Nuclease p1: a new biocatalyst for direct asymmetric aldol reaction under solvent-free conditions†
Hai-Hong
Li‡
,
Yan-Hong
He‡
,
Yi
Yuan
and
Zhi
Guan
*
School of Chemistry and Chemical Engineering, Southwest University, Chongqing, 400715, P. R. China. E-mail: guanzhi@swu.edu.cn; Fax: +86-23-68254091; Tel: +86-23-68254091
First published on 24th November 2010
Abstract
The unnatural ability of nuclease p1 from Penicillium citrinum was first discovered to catalyze asymmetric aldol reactions between aromatic aldehydes and cyclic ketones under solvent-free conditions. The excellent enantioselectivities of up to 99% ee and high diastereoselectivities of up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (anti/syn) were achieved. This nuclease p1 catalyzed reaction provided a novel case for new activities of existing enzymes, which widens the applicability of this biocatalyst in organic synthesis.
1 (anti/syn) were achieved. This nuclease p1 catalyzed reaction provided a novel case for new activities of existing enzymes, which widens the applicability of this biocatalyst in organic synthesis.
1. Introduction
Biocatalytic processes have often proven to be economically feasible, ecologically advantageous and more sustainable than current chemical technologies because of the intrinsic advantages of biocatalysts in higher reaction selectivity, milder reaction conditions and potential use of inexpensive regenerable resources.1 However, there are some drawbacks of biocatalysis. For example, there is only limited availability of enzymes, and enzymes only feature limited substrate specificity.2 Therefore, the discovery of novel unnatural activities of existing enzymes to widen the applicability of existing enzymes is significant. Recently, research in the area of biocatalytic promiscuity has attracted significant attention from chemists and biochemists.3 Hilvert et al. reported the use of minimal modifications at enzyme active sites to expand their catalytic repertoires, including targeted mutagenesis and the addition of new reactive functionalities.4 Danson and Bull et al. reported structurally informed site-directed mutagenesis of a stereochemically promiscuous aldolase to afford stereochemically complementary biocatalysts.5 Griengl et al. reported hydroxynitrile lyase from Hevea brasiliensis catalyzed nitroaldol reactions.6 More recently, Zhang and Chen et al. reported lipase-catalysed tandem Knoevenagel condensation and esterification with alcohol cosolvents.7 In addition, some other elegant works on enzyme catalyzed promiscuity have also been reported.8Catalytic aldol reactions are among the most useful synthetic methods for highly stereocontrolled asymmetric synthesis.9 There are many reports about enantioselective aldol reactions catalyzed by small organic molecules,10 but there are few examples of aldol reactions catalyzed by enzymes, besides aldolases11 that are the natural enzymes for aldol reactions. Berglund and co-workers once used mutant CAL-B (lipase from Candida antarctica) to catalyze aldol additions in 2003.12 Wang and Yu also reported lipase catalyzed aldol reactions in 2008.13 However, of those reactions reported to date for which ee values are reported, the best ee value so far was only 43.6% with low yield of 11.7%.13 To the best of our knowledge, other hydrolase catalyzed aldol addition has not been reported. Therefore, the development of highly enantioselective aldol reactions catalyzed by enzymes is still in great demand.
Nuclease P1 (EC 3.1.30.1) from Penicillium citrinum belongs to a family of zinc-dependent endonucleases consisting of 270 amino acid residues with two disulfide bonds which cleaves single-stranded RNA and DNA into 5-mononucleotide.14 We found that nuclease p1 also had the ability to catalyze asymmetric aldol reactions between aromatic aldehdes and cyclic ketones with excellent stereoselectivity and high diastereoselectivities under solvent-free conditions. This finding provided a novel example of unnatural activities of existing enzymes.
