DOI:
10.1039/C1FO10071H
(Paper)
Food Funct., 2011,
2, 603-610
Potential nutraceutical food beverage with antioxidant properties from banana plant bio-waste (pseudostem and rhizome)
Received
19th May 2011
, Accepted 16th August 2011
First published on 14th September 2011
Abstract
Banana plant biomass waste, viz.pseudostem (BPS) and rhizome (BR), contribute 30.81 and 12.67 per cent respectively. A negligible percentage of these were used for fresh consumption, otherwise they are waste and incinerated. In order to utilize these bio-wastes in a bioactive perspective, nutritional and nutraceutical components were studied from the juices and its Ready-To-Serve (RTS) beverage. When compared to BPS juice, BR juice showed higher total phenolic content (TPC) and total flavonoid content (TFC) of 341.44 mgGAE and 87.60 mgCE/200 μL, respectively, and concomitantly exhibited high antioxidant activity (AOA) in all the assays tested, viz.DPPH radical scavenging activity (82.93%), superoxide radical scavenging activity (49.45%), metal chelation activity (48.32%) total reducing power (0.846 OD at 700 nm) and total antioxidant capacity (928 mmol ascorbic acid equivalents). High quantity of polyphenols present in BR juice resulted high AOA. Strong positive correlations were observed between TPC and TFC of BPS and BR juice with AOA assays. Among the different concentrations of RTS beverages prepared, 25% BPS juice and 20% BR juice with 15°brix TSS and 0.3% acidity were adjudged as best by sensory panelists. Thus, BPS and BR juice can be effectively used to produce new generation functional beverages.
1. Introduction
Banana is a most important fruit crop that grows all over the tropical regions of the world and has an major commercial importance in many of these countries. The annual production of banana in India is 26.2 million tons, contributing about 23% of world banana production.1Pseudostem (BPS) and rhizome (BR) together constitute a major part of plant biomass, which are usually left in the plantation or incinerated and wasted. BPS appears to be a rich source of fibre, total carbohydrate and cellulose.2 Manimehalai (2005)3 observed moisture, protein, fat, minerals, fibre and carbohydrates content of BPS of 93.1, 0.3, 0.03, 1.04, 0.68 and 1.20 g/100 g, respectively. Though it is a reasonably rich source of nutrition, a negligible percentage of BPS is used for human consumption and for production of fiber.2 The nutritive value of BR includes moisture, protein, fat, minerals, crude fibre, carbohydrates, calcium, phosphorus, iron, and energy of 85.1, 0.4, 0.2, 1.4, 1.1, 11.8, 0.025, 0.010, 0.0011 g and 51 kcal/100 g of edible portion, respectively.4 BR is often cooked and eaten as a vegetable in India, mainly in tribal areas. In indigenous systems of medicine, BPS juice is a well-known remedy for urinary disorders, stomach troubles like diarrhoea, dysentery and flatulence, it helps in treatment for removal of stones in the kidney, gall bladder, and prostate and is also used as an antidote for snake-bite.5,6 BR extracts were used as a coolant, to treat diabetes, piles, intestinal worms, mental diseases, acidity, food poisoning, to cure pyorrhea and to heal wounds.5
Antioxidants are an important group of medicinal compounds as well as being food additives and can delay the oxidation of lipids or of other molecules by inhibiting the initiation or propagation of oxidative chain reactions. Polyphenols like phenolics, flavonoids and phenolic acids7 are considered to be the most active natural antioxidants. More recently, we reported the polyphenolic profile of pseudostem of eight major Indian banana cultivars and also the presence of phenolic acids such as gentisic acid, (+)-catechin, protocatechuic acid, caffeic acid, ferulic acid, and cinnamic acid in banana cultivar nanjanagudu rasabale by using RPHPLC-DAD and ESI-MS analysis.6 The antioxidant activities of polyphenols may be related to their redox properties, which allow them to act as reducing agents or hydrogen/electron donors, scavenge free radicals, terminate the radical chain reactions and chelate transition metals8 and thereby promote possible beneficial roles in human health, such as reducing the risk of cancer, cardiovascular disease, may prevent or repair cell damage caused by reactive oxygen species (ROS).9 Recently, interest in polyphenol antioxidants from plant sources has increased remarkably over the last decade because of their protective effects against different degenerative diseases and due to toxicity and carcinogenicity of synthetic antioxidants.10
Despite rich nutritional and nutraceutical properties of BPS and BR, they are wasted and incinerated in India and elsewhere. Extensive literature surveys also supported that no work has been reported regarding utilization of banana pseudostem and rhizome for food, nutraceutical or pharmaceutical purposes. Hence, the present study is focused on utilizing these vast quantities of banana plant bio-waste as a source of polyphenol-rich RTS beverage and elucidate their antioxidant properties for future use.
2. Materials and methods
2.1 Chemicals
All the reagents and chemicals used in this study were of analytical grade and obtained from Loba Chemie Pvt. Ltd., Sisco Research Laboratories, E. Merck and Ranbaxy fine chemicals (India), and M/s Sigma Chemicals Co. (St. Louis, MO, USA).
2.2 Plant material
Banana (Musa AAB cv. Nanjanagudu Rasabale) plants11,12 were identified and harvested from plantations in Mysore district of Karnataka, India. After harvesting of fruit bunches, pseudostem and rhizome were separated from plant, transferred to the lab and used for study.
