS/Charles River Laboratories and were staged according to Hamburger and Hamilton (1951).98Embryonic skin explant culture
Embryonic skin from the indicated days were dissected from the body, placed on a culture insert (Falcon, Becton Dickinson) and grown for the indicated time in DMEM containing 10% fetal bovine serum.Epidermis and dermis recombination
Dorsal skin from E7–E9 and metatarsal skin from E9–E12 were used for epidermis/dermis recombination. Skins were dissected in HBSS. 2XCMF in 0.25% EDTA, pH 7.5 was used to treat the skin for 10 min on ice to separate the epidermis and dermis. The recombined epidermis and dermis were cultured on inserts (Falcon, Becton Dickinson) for 4–6 days in DMEM containing 10% fetal bovine serum.Whole mount in situ hybridization
Hox RNA probes are from Dr Tabin.99 Our whole mount in situ hybridization method was performed according to the method of Jiang et al. (1998).100 Paraffin sections (15 μm) were prepared from the whole mount in situ hybridization samples and faintly counterstained with eosin.Time lapse video microscopy
An E9 chicken skin explant was grown on a culture insert (Falcon, Becton Dickinson) within a culture chamber (SmartSlide, Wafergen) for 17 h. Brightfield images were collected every 15 min with an inverted microscope (Olympus) and assembled into a time lapse movie. Explants were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum plus gentamycin (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). The bottom plate of the culture chamber was set to 37 °C. The cover plate was heated to 37.5 °C to avoid condensation which would obscure our view.
1000). The bottom plate of the culture chamber was set to 37 °C. The cover plate was heated to 37.5 °C to avoid condensation which would obscure our view.
Multiphoton microscopy
Multiphoton microscopy was performed as described.59 Imaging was performed on E6 and E7 skin explants.RNA collection
Dorsal feather forming and ventral scale forming skins from embryonic day 7, day 9, and day 11 (E7, E9 and E11) white leghorn embryos were dissected in HBSS. The epithelium and mesenchyme were separated as described above. Total RNA was extracted from the specific tissue types E7 feather forming epithelium and mesenchyme, E9 feather forming epithelium and mesenchyme, E9 scale forming epithelium and mesenchyme, and E11 scale forming epithelium and mesenchyme. Each RNA sample contains five (5) pooled tissues.Microarray analysis
The chicken RNA samples were probed onto GeneChip Chicken Genome Arrays (Affymetrix). Replicates were performed for each tissue type for a sample size of three (n = 3). The raw data was uploaded into Partek Genomic Suite (PGS) using the .cel files. Robust Multi-chip Analysis for background adjustment, Quantile normalization and Robust Linear Model summarization of raw data were performed using the default mode of PGS. Each of the cluster figures (Fig. 3D and D′, 4D and D′) are actually subsets of the same, single, and much larger cluster that is based on the initial normalized (RMA) data and not on differential expression gene lists. The cluster was generated with the Pearson Centered distance metric and Centroid linkage. A 4 way Analysis of Variance (ANOVA) was performed to detect statistically different gene expression patterns and generate gene lists. The gene lists were uploaded into Ingenuity Pathway Analysis (IPA) to examine gene enrichment in pathways and gene ontology. Candidate genes were verified by whole mount in situ hybridization and by qPCR.Quantitative PCR
Quantitative PCR (qPCR) was performed using the Applied Biosciences Power SYBR® Green RNA-to-CT™ 1-Step Kit following the manufacturers protocol. Briefly, replicate RNA samples from the microarray assays were aliquoted onto a 96 well plate. The RT-PCR (reverse transcriptase) master mix, QPCR (quantitative) master mix, and dH20 were added to each sample replicate. Primers designed (QuantPrime) for specific genes were added to the samples.101 The qPCR machine (Stratagene Mx3000p) was programmed with an annealing temperature of 60 degrees for 40 amplification cycles. The threshold cycle (Ct) was used to determine gene expression differences.Acknowledgements
This work is supported by US NIH grant AR42177, AR47364, (to CMC), AR 060306 (CMC, TXJ, RW), and Taiwan National Science Council (to SJL). SJL is also supported by physician scientist award PS9803 from National Health Research Institutes (NHRI), Taiwan. AL is supported by pre-doctoral fellowship of California Institute of Regenerative Medicine training grant. Microscopy was performed by the Cell and Tissue Imaging Core of the USC Research Center for Liver Diseases, NIH grant No. P30 DK048522.References
- C. M. Chuong, in Molecular Basis of Epithelial Appendage Morphogenesis, ed. C. M. Chuong, Landes Bioscience, Austin, 1998, pp. 3–14 Search PubMed.