2. Results and discussion
Asymmetric direct aldol reaction of 4-cyanobenzaldehyde and cyclohexanone catalyzed by nuclease p1 was first carried out as a model experiment to find out the optimum reaction conditions, such as the solvent, the ratio of substrates, the enzyme loading, the water content and the temperature. Based on the concept that not only do enzymes work in anhydrous organic media, but they acquire remarkable properties such as enhanced stability, altered substrate and enantiomeric specificities, molecular memory, and the ability to catalyze unusual reactions which are impossible in aqueous media,15 the catalytic activity of nuclease p1 was evaluated in different solvents including solvent-free conditions (Table 1). The results clearly indicated that the catalytic activity and the stereoselectivity of nuclease p1 were significantly influenced by the reaction media. Among the catalytic systems examined, the nuclease p1 exhibited the best catalytic activity and moderate stereoselectivity in DMSO (Table 1, entry 1). However, the enzyme showed the best diastereo- and enantio- selectivities under solvent-free conditions (Table 1, entry 3). Thus, we chose solvent-free as the optimum conditions for the asymmetric direct aldol reaction.|
|
|||||
|---|---|---|---|---|---|
| Entry | Solvent | Time/h | Yield [%]b | d.r.c | ee[%]d |
| a All reactions except the reactions under solvent-free conditions were carried out using 4-cyanobenzaldehyde (200 mg, 1.5 mmol), cyclohexanone (1.0 ml, 10 mmol), nuclease p1 (200 mg), H2O (0.75 ml) and organic solvent (5 ml) at 20 °C. b Yield of the isolated product after chromatography on silica gel. c The d.r. was the anti/syn ratio, which was determined by 1H NMR analysis of the diastereomeric isomers. d Enantiomeric excess (anti) was determined by HPLC analysis using a chiral column; relative and absolute configurations of the products were determined by comparison with the known 1H NMR and chiral HPLC analysis. e Reaction conditions: 4-cyanobenzaldehyde (200 mg, 1.5 mmol), cyclohexanone (1.0 ml, 10 mmol) and nuclease p1 (200 mg) at 20 °C. f Pre-treated with EDTA at 30 °C for 24 h. | |||||
| 1 | DMSO | 96 | 72 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
54 |
| 2 | DMF | 96 | 53 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
55 |
| 3 | Solvent-freee | 96 | 30 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
81 |
| 4 | THF | 96 | 28 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
64 |
| 5 | CH3CN | 96 | 27 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
67 |
| 6 | Cyclohexane | 96 | 21 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 58 |
65 |
| 7 | CH2Cl2 | 96 | 20 | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
61 |
| 8 | H2O | 96 | 18 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
42 |
| 9 | Solvent-free (nuclease p1 denatured with EDTAf) | 96 | 3 | — | — |
| 10 | Solvent-free (no enzyme) | 168 | nr | — | — |
In order to verify the specific catalytic effect of nuclease p1 on the aldol reaction, we performed some control experiments under solvent-free conditions (Table 1). Just as we expected, the aldol reaction between 4-cyanobenzaldehyde and cyclohexanone in the absence of enzyme under solvent-free conditions showed no adduct after 168 h (Table 1, entry 10). In addition, since nuclease p1 is a zinc-dependent enzyme, EDTA (ethylene diamine tetraacetic acid) was used to denature the enzyme. We found that the aldol reaction with EDTA-denatured nuclease p1 only gave 3% yield of aldol product after 96 h, which excluded the possibility that the reaction was caused by impurities of the enzyme or the catalysis simply arose from amino acids of the protein. On the other hand, it can be assumed that the tertiary structure of the enzyme was essential in the process. Thus, we validated that nuclease p1 catalyzed the direct aldol reaction.
Next, the effect of molar ratio of substrates on the nuclease p1 catalyzed aldol reaction was investigated under solvent-free conditions (Fig. 1). The best ee value of 82% with yield of 32% was obtained when the molar ratio of cyclohexanone to 4-cyanobenzaldehyde was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. No positive effect was observed on ee as well as on yield while increasing the amount of cyclohexanone. Thus, the 5
1. No positive effect was observed on ee as well as on yield while increasing the amount of cyclohexanone. Thus, the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of ketone to aldehyde was chosen as the optimal ratio for further studies.