2.3 Juice extraction and standardization of RTS beverage
Pseudostem is an actively growing aerial stem with closely packed leaf sheaths. It functions as a vascular bridge for the flow of water and nutrients from underground rhizome to leaves and banana fruit bunch.6 Rhizome is a modified stem of banana plant which remains underground and bears the banana plant on surface and roots below. After harvesting the fruit bunch, the pseudostem was obtained after removal of surrounding leaf sheaths. The pseudostem and rhizomes were cleaned, washed thoroughly in water and juices were extracted after passing through the pulper and strained by using muslin cloth. Control of astringency browning was done by pretreatment of pseudostem and rhizome juice by 25 ppm of L-cystein. Juices were cooled immediately to 4 °C later centrifuged for 10 min at 3000 rpm. RTS beverages with various strengths of juice (10, 15, 20 and 25%), sugar (10 and 15°brix) and acidity (0.25 and 0.30%) were mixed and filled into pre-sterilized bottles.13 Sealed bottles were pasteurized for 25 min in boiling water, cooled and stored at room temperature for further analysis.
2.4 Sensory analysis
Sensory evaluation (colour, appearance, body, taste, flavor and overall quality) for the pseudostem and rhizome RTS beverages was done with trained panel consisting of departmental staff and research workers both male and female aged 22 to 58 years, who had very well experience in judging fruit and vegetable products and their sensory quality.13
2.5 Chemical composition
Total soluble solids (°Brix) and pH of juices were observed using digital refractometer (ATAGO RX–5000 as % sucrose content) and pH meter respectively. Total acidity (% citric acid) and tannins were estimated as given by Ranganna (2001).13 Total, non-reducing and reducing sugars were determined according to Lane and Eynon’s method by using Fehling's solution.13 The protein and amino acid content were estimated using Folins-Ciocalteu reagent14 and ninhydrin,13 respectively.
2.6 Bioactive constituents
2.6.1 Determination of total phenolic content (TPC).
The TPC of BPS, BR juice and its RTS beverages were determined by the Folin-Ciocalteau colorimetric method.15,16 In brief, different concentration (10–200 μL) of juice/RTS beverage/gallic acid was mixed with 2 mL of 2% aqueous sodium carbonate solution. After 3 min, 100 μL of 50% Folin-Ciocalteau's phenol reagent was added to the mixture. After 30 min of incubation at room temperature, absorbance was measured at 760 nm against a blank using UV-visible spectrophotometer (UV-160A, Shimadzu, Japan). TPC was calculated from calibration curve of gallic acid and expressed as the means (±SD) mg of gallic acid equivalents (GAE) per 200 μL of juice or RTS beverages.
2.6.2 Determination of total flavonoid content (TFC).
The TFC of BPS, BR juice and its RTS beverages were measured by following aluminum chloride colorimetric method.17,18 For the analysis, different concentration (10–200 μL) of juice/RTS beverage/catechin was placed in a 10 mL volumetric flask and volume was made to 5 mL with distilled water, followed by 0.3 mL of 5% NaNO2. After 5 min, 0.6 mL of 10% AlCl3 was added. After 5 min 2 mL of 1 M NaOH was added and volume made up with distilled water. The solution was mixed and absorbance measured at 510 nm. TFC amounts were expressed as catechin equivalents (CE) per 200 μL of juice or RTS beverages using a standard curve.
2.7
Antioxidant activities measurement
No single method can fully evaluate the antioxidant capacity of foods, due to the diversity of chemical structure, synergic and redox interactions among the different antioxidant molecules found in food. Until now, many methods have been developed based on different mechanisms that generated different radicals and/or target molecules and measured different endpoints.16 Considering the above facts, five different antioxidant assays were used viz., DPPH radical scavenging activity, superoxide radical scavenging activity, metal chelating activity, total reducing power and total antioxidant capacity.
2.7.1
DPPH radical scavenging activity (DPPH RSA).
Tris-HCl buffer (pH 7.4) was added to BPS, BR juice and its RTS beverages/standard (gallic acid and catechin) of different concentration (10–200 μL) to make 1 mL to which 1 mL of 500 μM DPPH was added. The mixture was shaken vigorously and left to stand for 30 min. Absorbance of the resulting solution was measured at 517 nm in a UV-visible spectrophotometer. All measurements were made in triplicates and radical scavenging potential was expressed as % antioxidant activity and also IC50 value, which represents the sample concentration at which 50% of the DPPH radicals scavenged.19,6
2.7.2
Superoxide radical scavenging activity (SRSA).
Superoxide radicals were generated in 1 mL of Tris-HCl buffer (0.02 M, pH 8.3) containing 0.1 mM NADH, 0.1 mM NBT, 10 μM PMS and different concentrations (10–200 μL) of BPS, BR juice and its RTS beverages/standard (gallic acid and catechin). The colour reaction of superoxide radicals and NBT was detected at 560 nm, using a UV-Vis Spectrophotometer. Results from triplicate measurements were expressed as percentageas well as IC50 value, which represents the sample concentration at which 50% of the superoxide radicals scavenged.20,6
2.7.3
Metal chelating activity (MCA).