- R. B. Widelitz, J. M. Veltmaat, J. A. Mayer, J. Foley and C. M. Chuong, Semin. Cell Dev. Biol., 2007, 18, 255–266 CrossRef CAS.
- M. Plikus, W. P. Wang, J. Liu, X. Wang, T. X. Jiang and C. M. Chuong, Am. J. Pathol., 2004, 164, 1099–1114 CrossRef CAS.
- J. A. Mayer, J. Foley, D. De La Cruz, C. M. Chuong and R. Widelitz, Am. J. Pathol., 2008, 173, 1339–1348 CrossRef CAS.
- C. M. Chuong and M. K. Richardson, Int. J. Dev. Biol., 2009, 53, 653–658 CrossRef.
- P. K. Maini, R. E. Baker and C. M. Chuong, Science, 2006, 314, 1397–1398 CrossRef CAS.
- A. M. Turing, Bull. Math. Biol., 1990, 52, 153–197 CAS.
- S. Kondo and T. Miura, Science, 2010, 329, 1616–1620 CrossRef CAS.
- C. M. Lin, T. X. Jiang, R. E. Baker, P. K. Maini, R. B. Widelitz and C. M. Chuong, Dev. Biol., 2009, 334, 369–382 CrossRef CAS.
- Z. Yue, T. X. Jiang, R. B. Widelitz and C. M. Chuong, Nature, 2005, 438, 1026–1029 CrossRef CAS.
- T. X. Jiang, H. S. Jung, R. B. Widelitz and C. M. Chuong, Development, 1999, 126, 4997–5009 CAS.
- H. S. Jung, P. H. Francis-West, R. B. Widelitz, T. X. Jiang, S. Ting-Berreth, C. Tickle, L. Wolpert and C. M. Chuong, Dev. Biol., 1998, 196, 11–23 CrossRef CAS.
- T. Schlake and S. Sick, Cell Adhes. Migr., 2007, 1, 149–151 Search PubMed.
- N. Turner and R. Grose, Nat. Rev. Cancer, 2010, 10, 116–129 CrossRef CAS.
- I. L. Blitz and K. W. Cho, Dev. Dyn., 2009, 238, 1321–1331 CrossRef CAS.
- R. van Amerongen and R. Nusse, Development, 2009, 136, 3205–3214 CrossRef CAS.
- T. X. Jiang, R. B. Widelitz, W. M. Shen, P. Will, D. Y. Wu, C. M. Lin, H. S. Jung and C. M. Chuong, Int. J. Dev. Biol., 2004, 48, 117–135 CrossRef CAS.
- W. Shen, P. Will, A. Galstyan and C.-M. Chuong, Autonomous Robots, 2004, 17, 93 CrossRef.
- M. Rubenstein, Y. Sai, C. M. Chuong and W. M. Shen, Int. J. Dev. Biol., 2009, 53, 869–881 CrossRef.
- C. M. Chuong, P. Wu, M. Plikus, T. X. Jiang and R. Bruce Widelitz, Curr. Top. Dev. Biol., 2006, 72, 237–274 CAS.
- M. Yu, P. Wu, R. B. Widelitz and C. M. Chuong, Nature, 2002, 420, 308–312 CrossRef CAS.
- Z. Yue, T. X. Jiang, R. B. Widelitz and C. M. Chuong, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 951–955 CrossRef CAS.
- K. S. Stenn and R. Paus, Physiol. Rev., 2001, 81, 449–494 CAS.
- M. V. Plikus, J. A. Mayer, D. de la Cruz, R. E. Baker, P. K. Maini, R. Maxson and C. M. Chuong, Nature, 2008, 451, 340–344 CrossRef CAS.
- M. V. Plikus, R. B. Widelitz, R. Maxson and C. M. Chuong, Int. J. Dev. Biol., 2009, 53, 857–868 CrossRef.
- D. S. Dolberg and M. J. Bissell, Nature, 1984, 309, 552–556 CrossRef CAS.
- A. W. Stoker, C. Hatier and M. J. Bissell, J. Cell Biol., 1990, 111, 217–228 CrossRef CAS.
- B. Mintz and W. K. Silvers, Cancer Res., 1996, 56, 463–466 CAS.
- M. D. Sternlicht, H. Kouros-Mehr, P. Lu and Z. Werb, Differentiation, 2006, 74, 365–381 CrossRef CAS.