1 molar ratio of ketone to aldehyde was chosen as the optimal ratio for further studies.
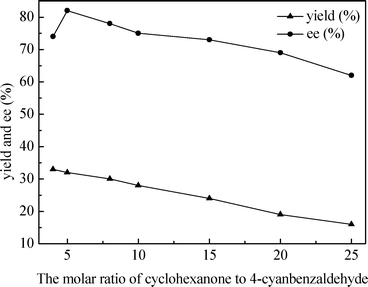 | ||
| Fig. 1 Effect of the molar ratio of substrates on the nuclease p1 catalyzed aldol reaction. Conditions: 4-cyanobenzaldehyde (1.0 mmol), cyclohexanone (4–25 mmol) and nuclease p1 (200 mg) at 20 °C for 90 h. | ||
We then examined the influence of enzyme loading on the aldol reaction of cyclohexanone (5.0 mmol) and 4-cyanobenzaldehyde (1.0 mmol) under solvent-free conditions. From Fig. 2, it can be seen that the catalytic activity and the stereoselectivity of nuclease p1 were significantly influenced by the enzyme loading. When the enzyme loading was increased to 200 mg, a major enhancement in ee and yield was observed. However, no evident improvement of ee was observed though there was a slight enhancement in yield on increasing further the amount of enzyme. So the enzyme loading of 200 mg was chosen for the further studies under solvent-free conditions.
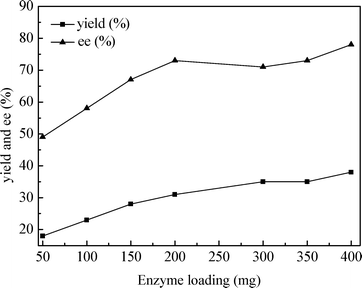 | ||
| Fig. 2 Influence of the enzyme loading on the nuclease p1 catalyzed aldol reaction. Conditions: 4-cyanobenzaldehyde (1.0 mmol), cyclohexanone (5.0 mmol) and nuclease p1 (50–400 mg) at 25 °C for 96 h. | ||
Water concentration affects both the enantioselectivity and activity of enzymes.16 In order to ascertain the role played by water, we carried out the reaction in different water content under solvent-free conditions. As shown in Fig. 3, the yield and ee value of the aldol reaction could be evidently effected by water content. The nuclease p1 exhibited the highest enantioselectivity at water content of 0.15 (wwater/wenzyme), and under this conditions the enzymatic reaction reached the highest ee value of 84% (yield of 35%). Once the concentration of water surpassed 0.15 (wwater/wenzyme), the ee value decreased. However, the highest enzyme activity was reached at water content of 0.70 (wwater/wenzyme), which gave the best yield of 46% (76% ee), and higher water content led to a decrease of yield. Therefore, to obtain the best ee, we chose the water content of 0.15 (wwater/wenzyme) for the aldol reaction.
 | ||
| Fig. 3 Influence of water content on the nuclease p1 catalyzed aldol reaction. Conditions: 4-cyanobenzaldehyde (1.0 mmol), cyclohexanone (5.0 mmol), nuclease p1 (200 mg), deionized water (0–2.0, wwater/wenzyme) at 25 °C for 107 h. | ||
Temperature plays an important role in enzyme catalyzed reactions, due to its effects on the selectivity and rate of the reaction, and also on the stability of the enzyme. We then studied the effect of temperature on the reaction (Fig. 4). The enzyme showed the best enantioselectivity of 91% ee at 15 °C, however, it required a higher temperature (45 °C) to exhibit its best activity. In order to get the best enantioselectivity, we chose 15 °C as the optimal temperature.