Different concentrations (10–200 μL) of BPS, BR juice and its RTS beverages/standard (EDTA-ethylenediamine tetra-acetic acid) were mixed with 2 mM FeCl2·4H2O and 5 mM ferrozine at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the mixture were shaken. After 10 min, the Fe++ was monitored by measuring the formation of ferrous ion-ferrozine complex at 562 nm, expressed as percentage Fe2+ chelating activity. IC50 value represents the sample concentration at which 50% of the metal chelation occurred.21,22
2 and the mixture were shaken. After 10 min, the Fe++ was monitored by measuring the formation of ferrous ion-ferrozine complex at 562 nm, expressed as percentage Fe2+ chelating activity. IC50 value represents the sample concentration at which 50% of the metal chelation occurred.21,22
2.7.4 Total reducing power (TRP) assay.
Reaction mixture, containing 10–200 μL of BPS, BR juice and its RTS beverages/ascorbic acid (standard) in phosphate buffer (0.2 M, pH 6.6), was incubated with K3Fe(CN)6 (1% w/v) at 50 °C for 20 min. The reaction was terminated by the addition of trichloroacetic acid solution (10% w/v) and the mixture was centrifuged (3000 g) for 10 min. The supernatant was mixed with distilled water and ferric chloride (0.1% w/v) solution and left to incubate for another 10 min, and the formation of ferrous ion (Fe2+) was measured spectrophotometrically at 700 nm. Higher absorbance values indicative of greater reducing capacity of ferric (Fe3+) to ferrous (Fe2+) ions The sample concentration providing 0.5 of absorbance (IC50) was calculated from the graph of absorbance at 700 nm against standard ascorbic acid.23,17
2.7.5 Determination of total antioxidant capacity (TAC).
For the experiment, different concentration (10–200 μL) of BPS, BR juice and its RTS beverages/standard ascorbic acid was combined with 3 mL of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95 °C for 90 min. Then the absorbance of the solution was measured at 695 nm using UV-visible spectrophotometer against blank after cooling to room temperature. The antioxidant activity is expressed as the number of equivalents of ascorbic acid.24
2.8 Statistical analysis
Results were expressed as mean ± standard deviation of triplicate analyzes. Data are analyzed by one-way analysis of variance (ANOVA) and post-hoc mean separations were performed by Duncan's Multiple Range Test (DMRT) at p < 0.05. Logarithmic regressions (R2) between TPC, TFC and AOA assay results and also correlation coefficient (R) between the tested parameters was assessed by using Microsoft Excel XPR (Microsoft Corporation, USA).
3. Results and discussion
In the present investigation different concentrations of RTS beverages were prepared and studied for their chemical composition, bioactive contents, antioxidant activity and sensory acceptability.
3.1 Banana plant biomass
Pseudostem (30.81%) and rhizome (12.67%) of banana plant together contributes 43.48 per cent of the biomass (Fig. 1). Currently <2% of pseudostem production is used for human consumption and for production of fiber,2 the remaining are incinerated and wasted.
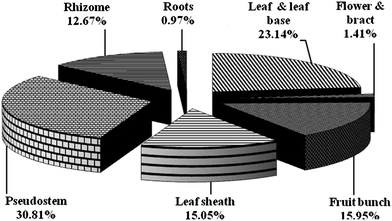 |
| | Fig. 1 Biomass composition of different parts of banana plant cv. Nanjanagudu Rasabale. | |
3.2 Sensory evaluation of different treatments
RTS beverage with twenty percent BR (T7) juice and twenty five per cent BPS (T8) juice with 15°brix TSS and 0.30% acidity content showed higher overall quality score of 8.0 and 7.1 respectively (Table 1). Therefore, these concentrations of juices were subjected for further study.
Table 1 Sensory evaluation of different treatments of banana pseudostem and rhizome RTS beveragesa
| Treatment no. |
Juice content (%) |
TSS (°Brix) |
Acidity (%) |
Overall quality |
|
Mean values in a column with different superscripts differ significantly at P < 0.05.
|
|
Pseudostem juice |
| 1 |
10 |
10.16 ± 0.26 |
0.252 ± 0.01 |
4.0 ± 0.11a |
| 2 |
15 |
10.32 ± 0.15 |
0.250 ± 0.03 |
4.3 ± 0.34b |
| 3 |
20 |
10.23 ± 0.19 |
0.252 ± 0.02 |
5.4 ± 0.29c |
| 4 |
25 |
10.17 ± 0.21 |
0.255 ± 0.03 |
6.0 ± 0.33d |
| 5 |
10 |
15.04 ± 0.28 |
0.300 ± 0.02 |
5.5 ± 0.20a |
| 6 |
15 |
15.09 ± 0.39 |
0.306 ± 0.02 |
5.9 ± 0.25b |
| 7 |
20 |
15.14 ± 0.65 |
0.303 ± 0.04 |
6.6 ± 0.12c |
| 8 |
25 |
15.29 ± 0.19 |
0.302 ± 0.06 |
7.1 ± 0.21d |
| |
| Rhizome juice |
| 1 |
10 |
10.66 ± 0.09 |
0.257 ± 0.05 |
4.5 ± 0.20a |
| 2 |
15 |
10.22 ± 0.12 |
0.252 ± 0.02 |
5.5 ± 0.25b |
| 3 |
20 |
10.24 ± 0.14 |
0.255 ± 0.04 |
6.0 ± 0.16c |
| 4 |
25 |
10.07 ± 0.18 |
0.253 ± 0.02 |
6.0 ± 0.13c |
| 5 |
10 |
15.40 ± 0.22 |
0.310 ± 0.02 |
6.5 ± 0.25a |
| 6 |
15 |
15.10 ± 0.44 |
0.311 ± 0.09 |
7.0 ± 0.21b |
| 7 |
20 |
15.34 ± 0.53 |
0.306 ± 0.05 |
8.0 ± 0.26c |
| 8 |
25 |
15.59 ± 0.33 |
0.309 ± 0.08 |
7.2 ± 0.20b |
3.3 Chemical composition of BPS juice, BR juice and its RTS beverages
The chemical composition of banana pseudostem juice, rhizome juice and its RTS beverage was presented in Table 2. BPS and BR juice showed low TSS (2.9 and 2.54°brix) and acidity (0.0069 and 0.07%) respectively. The pH of the BPS (6.3) and BR (6.7) juice were near to neutral pH, which were suitable for preparing the beverages. This value (pH) is of importance as measure of the active acidity which influence the flavor or palatability of a product.13 Total sugar, reducing sugar, non-reducing sugar, protein, amino acid and tannin content (TAE-tannic acid equivalents) of BR juice (18.07, 14.12, 3.95, 127.99, 12.34 and 3.12 mg/100 mL respectively) and its RTS (T7) beverage (14.56, 5.17, 9.39, 25.21, 3.63 and 1.08 mg/100 mL respectively) was higher than BPS juice (8.3, 6.2, 2.1, 60, 8.3 and 1.95 mg/100 mL respectively) and its RTS (T8) beverage (13.61, 4.87, 8.74, 16.61, 2.15, 0.72 mg/100 mL, respectively).