- M. D. Sternlicht, S. W. Sunnarborg, H. Kouros-Mehr, Y. Yu, D. C. Lee and Z. Werb, Development, 2005, 132, 3923–3933 CrossRef CAS.
- A. Mantovani, P. Allavena, A. Sica and F. Balkwill, Nature, 2008, 454, 436–444 CrossRef CAS.
- L. M. Coussens and Z. Werb, Nature, 2002, 420, 860–867 CrossRef CAS.
- G. R. Cunha, A. A. Donjacour, P. S. Cooke, S. Mee, R. M. Bigsby, S. J. Higgins and Y. Sugimura, Endocr. Rev., 1987, 8, 338–362 CrossRef CAS.
- S. W. Hayward, P. C. Haughney, M. A. Rosen, K. M. Greulich, H. U. Weier, R. Dahiya and G. R. Cunha, Differentiation, 1998, 63, 131–140 CrossRef CAS.
- S. Aboseif, A. El-Sakka, P. Young and G. Cunha, Differentiation, 1999, 65, 113–118 CrossRef CAS.
- W. A. Ricke, K. Ishii, E. A. Ricke, J. Simko, Y. Wang, S. W. Hayward and G. R. Cunha, Int. J. Cancer, 2006, 118, 2123–2131 CrossRef CAS.
- J. A. Mayer, C. M. Chuong and R. Widelitz, Differentiation, 2004, 72, 474–488 CrossRef CAS.
- B. Weigelt and M. J. Bissell, Semin. Cancer Biol., 2008, 18, 311–321 CrossRef CAS.
- M. A. LaBarge, C. M. Nelson, R. Villadsen, A. Fridriksdottir, J. R. Ruth, M. R. Stampfer, O. W. Petersen and M. J. Bissell, Integr. Biol., 2009, 1, 70–79 RSC.
- M. Bissell, Integr. Biol., 2010, 2, 9 RSC.
- F. Prin and D. Dhouailly, Int. J. Dev. Biol., 2004, 48, 137–148 CrossRef CAS.
- D. Dhouailly, Int. J. Dev. Biol., 2009, 53, 775–782 CrossRef.
- I. Kupershmidt, Q. J. Su, A. Grewal, S. Sundaresh, I. Halperin, J. Flynn, M. Shekar, H. Wang, J. Park, W. Cui, G. D. Wall, R. Wisotzkey, S. Alag, S. Akhtari and M. Ronaghi, PLoS One, 2010, 5, e13066 CrossRef.
- M. Rendl, L. Lewis and E. Fuchs, PLoS Biol., 2005, 3, e331 CrossRef.
- R. J. Morris, Y. Liu, L. Marles, Z. Yang, C. Trempus, S. Li, J. S. Lin, J. A. Sawicki and G. Cotsarelis, Nat. Biotechnol., 2004, 22, 411–417 CrossRef CAS.
- E. B. Lewis, Nature, 1978, 276, 565–570 CrossRef CAS.
- M. Kessel and P. Gruss, Science, 1990, 249, 374–379 CrossRef CAS.
- B. Kanzler, J. P. Viallet, H. Le Mouellic, E. Boncinelli, D. Duboule and D. Dhouailly, Int. J. Dev. Biol., 1994, 38, 633–640 CAS.
- A. I. Reid and S. J. Gaunt, Int. J. Dev. Biol., 2002, 46, 209–215 CAS.
- E. B. Brown, R. B. Campbell, Y. Tsuzuki, L. Xu, P. Carmeliet, D. Fukumura and R. K. Jain, Nat. Med., 2001, 7, 864–868 CrossRef CAS.
- A. Zoumi, A. Yeh and B. J. Tromberg, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11014–11019 CrossRef CAS.
- W. R. Zipfel, R. M. Williams, R. Christie, A. Y. Nikitin, B. T. Hyman and W. W. Webb, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 7075–7080 CrossRef CAS.
- J. N. Lee, S. H. Jee, C. C. Chan, W. Lo, C. Y. Dong and S. J. Lin, J. Invest. Dermatol., 2008, 128, 2240–2247 CrossRef CAS.
- F. C. Li, Y. Liu, G. T. Huang, L. L. Chiou, J. H. Liang, T. L. Sun, C. Y. Dong and H. S. Lee, Am. J. Physiol.: Gastrointest. Liver Physiol., 2009, 296, G1091–7 CrossRef CAS.