 | ||
| Fig. 4 Influence of temperature on the nuclease p1 catalyzed aldol reaction. Conditions: 4-cyanobenzaldehyde (1.0 mmol), cyclohexanone (5.0 mmol), nuclease p1 (200 mg), temperature (5–70 °C) and deionized water (30 mg) for 96 h. | ||
With the optimal reaction conditions in hand, we further studied the substrate scope and the generality of the nuclease p1 catalyzed asymmetric direct aldol reaction. Various cyclic ketones and substituted benzaldehydes were investigated under solvent-free conditions (Table 2). It can be seen that a wide range of substrates could participate in the reaction. Five-, six- and seven-membered cyclic ketones as aldol donors could be accepted by the enzyme. Generally, the enzyme exhibited better diastereoselectivity and enantioselectivity with cyclohexanone (Table 2, entries 1–12) than with cyclopentanone and cycloheptanone (Table 2, entries 13–15). Furthermore, both electron-donating and electron-withdrawing substituents of aromatic aldehydes were tolerated. Notably, this nuclease p1 catalyzed asymmetric direct aldol reaction exhibited high selectivity. The best enantioselectivity of >99% ee (Table 2, entry 1) and the best diastereoselectivity of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (anti/syn) (Table 2, entry 7) were achieved. Moreover, the effect of sterically hindered substituents on benzaldehydes had a great impact on the diastereoselectivity of the reaction. When reacting with cyclohexanone, substituted benzaldehydes (Table 2, entries 1 and 3–12) gave better diastereoselectivity than benzaldehyde (Table 2, entry 2), substituents in the 2-position gave higher dr values (Table 2, entries 4, 6, 7 and 11), and the best diastereoselectivity of >99
1 (anti/syn) (Table 2, entry 7) were achieved. Moreover, the effect of sterically hindered substituents on benzaldehydes had a great impact on the diastereoselectivity of the reaction. When reacting with cyclohexanone, substituted benzaldehydes (Table 2, entries 1 and 3–12) gave better diastereoselectivity than benzaldehyde (Table 2, entry 2), substituents in the 2-position gave higher dr values (Table 2, entries 4, 6, 7 and 11), and the best diastereoselectivity of >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was achieved by using the most hindered substrate 2,6-dichlorobenzaldehyde (Table 2, entry 7). Interestingly, anti isomers were received as the major products by using cyclohexanone and cyclopentanone, but no diastereoselectivity was observed by using cycloheptanone. The nuclease p1 catalyzed-aldol reaction seems to prefer cyclohexanone than cyclopentanone and cycloheptanone. It is also worthy to note that this enzyme had a moderate to excellent enantioselectivity for anti isomers, but low or no enantioselectivity for syn isomers. Maybe the catalytic site of the nuclease p1 had a specific selectivity for the aldol reaction. The yield of the aldol reaction catalyzed by nuclease p1 is still low and the reaction mechanism is unclear at the moment. Further efforts to deal with this problem are currently underway in our laboratory.