Table 2 Chemical composition of banana pseudostem juice, rhizome juice and its RTS beveragea
| Parameters |
Pseudostem juice |
Rhizome juice |
Pseudostem RTS beverage (T8) |
Rhizome RTS beverage (T7) |
|
Mean values in a row with different superscripts differ significantly at P < 0.05.
|
| Juice yield (%) |
65 ± 2.2b |
25 ± 0.15a |
— |
— |
|
TSS (°Brix) |
2.9 ± 0.39b |
2.54 ± 0.25a |
15.29 ± 0.19c |
15.34 ± 0.53c |
| Acidity (%) |
0.0069 ± 0.01a |
0.07 ± 0.02b |
0.30 ± 0.06c |
0.30 ± 0.05c |
| pH |
6.3 ± 0.15b |
6.7 ± 0.22b |
2.94 ± 0.02a |
2.90 ± 0.06a |
| Total sugars (mg/100ml) |
8.3 ± 0.47a |
18.07 ± 0.32d |
13.61 ± 0.56b |
14.56 ± 0.24c |
| Reducing sugar (mg/100ml) |
6.2 ± 0.26c |
14.12 ± 0.42d |
4.87 ± 0.51a |
5.17 ± 0.20b |
| Non-reducing sugar (mg/100ml) |
2.1 ± 0.20a |
3.95 ± 0.12b |
8.74 ± 0.67c |
9.39 ± 0.55d |
|
Protein (mg/100ml) |
60 ± 0.45c |
127.99 ± 0.52d |
16.61 ± 2.67a |
25.21 ± 0.67b |
|
Amino acids (mg/100ml) |
8.3 ± 0.26c |
12.34 ± 0.42d |
2.15 ± 0.84a |
3.63 ± 1.01b |
|
Tannins (mg/100ml) |
1.95 ± 0.15c |
3.12 ± 0.20d |
0.72 ± 0.51a |
1.08 ± 0.67b |
3.4 Bioactive components of BPS juice, BR juice and its RTS beverages
The amount of TPC and TFC were higher in BR juice (341.44 mgGAE and 87.60 mgCE/200 μL respectively). Comparatively, BPS juice showed lower TPC and TFC (115.40 mgGAE and 42.86 mgCE/200 μL respectively) (Fig. 2a). The polyphenolic content present in plant can vary significantly due to different factors, such as plant genetics and cultivar, soil composition and growing conditions, maturity state, and post harvest conditions, and others.25,6 In RTS beverage also the TPC and TFC in T7 from BR juice (62.41 mgGAE and 22.01 mgCE/200 μL of juice respectively) were higher than the RTS from BPS (T8) juice (58.11 mgGAE and 12.59 mgCE/200 μL of juice respectively) (Table 3). The high polyphenolic content (TPC and TFC) present in these juices might be responsible for high overall quality score. Polyphenolic antioxidants are often added to foods for stabilization, prevent off-flavour formation, minimise rancidity, retard the formation of toxic oxidation products, maintain nutritional quality, and increase shelf life.26
Table 3 Bioactive components and antioxidant activity of banana pseudostem juice, rhizome juice and its RTS beveragea
| Parameters |
Pseudostem RTS beverage (T8) |
Rhizome RTS beverage (T7) |
|
Mean values in a row with different superscripts differ significantly at P < 0.05.