- T. H. Tsai, S. H. Jee, J. Y. Chan, J. N. Lee, W. R. Lee, C. Y. Dong and S. J. Lin, J. Biomed. Opt., 2009, 14, 024034 CrossRef.
- T. H. Tsai, S. H. Jee, C. Y. Dong and S. J. Lin, J. Dermatol. Sci., 2009, 56, 1–8 CrossRef.
- W. Denk, J. H. Strickler and W. W. Webb, Science, 1990, 248, 73–76 CrossRef CAS.
- S. J. Lin, C. Y. Hsiao, Y. Sun, W. Lo, W. C. Lin, G. J. Jan, S. H. Jee and C. Y. Dong, Opt. Lett., 2005, 30, 622–624 Search PubMed.
- S. J. Lin, R. Wu Jr, H. Y. Tan, W. Lo, W. C. Lin, T. H. Young, C. J. Hsu, J. S. Chen, S. H. Jee and C. Y. Dong, Opt. Lett., 2005, 30, 2275–2277 Search PubMed.
- P. J. Campagnola and L. M. Loew, Nat. Biotechnol., 2003, 21, 1356–1360 CrossRef CAS.
- S. J. Lin, S. H. Jee, C. J. Kuo, R. J. Wu, W. C. Lin, J. S. Chen, Y. H. Liao, C. J. Hsu, T. F. Tsai, Y. F. Chen and C. Y. Dong, Opt. Lett., 2006, 31, 2756–2758 Search PubMed.
- S. J. Lin, W. Lo, H. Y. Tan, J. Y. Chan, W. L. Chen, S. H. Wang, Y. Sun, W. C. Lin, J. S. Chen, C. J. Hsu, J. W. Tjiu, H. S. Yu, S. H. Jee and C. Y. Dong, J. Biomed. Opt., 2006, 11, 34020 CrossRef.
- D. J. Pearton, Y. Yang and D. Dhouailly, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 3714–3719 CrossRef CAS.
- Y. Chen, Y. Zhang, T. X. Jiang, A. J. Barlow, T. R. St Amand, Y. Hu, S. Heaney, P. Francis-West, C. M. Chuong and R. Maas, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 10044–10049 CrossRef CAS.
- M. H. Hardy, D. Dhouailly, H. Torma and A. Vahlquist, J. Exp. Zool., 1990, 256, 279–289 CrossRef CAS.
- D. Dhouailly, M. H. Hardy and P. Sengel, J. Embryol. Exp. Morphol., 1980, 58, 63–78 Search PubMed.
- R. Crowe and L. Niswander, Dev. Biol., 1998, 195, 70–74 CrossRef CAS.
- H. Zou and L. Niswander, Science, 1996, 272, 738–741 CrossRef CAS.
- R. B. Widelitz, T. X. Jiang, J. Lu and C. M. Chuong, Dev. Biol., 2000, 219, 98–114 CrossRef CAS.
- S. M. Brugger, A. E. Merrill, J. Torres-Vazquez, N. Wu, M. C. Ting, J. Y. Cho, S. L. Dobias, S. E. Yi, K. Lyons, J. R. Bell, K. Arora, R. Warrior and R. Maxson, Development, 2004, 131, 5153–5165 CrossRef CAS.
- A. D. Reginelli, Y. Q. Wang, D. Sassoon and K. Muneoka, Development, 1995, 121, 1065–1076 CAS.
- S. L. Cheng, J. S. Shao, J. Cai, O. L. Sierra and D. A. Towler, J. Biol. Chem., 2008, 283, 20505–20522 CrossRef CAS.
- S. M. Hussein, E. K. Duff and C. Sirard, J. Biol. Chem., 2003, 278, 48805–48814 CrossRef CAS.
- B. H. van den Berg, F. M. McCarthy, S. J. Lamont and S. C. Burgess, PLoS One, 2010, 5, e10642 CrossRef.
- T. Iimura and O. Pourquie, Nature, 2006, 442, 568–571 CrossRef CAS.
- B. A. Morgan and C. J. Tabin, Curr. Opin. Genet. Dev., 1993, 3, 668–674 CrossRef CAS.
- J. Zakany and D. Duboule, Curr. Opin. Genet. Dev., 2007, 17, 359–366 CrossRef CAS.
- C. M. Chuong, G. Oliver, S. A. Ting, B. G. Jegalian, H. M. Chen and E. M. De Robertis, Development, 1990, 110, 1021–1030 CAS.
- C. M. Chuong, BioEssays, 1993, 15, 513–521 CrossRef CAS.