1 was achieved by using the most hindered substrate 2,6-dichlorobenzaldehyde (Table 2, entry 7). Interestingly, anti isomers were received as the major products by using cyclohexanone and cyclopentanone, but no diastereoselectivity was observed by using cycloheptanone. The nuclease p1 catalyzed-aldol reaction seems to prefer cyclohexanone than cyclopentanone and cycloheptanone. It is also worthy to note that this enzyme had a moderate to excellent enantioselectivity for anti isomers, but low or no enantioselectivity for syn isomers. Maybe the catalytic site of the nuclease p1 had a specific selectivity for the aldol reaction. The yield of the aldol reaction catalyzed by nuclease p1 is still low and the reaction mechanism is unclear at the moment. Further efforts to deal with this problem are currently underway in our laboratory.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Prod. | n | Time/h | Yield [%]b | d.r.c | ee[%]d (anti) |
| a For the general procedure see ref. [17]. b Yield of the isolated product (anti + syn) after chromatography on silica gel. c The d.r. was the anti/syn ratio, which was determined by 1H NMR analysis. d Enantiomeric excess was determined by HPLC analysis using a chiral column; absolute configurations of the products were determined by comparison with the known chiral HPLC analysis 18 (see the ESI†). e anti (79% ee), syn (33% ee). f anti (82% ee), syn (47% ee). | |||||||
| 1 | 4-MeC6H4 | 3a | 2 | 144 | 25 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
>99 |
| 2 | C6H5 | 3b | 2 | 144 | 20 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
85 |
| 3 | 4-ClC6H4 | 3c | 2 | 144 | 21 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
91 |
| 4 | 2-ClC6H4 | 3d | 2 | 240 | 38 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
93 |
| 5 | 3-ClC6H4 | 3e | 2 | 158 | 24 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
92 |
| 6 | 2,4-Cl2C6H3 | 3f | 2 | 240 | 31 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
90 |
| 7 | 2,6-Cl2C6H3 | 3g | 2 | 240 | 37 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 |
| 8 | 4-BrC6H4 | 3h | 2 | 144 | 17 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
93 |
| 9 | 4-CNC6H4 | 3i | 2 | 165 | 43 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
91 |
| 10 | 4-CF3C6H4 | 3j | 2 | 158 | 26 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
90 |
| 11 | 2-NO2C6H4 | 3k | 2 | 165 | 21 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
89 |
| 12 | 3-NO2C6H4 | 3l | 2 | 165 | 28 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
92 |
| 13 | 4-NO2C6H4 | 3m | 1 | 165 | 55 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
79/33e |
| 14 | 4-CNC6H4 | 3n | 1 | 144 | 39 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
82/47f |
| 15 | 4-NO2C6H4 | 3o | 3 | 165 | 39 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
49 |
3. Conclusion
In summary, we have succeeded in obtaining enantiomeric aldol products by using enzyme nuclease p1 as a new biocatalyst. A wide range of cyclic ketones and substituted benzaldehydes could be accepted by the enzyme. In most cases, excellent ee values and good d.r. were obtained without any additive. Notably, this is the first highly stereoselective aldol reaction catalyzed by enzymes besides aldolases. This biocatalytic reaction was performed under mild and solvent-free conditions. Compared with current chemical technologies, the nuclease p1 catalyzed direct asymmetric aldol reaction is more economically feasible, ecologically advantageous and sustainable by using inexpensive regenerable resources. It is also a novel case of unnatural activities of existing enzymes, and might be a potential synthetic method for organic chemistry.Acknowledgements
Financial support from Natural Science Foundation Project of CQ CSTC (2009BA5051) is gratefully acknowledged.References
- (a) J. R. Knowles, Nature, 1991, 350, 121–124 CAS; (b) W. D. Fessner and T. Anthonsen, in Modern Biocatalysis: Stereoselective and Environmentally Friendly Reactions, Wiley-VCH, Weinheim, 2009, pp. XV–XVI Search PubMed.
- A. S. Bommarius and B. R. Riebel, in Enzyme Biocatalysis: Principles and Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2004, pp. 1–2 Search PubMed.
- (a) O. Khersonsky, C. Roodveldt and D. S. Tawfik, Curr. Opin. Chem. Biol., 2006, 10, 498–508 CrossRef CAS; (b) K. Hult and P. Berglund, Trends Biotechnol., 2007, 25, 231–238 CrossRef CAS.
- (a) M. D. Toscano, K. J. Woycechowsky and D. Hilvert, Angew. Chem., Int. Ed., 2007, 46, 3212–3236 CrossRef CAS; (b) F. P. Seebeck, A. Guainazzi, C. Amoreira, K. K. Baldridge and D. Hilvert, Angew. Chem., Int. Ed., 2006, 45, 6824–6826 CrossRef CAS; (c) M. D. Toscano, M. M. Müller and D. Hilvert, Angew. Chem., Int. Ed., 2007, 46, 4468–4470 CrossRef CAS.