|
| Bioactive components |
| Total phenolics (mgGAE/200 μl of juice) |
58.11 ± 2.16a |
62.41 ± 0.86b |
| Total flavonoids (mgCE/200 μl of juice) |
12.59 ± 1.57a |
22.01 ± 2.47b |
| |
|
Antioxidant activity at 200 μl of RTS beverage |
|
DPPH radical scavenging activity (%) |
40.22 ± 2.24a |
53.31 ± 3.11b |
|
Superoxide radical scavenging activity (%) |
26.75 ± 0.94a |
30.54 ± 2.48b |
|
Metal chelating activity (%) |
24.45 ± 0.18a |
32.38 ± 0.47b |
| Total reducing power (OD at 700 nm) |
0.249 ± 0.62a |
0.291 ± 0.24b |
| Total antioxidant capacity (mmol ascorbic acid equivalents) |
58 ± 2.57a |
194 ± 2.66b |
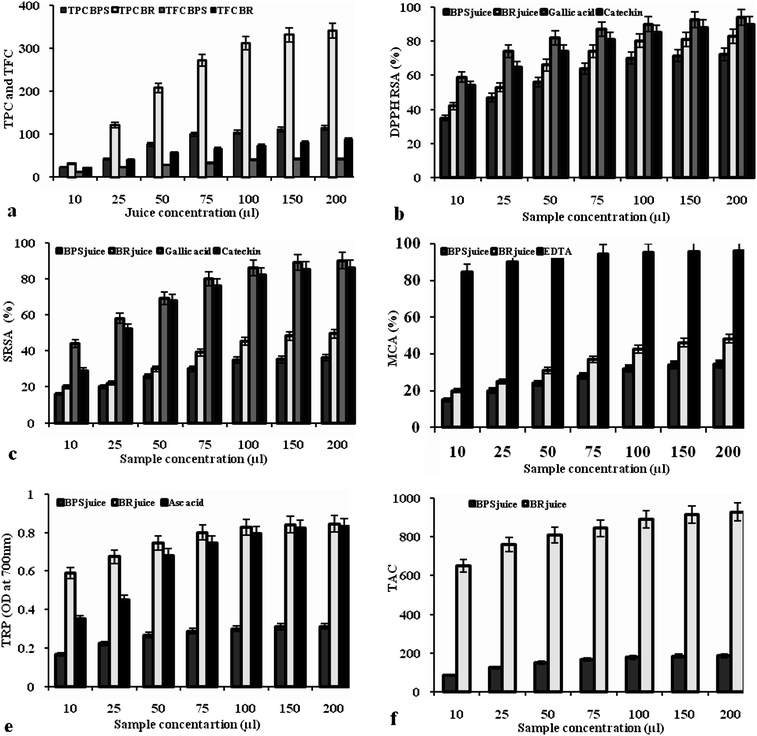 |
| | Fig. 2 Bioactive components and AOA of BPS and BR juice. (a) Bioactive components (TPC: total phenolic content in mg gallic acid equivalents; TFC: total flavonoid content in catechin equivalents) of BPS and BR juice; (b) DPPH RSA-1: 1-diphenyl-2-picrylhydrazyl radical scavenging activity; (c) SRSA: superoxide radical scavenging activity; (d) MCA: metal chelating activity; (e)TRP: total reducing power; (f) TAC: total antioxidant capacity in mmol ascorbic acid equivalents of BPS and BR juice. BPS: banana pseudostem; BR: banana rhizome; EDTA: ethylenediamine tetra-acetic acid; Asc. acid: ascorbic acid). | |
3.5
Antioxidant activities of banana pseudostem juice, rhizome juice and its RTS beverages
3.5.1
DPPH radical scavenging activity (DPPH RSA).
DPPH is a free radical, frequently used to determine radical-scavenging activity of natural compounds to act as free radical scavengers or hydrogen donors. In its radical form, DPPH absorbs at 517 nm, but upon reduction with an antioxidant, its absorption decreases due to the formation of its non-radical form, DPPH-H.19 Concentration dependent DPPH RSA was observed in both juices and standards (Fig. 2b). BR juice displayed highest activity of 82.93% at the concentration of 200 μl, which is comparable to the activity of standard gallic acid (93.8%) and catechin (90%) at 200 μg mL−1 (1 mg mL−1 stock). Whereas, its RTS beverage also showed good DPPH RSA of 53.31% (T7) and 40.22% (T8) at 200 μL concentration (Table 3). Increase in polyphenolic concentration in juices results concurrent increase in AOA. This radical scavenging activity of juices/RTS beverages could be related to the nature of polyphenolic compounds and their electron transfer/hydrogen donating ability.
3.5.2
Superoxide radical scavenging activity (SRSA).
Superoxide is a relatively weak oxidant, and exhibits limited chemical reactivity, but can generate more dangerous species, including singlet oxygen and hydroxyl radicals27 and these active free radicals have the potential for reacting with biological macromolecules, and thereby, inducing tissue damage.28 At 200 μL concentration (standard 1 mg mL−1 stock) the scavenging effect of juices, RTS beverages and standards were in the following order: gallic acid (90%) > catechin (86%) > BR juice (49.45%) > BPS juice (36.22%) > T7 (30.54%) > T8 (26.75%) (Fig. 2c and Table 3). The results suggest that the juices of BR and BPS and its RTS beverages display scavenging effect on superoxide anion radical generation that could help prevent or ameliorate oxidative damage.
3.5.3
Metal chelating activity (MCA).
Elemental species, such as ferrous iron (Fe2+), can facilitate the production of ROS within living systems and these reduced metals may form highly reactive hydroxyl radicals, and thereby contribute to oxidative stress by Fenton reaction.29 The resulting oxy radicals cause damage to cellular lipids, nucleic acids, proteins, and carbohydrates, leading to cellular impairment. Minimizing ferrous ion may afford protection against oxidative damage by inhibiting production of ROS and molecular damage.18 In this study BPS and BR juices and its RTS beverages showed good MCA and it was dose dependent (Fig. 2d and Table 3). At 200 μL concentration, BPS and BR juices showed 34.47 and 48.32% activity and its RTS beverages showed 32.38 and 24.45% activity respectively. Whereas, standard EDTA showed high activity of 96% at 200 μg mL−1 (1 mg mL−1 stock) concentration. Polyphenolic compounds enriched in the juices/RTS beverages are capable of complexing with and stabilizing transition metal ions, rendering them unable to participate in metal catalyzed reaction.30
3.5.4 Total reducing power (TRP) assay.
Fig. 2e and Table 3 shows the reductive capability of juices and its RTS beverages relative to ascorbic acid, a well known antioxidant. Similar to the results of other AOA assays, the reducing potential of juices observed to be dose dependent. TRP assay may indicate how easily a given antioxidant donates electrons to reactive free radical species, thus promoting the termination of free radical chain reactions. Phenols and flavonoids are well known for their ability to donate electron.7 The high reducing ability (to reduce Fe3+ to its more active Fe2+) of BR juice (0.846 OD at 700 nm at 200 μL) is comparable to ascorbic acid (0.832 OD at 700 nm at 200 μg mL−1 from 1 mg mL−1 stock), commonly used as a antioxidant in foods, may be attributed to their high polyphenol content.