- H. Y. Chang, J. T. Chi, S. Dudoit, C. Bondre, M. van de Rijn, D. Botstein and P. O. Brown, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12877–12882 CrossRef CAS.
- H. Y. Chang, Science, 2009, 326, 1206–1207 CrossRef CAS.
- A. R. Godwin and M. R. Capecchi, J. Invest. Dermatol. Symp. Proc., 1999, 4, 244–247 Search PubMed.
- E. Rieger, J. J. Bijl, J. W. van Oostveen, H. P. Soyer, C. B. Oudejans, N. M. Jiwa, J. M. Walboomers and C. J. Meijer, J. Invest. Dermatol., 1994, 103, 341–346 CrossRef CAS.
- A. R. Godwin and M. R. Capecchi, Genes Dev., 1998, 12, 11–20 CrossRef CAS.
- C. M. Chuong, H. S. Jung, D. Noden and R. B. Widelitz, Biochem. Cell Biol., 1998, 76, 1069–1077 Search PubMed.
- N. K. Wessells, Dev. Biol., 1965, 12, 131–153 CrossRef CAS.
- E. S. Stuart and A. A. Moscona, Science, 1967, 157, 947–948 CrossRef CAS.
- A. Mauger, M. Demarchez, D. Herbage, J. A. Grimaud, M. Druguet, D. Hartmann and P. Sengel, Dev. Biol., 1982, 94, 93–105 CrossRef CAS.
- Y. Al-Nuaimi, G. Baier, R. E. Watson, C. M. Chuong and R. Paus, Exp. Dermatol., 2010, 19, 707–713 CrossRef.
- P. Wu, L. Hou, M. Plikus, M. Hughes, J. Scehnet, S. Suksaweang, R. Widelitz, T. X. Jiang and C. M. Chuong, Int. J. Dev. Biol., 2004, 48, 249–270 CrossRef CAS.
- R. O. Prum and A. H. Brush, Q. Rev. Biol., 2002, 77, 261–295 Search PubMed.
- L. Hou, C. Chuong, A. Yang, X. L. Zheng and J. F. Hou, Fossil Birds of China, Yunnan Science and Technology, China, 2003 Search PubMed.
- C. M. Chuong, P. Wu, F. C. Zhang, X. Xu, M. Yu, R. B. Widelitz, T. X. Jiang and L. Hou, J. Exp. Zool., Part B, 2003, 298, 42–56 Search PubMed.
- R. O. Prum, J. Exp. Zool., Part B, 2005, 304B, 570–579 Search PubMed.
- R. Chodankar, C. H. Chang, Z. Yue, T. X. Jiang, S. Suksaweang, L. Burrus, C. M. Chuong and R. Widelitz, J. Invest. Dermatol., 2003, 120, 20–26 CrossRef CAS.
- C. H. Chang, M. Yu, P. Wu, T. X. Jiang, H. S. Yu, R. B. Widelitz and C. M. Chuong, J. Invest. Dermatol., 2004, 122, 1348–1355 CrossRef CAS.
- D. Duboule, Int. J. Dev. Biol., 2009, 53, 717–723 CrossRef.
- V. Hamburger and H. L. Hamilton, J. Morphol., 1951, 88, 49–92 CrossRef.
- C. E. Nelson, B. A. Morgan, A. C. Burke, E. Laufer, E. DiMambro, L. C. Murtaugh, E. Gonzales, L. Tessarollo, L. F. Parada and C. Tabin, Development, 1996, 122, 1449–1466 CAS.
- T. X. Jiang, S. Stott, R. B. Widelitz and C. M. Chuong, in Molecular Basis of Epithelial Appendage Morphogenesis, ed. C. M. Chuong, Landes Bioscience, Austin, TX, 1998, pp. 359 Search PubMed.
- S. Arvidsson, M. Kwasniewski, D. M. Riano-Pachon and B. Mueller-Roeber, BMC Bioinformatics, 2008, 9, 465 CrossRef.
- C. M. Chuong, R. Chodankar, R. B. Widelitz and T. X. Jiang, Curr. Opin. Genet. Dev., 2000, 10, 449–456 CrossRef CAS.
Footnotes |
| † Published as part of an Integrative Biology themed issue in honour of Mina J. Bissell: Guest Editor Mary Helen Barcellos-Hoff. |
| ‡ Electronic supplementary information (ESI) available. See DOI: 10.1039/c0ib00108b |
| This journal is © The Royal Society of Chemistry 2011 |