- S. F. Royer, L. Haslett, S. J. Crennell, D. W. Hough, M. J. Danson and S. D. Bull, J. Am. Chem. Soc., 2010, 132, 11753–11758 CrossRef CAS.
- T. Purkarthofer, K. Gruber, M. Gruber-Khadjawi, K. Waich, W. Skranc, D. Mink and H. Griengl, Angew. Chem., Int. Ed., 2006, 45, 3454–3456 CrossRef CAS.
- Y.-F. Lai, H. Zheng, S.-J. Chai, P.-F. Zhang and X.-Z. Chen, Green Chem., 2010, 12, 1917–1918 RSC.
- (a) S. G. Burton, D. A. Cowan and J. M. Woodley, Nat. Biotechnol., 2002, 20, 37–45 CrossRef CAS; (b) U. T. Bornscheuer and R. J. Kazlauskas, Angew. Chem., Int. Ed., 2004, 43, 6032–6040 CrossRef CAS; (c) R. Kourist, S. Bartch, L. Fransson, K. Hult and U. T. Bornscheuer, ChemBioChem, 2008, 9, 67–69 CrossRef CAS; (d) J.-M. Xu, F. Zhang, B.-K. Liu, Q. Wu and X.-F. Lin, Chem. Commun., 2007, 20, 2078–2080 Search PubMed; (e) Q. Wu, J.-M. Xu, L. Xia, J.-L. Wang and X.-F. Lin, Adv. Synth. Catal., 2009, 351, 1833–1841 CrossRef CAS; (f) E. Champion, I. André, C. Moulis, J. Boutet, K. Descroix, S. Morel, P. Monsan, L. A. Mulard and M. Remaud-Siméon, J. Am. Chem. Soc., 2009, 131, 7379–7389 CrossRef CAS.
- W.-D. Fessner, M. Petersen and M. T. Zannetti, in Top. Curr. Chem. Vol. 186 Carbohydrates, eds. J. Thiem, H. Driguez, Springer, Heidelberg 1997, pp 87–117 Search PubMed.
- (a) K. Sakthivel, W. Notz, T. Bui and C. F. Barbas III, J. Am. Chem. Soc., 2001, 123, 5260–5267 CrossRef CAS; (b) A. B. Northrup and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 6798–6799 CrossRef CAS; (c) W. Notz, F. Tanaka and C. F. Barbas III, Acc. Chem. Res., 2004, 37, 580–591 CrossRef CAS; (d) J. T. Suri, D. B. Ramachary and C. F. Barbas III, Org. Lett., 2005, 7, 1383–1385 CrossRef CAS.
- (a) O. Eyrisch and W.-D. Fessner, Angew. Chem., 1995, 107, 1738–1740 ( Angew. Chem., Int. Ed. Engl. , 1995 , 34 , 1639–1641 ) CrossRef; (b) T. Kimura, V. P. Vassilev, G.-J. Shen and C.-H. Wong, J. Am. Chem. Soc., 1997, 119, 11734–11742 CrossRef CAS; (c) T. D. Machajewski and C. H. Wong, Angew. Chem., 2000, 112, 1406–1430 ( Angew. Chem. Int. Ed. , 2000 , 39 , 1353–1374 ) CrossRef; (d) A. Heine, G. DeSantis, J. G. Luz, M. Mitchell, C. H. Wong and I. A. Wilson, Science, 2001, 294, 369–374 CrossRef CAS; (e) W. D. Fessner and V. Helaine, Curr. Opin. Biotechnol., 2001, 12, 574–586 CrossRef CAS (and references therein); (f) W. A. Greenberg, A. Varvak, S. R. Hanson, K. Wong, H. J. Huang, P. Chen and M. J. Burk, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5788–5793 CrossRef CAS.