3.5.5 Total antioxidant capacity (TAC).
Phosphomolybdenum assay is a simple, independent and commonly employed to measure antioxidant activity of foods. Moreover, it is a quantitative one, since the antioxidant activity is expressed as the number of equivalents of ascorbic acid. This method is based on the reduction of Mo(VI) to Mo(V) by the juices and subsequent formation of a green phosphate/Mo(V) or phosphomolybdenum complex at acid pH.24TAC of BPS juice, BR juice and its RTS beverages were given in Fig. 2f and Table 3. In this study BR juice and its RTS (T7) beverage showed highest TAC (928 and 194 mmol ascorbic acid equivalents/200 μL of juice respectively), followed by BPS juice and its RTS (T8) beverage (186 and 58 mmol ascorbic acid equivalents/200 μL of juice respectively). Difference in AOA was found to be depending on concentration juice. Mo reduction capacity of these juices and RTS beverages seems to be depending mainly on their polyphenolic content (phenolics and flavonoids). Thus, these results indicated that banana plant bio-wastes with high polyphenolics were characterized as good antioxidants by their greatest reducing potential.
3.5.6
EC50 values for antioxidant activities.
Concentration of sample at which the inhibition percentage reaches 50% is the IC50 value. IC50 value is negatively related to the AOA, lower the IC50 value, the higher is the AOA of the tested sample. The IC50 values of BPS juice, BR juice and reference standards for different AOA assays studied are summarized in Table 4. There was a significant difference (P < 0.05) between IC50 values of the BR juice with different AOA tested. BR juice showed lowest IC50 value of 8.4 μL for TRP assay followed by 24 μL for DPPH RSA, 210 μL for SRSA and 220 μL for MCA. Whereas, BPS juice showed IC50 value only for DPPH RSA (47 μL). The IC50 value of BR juice for TRP assay (8.4 μL) and DPPH RSA (24 μL) is much lower than the reference standard ascorbic acid (33.5 μL) and catechin (39.67 μL) respectively. However, in other standards like gallic acid and EDTA showed lower IC50 values than the juices. Higher polyphenol content in BR juice contributed strongest AOA.
Table 4
Antioxidant activity (IC50 value in μl) of banana pseudostem juice, rhizome juice and standardsa
|
Sl. No |
AOA assays |
BPS juice |
BR juice |
Gallic acid
|
Catechin
|
EDTA
|
Asc acid
|
|
Mean values in a column with different superscripts differ significantly at P < 0.05. IC50: Effective concentration of the sample to show 50% of antioxidant activity. NT: Not tested. BPS: banana pseudostem, BR: banana rhizome, DPPH RSA-1: 1-diphenyl-2-picrylhydrazyl radical scavenging activity, SRSA: superoxide radical scavenging activity, MCA: metal chelating activity, TRP: total reducing power.
Juice or ascorbic acid concentration to get 0.5 of absorbance at 700 nm.
|
| 1 |
DPPH RSA |
47 ± 1.3 |
24 ± 1.4b |
6.44 ± 0.6a |
39.67 ± 1.2b |
NT
|
NT
|
| 2 |
SRSA
|
— |
210 ± 4.6c |
15.23 ± 0.8b |
24.34 ± 1.0a |
NT
|
NT
|
| 3 |
MCA
|
— |
220 ± 4.8c |
NT
|
NT
|
6.5 ± 0.3 |
NT
|
| 4 |
TRP
b |
— |
8.4 ± 0.6a |
NT
|
NT
|
NT
|
33.5 ± 2.2 |
3.6 Correlation between BPS and BR juice polyphenols with their antioxidant activities
The TPC and TFC of BPS and BR juice were correlated with AOA assays (Table 5). Strong positive correlation was observed with TPC of BR juice and TAC (R2 = 0.996), followed by TPC of BPS juice and TRP (R2 = 0.992). Whereas, weak correlation were recorded with TPC of BR juice and SRSA (R2 = 0.743) and MCA (R2 = 0.830). Strong positive correlation was also found between the AOA assays viz., TRP and TAC (R = 0.998) of BPS juice followed by DPPH RSA and TRP (R = 0.997) of BR juice. Whereas, poor correlation was observed between TAC and SRSA (R = 0.951) and also TRP and SRSA (R = 0.959) of BR juice (Table 6). The positive correlation between polyphenolic content and antioxidant potential of various plant extracts have been well demonstrated in prior reports.17,20,15
Table 5 Relationship between measured attributes of banana pseudostem and rhizome juicea
|
AOA assays |
TPC of BPS juice |
TFC of BPS juice |
TPC of BR juice |
TFC of BR juice |
|
TPC: total phenolic content, TFC: total flavonoid content, BPS: banana pseudostem, BR: banana rhizome, DPPH RSA-1: 1-diphenyl-2-picrylhydrazyl radical scavenging activity, SRSA: superoxide radical scavenging activity, MCA: metal chelating activity, TRP: total reducing power, TAC: total antioxidant capacity.
|
|
DPPH RSA |
y = 22.65ln(x) − 37.45 |
y = 10.74ln(x) − 12.16 |
y = 17.51ln(x) − 23.06 |
y = 31.89ln(x) − 59.92 |
| R2 = 0.962 |
R2 = 0.984 |
R2 = 0.911 |
R2 = 0.961 |
|
SRSA
|
y = 12.42ln(x) − 24.83 |
y = 16.61ln(x) − 27.95 |
y = 12.39ln(x) − 28.49 |
y = 23.35ln(x) − 57.74 |
| R2 = 0.921 |
R2 = 0.908 |
R2 = 0.743 |
R2 = 0.839 |
|
MCA
|
y = 11.56ln(x) − 22.65 |
y = 15.66ln(x) − 26.23 |
y = 10.46ln(x) − 19.77 |
y = 19.41ln(x) − 43.21 |
| R2 = 0.906 |
R2 = 0.916 |
R2 = 0.830 |
R2 = 0.908 |
|
TRP
|
y = 0.086ln(x) − 0.103 |
y = 0.116ln(x) − 0.126 |
y = 0.110ln(x) + 0.185 |
y = 0.199ln(x) − 0.041 |
| R2 = 0.992 |
R2 = 0.982 |
R2 = 0.938 |
R2 = 0.977 |
|
TAC
|
y = 60.34ln(x) − 104.1 |
y = 81.33ln(x) − 121.5 |
y = 143.7ln(x) + 653.2 |
y = 202.0ln(x) + 14.83 |
| R2 = 0.984 |
R2 = 0.986 |
R2 = 0.996 |
R2 = 0.981 |
Table 6 Correlation between measured attributes of banana pseudostem and rhizome juicea
| |
TPC BPS |
TFC BPS |
DPPH
|
SRSA
|
MCA
|
TRP
|
TAC
|
|
BPS: banana pseudostem, BR: banana rhizome, TPC: total phenolic content, TFC: total flavonoid content, DPPH RSA-1: 1-diphenyl-2-picrylhydrazyl radical scavenging activity, SRSA: superoxide radical scavenging activity, MCA: metal chelating activity, TRP: total reducing power, TAC: total antioxidant capacity.
|
| TP BPS |
1 |
|
|
|
|
|
|
|
TF BPS |
0.980 |
1 |
|
|
|
|
|
|
DPPH
|
0.990 |
0.997 |
1 |
|
|
|
|
|
SRSA
|
0.988 |
0.989 |
0.992 |
1 |
|
|
|
|
MCA
|
0.980 |
0.993 |
0.991 |
0.995 |
1 |
|
|
|
TRP
|
0.985 |
0.987 |
0.991 |
0.973 |
0.971 |
1 |
|
|
TAC
|
0.985 |
0.992 |
0.994 |
0.977 |
0.978 |
0.998 |
1 |
| |
TPC BR |
TFC BR |
DPPH
|
SRSA
|
MCA
|
TRP
|
TAC
|
| TP BR |
1 |
|
|
|
|
|
|
|
TF BR |
0.992 |
1 |
|
|
|
|
|
|
DPPH
|
0.999 |
0.989 |
1 |
|
|
|
|
|
SRSA
|
0.972 |
0.969 |
0.970 |
1 |
|
|
|
|
MCA
|
0.990 |
0.986 |
0.989 |
0.993 |
1 |
|
|
|
TRP
|
0.998 |
0.988 |
0.997 |
0.959 |
0.982 |
1 |
|
|
TAC
|
0.990 |
0.993 |
0.986 |
0.951 |
0.978 |
0.989 |
1 |
The results also indicated that the superior AOA of BR juice and its RTS beverage as compare to BPS juice and its RTS beverage may be explained by its high polyphenolic (TPC and TFC) concentration, structural differences of polyphenolic compounds and its derivatives, and their synergistic interactions and also number of hydroxyl groups in the molecules, as reported earlier in various plant extracts.21,15,31,7,6 The quantitative and qualitative difference in polyphenolic compounds of pseudostem of different banana cultivars were recorded in our earlier studies.6
4. Conclusion
For the first time nutritional and nutraceutical components of banana plant waste, viz. pseudostem and rhizome were enumerated and also highly acceptable RTS beverages were standardized. High concentration of bioactive constituents like polyphenols (phenolics and flavonoids) were concurrent to its high antioxidant activities was demonstrated. Thus, banana plant biomass, which is a bio-waste, can be effectively used to produce new age functional beverages.
Acknowledgements
We thank Dr V. Prakash, Director, Central Food Technological Research Institute, Mysore, for his keen interest in the work and encouragement. Mr. Saravanan thanks University Grants Commission, New Delhi, India, for awarding the Junior Research and Senior Research Fellowship.
References
-
FAOSTAT (Food and agricultural organization statistics) ( 2008) Food and Agricultural Commodities Production. Food and Agriculture Organization of the United Nations, Rome, Italy (http://faostat.fao.org) Search PubMed.
- S. Uma, S. Kalpana, S. Sathiamoorthy and V. Kumar, Evaluation of commercial cultivars of banana (Musa spp.) for their suitability for the fibre industry, PGR newsletter (FAO bioversity)., 2005, 142, 29–35 Search PubMed.
- N. Manimehalai, Fruit and Vegetable waste utilization, Bev. Food World, 2005, 58–59 Search PubMed.
-
C. Gopalan, B. V. Rama sastri, S. C. Balasubramanian, Nutritive value of Indian foods, National Institute of nutrition, Indian Council of Medical Research, Hydrabad, India, 1989, pp. 50 Search PubMed.
- P. Pushpangadan, J. Kaur and J. Sharma, Plantain or edible banana (Musa x paradisica var - sapiemtum) some lesser known folk uses in India, Ancient Sci. Life., 1989, IX(1), 20–24 Search PubMed.
- K. Saravanan and S. M. Aradhya, Polyphenols of pseudostem of different banana cultivars and their antioxidant activities, J. Agric. Food Chem., 2011, 59(8), 3613–3623 CrossRef CAS.