- C. Branneby, P. Carlqvist, A. Magnusson, K. Hult, T. Brinck and P. Berglund, J. Am. Chem. Soc., 2003, 125, 874–875 CrossRef.
- C. Li, X.-W. Feng, N. Wang, Y.-J. Zhou and X.-Q. Yu, Green Chem., 2008, 10, 616–618 RSC.
- (a) A. Lahm, A. Volbeda and D. Suck, J. Mol. Biol., 1990, 215, 207–210 CAS; (b) A. Volbeda, A. Lahm, F. Sakiyama and D. Suck, EMBO J., 1991, 10, 1607–1618 CAS; (c) W. N. Lipscomb and N. Strater, Chem. Rev., 1996, 96, 2375–2433 CrossRef CAS.
- (a) A. M. Klibanov, Trends Biochem. Sci., 1989, 14, 141–144 CrossRef CAS; (b) S. Tawaki and A. M. Klibanov, J. Am. Chem. Soc., 1992, 114, 1882–1884 CrossRef CAS; (c) K. Griebenow and A. M. Klibanov, J. Am. Chem. Soc., 1996, 47, 11695–11700 CrossRef; (d) A. M. Klibanov, Nature, 2001, 409, 241–246 CrossRef CAS.
- (a) C. Orrenius, T. Norin, K. Hult and G. Carrea, Tetrahedron: Asymmetry, 1995, 6, 3023–3030 CrossRef CAS; (b) E. Wehtje, J. Kaur, P. Adlercreutz, S. Chand and B. Mattiasson, Enzyme Microb. Technol., 1997, 21, 502–510 CrossRef; (c) D. Costes, E. Wehtje and P. Adlercreutz, Enzyme Microb. Technol., 1999, 25, 384–391 CrossRef CAS; (d) E. P. Hudson, R. K. Eppler, J. M. Beaudoin, J. S. Dordick, J. A. Reimer and D. S. Clark, J. Am. Chem. Soc., 2009, 131, 4294–4300 CrossRef CAS.
- General procedure: A test tube was charged with nuclease p1 (5 U/mg) (200 mg), aldehyde (1.0 mmol), to which the deionized water (30 mg) and ketone (5.0 mmol) were introduced. The resulting mixture was stirred for the specified amount of time at 15 °C. The, reaction was terminated by filtering the enzyme. CH2Cl2 was used to wash the filter paper to assure that products obtained were all dissolved in the filtrate. 20 ml of water was then added to the filtrate, and the filtrate was extracted three times with 20 ml of CH2Cl2. The combined extracts were dried over anhydrous Na2SO4, and the solvents were then removed under reduced pressure. The crude products were purified by column chromatography with petroleum ether/ethyl acetate as eluent.
- (a) J. R Chen, H. H. Lu, X. Y. Li, L. Cheng, J. Wan and W. J. Xiao, Org. Lett., 2005, 7, 4543–4545 CrossRef CAS; (b) Y. Y. Wu, Y. Z Zhang, M. L. Yu, G. Zhao and S. W. Wang, Org. Lett., 2006, 8, 4417–4420 CrossRef CAS; (c) N. Mase, Y. Nakai, N. Ohara, H. Yoda, K. Takabe, F. Tanaka and C. F. Barbas, J. Am. Chem. Soc., 2006, 128, 734–735 CrossRef CAS; (d) M. Gruttadauria, F. Giacalone, A. M. Marculescu, P. L. Meo, S. Riela and R. Noto, Eur. J. Org. Chem., 2007, 4688–4698 CrossRef; (e) H. Yang and R. G. Carter, Org. Lett., 2008, 10, 4649–4652 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General methods and the data of HPLC and 1H NMR. See DOI: 10.1039/c0gc00486c |
| ‡ Hai-Hong Li and Yan-Hong He contributed equally to this work |
| This journal is © The Royal Society of Chemistry 2011 |