- S. Karaman, E. Tutem, K. S. Baskan and R. Apak, Comparison of total antioxidant capacity and phenolic composition of some apple juices with combined HPLC–CUPRAC assay, Food Chem., 2010, 120, 1201–1209 CrossRef CAS.
- K. Le, F. Chiu and K. Ng, Identification and quantification of antioxidants in Fructuslycii, Food Chem., 2007, 105, 353–363 CrossRef CAS.
- B. Tepe, M. Sokmen, M. A. Akpulat and A. Sokmen, Screening of the antioxidant potentials of six salvia species from Turkey, Food Chem., 2006, 95, 200–204 CrossRef CAS.
- A. Moure, J. M. Cruz, D. Franco, J. M. Dominguez, J. Sineiro, H. Dominguez, M. J. Nuňez and J. C. Parajo, Natural antioxidants from residual sources, Food Chem., 2001, 72, 145–171 CrossRef CAS.
-
Wealth of India. Raw material, 1962, vol. 6 (L-M), New Delhi, CSIR.
- L. Venkatachalam, R. Thimmaraju, R. V. Sreedhar and N. Bhagyalakshmi, Direct shoot and cormlet regeneration from leaf explants of ‘silk’ banana (AAB), In Vitro Cell. Dev. Biol.: Plant, 2006, 42, 262–269 CrossRef.
-
S. Ranganna, Proximate constituents, in Handbook of analysis and qualitycontrol for fruit and vegetable Products, ed. S. Ranganna, vol. 2, Tata McGraw-Hill, New Delhi, 2001, pp. 12–17 Search PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the folin phenol reagent, J. Biol. Chem., 1951, 93, 265–275 Search PubMed.
- G. P. P. Kamatou, A. M. Viljoen and P. Steenkamp, Antioxidant, anti-inflammatory activities and HPLC analysis of South African Salvia species, Food Chem., 2010, 119, 684–688 CrossRef CAS.
- R. L. Prior, X. Wu and K. Schaich, Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements, J. Agric. Food Chem., 2005, 53(10), 4290–4302 CrossRef CAS.
- M. A. Esmaeili and A. Sonboli, Antioxidant, free radical scavenging activities of Salvia brachyantha and its protective effect against oxidative cardiac cell injury., Food Chem. Toxicol., 2010, 48, 846–853 CrossRef CAS.
- Gulcin, E. Bursal, M. H. Sehitoǧlu, M. Bilsel and A. C. Goren, Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey, Food Chem. Toxicol., 2010, 48, 2227–2238 CrossRef.
- M. S. Blois, Antioxidants determination by the use of a stable free radical, Nature, 1958, 181(4617), 1199–1200 CrossRef CAS.
- Y. Zhang, X. Li and Z. Wang, Antioxidant activities of leaf extract of Salvia miltiorrhiza Bunge and related phenolic constituents, Food Chem. Toxicol., 2010, 48, 2656–2662 CrossRef CAS.
- B. M. Lue, Z. Guo, N. S. Nielsen, C. Jacobsen, L. Hellgren, Z. Guo and X. Xu, Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays, Food Chem., 2010, 123, 221–230 CrossRef CAS.
- K. N. Prasad, S. Divakar, G. R. Shivamurthy and S. M. Aradhya, Isolation of a free radical-scavenging antioxidant from water spinach (Ipomoea aquatica Forsk), J. Sci. Food Agric., 2005, 85(9), 1461–1468 CrossRef CAS.
- M. Z. Končic', D. Kremer, K. Karlovic' and I. Kosalec, Evaluation of antioxidant activities and phenolic content of Berberis vulgarisL. and Berberis croatica Horvat, Food Chem. Toxicol., 2010, 48, 2176–2180 CrossRef.
- P. Prieto, M. Pineda and M. Aguilar, Spectrophotometer quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E, Anal. Biochem., 1999, 269, 337–341 CrossRef CAS.
- E. H. Jaffery, A. F. Brown, A. C. Kurilich, A. S. Keek, N. Matusheski and B. P. Klein, Variation in content of bioactive components in broccoli, J. Food Compos. Anal., 2003, 16, 323–330 CrossRef.
- C. Espin, M. T. Garciaconesa and F. A. Tomas barberan, Nutraceuricals: facts and fiction, Phytochemistry, 2007, 68, 2986–3008 CrossRef.
- B. Halliwell and S. Chirico, Lipid peroxidation: its mechanism, measurement and significance, Am. J. Clin. Nutr., 1993, 57(suppl), 715S CAS.
- B. Halliwell and J. M. C. Gutteridge, Oxygen toxicity, oxygen radicals, transition metals and disease, Biochem. J., 1984, 219, 1–4 CAS.
- S. Hippeli and E. F. Elstner, Transition metal ion-catalyzed oxygen activation during pathogenic processes, FEBS Lett., 1999, 443, 1–7 CrossRef CAS.
- S. Bourgou, R. Ksouri, A. Bellila, I. Skandrani, H. Falleh and B. Marzouk, Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots, C. R. Biol., 2008, 331, 48–55 CrossRef CAS.
- S. Shukla, A. Mehta, J. John, S. Sing, P. Mehta and S. P. Vyas, Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducellaseeds, Food Chem. Toxicol., 2009, 47, 1848–1851 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the mixture were shaken. After 10 min, the Fe++ was monitored by measuring the formation of ferrous ion-ferrozine complex at 562 nm, expressed as percentage Fe2+ chelating activity. IC50 value represents the sample concentration at which 50% of the metal chelation occurred.21,22
2 and the mixture were shaken. After 10 min, the Fe++ was monitored by measuring the formation of ferrous ion-ferrozine complex at 562 nm, expressed as percentage Fe2+ chelating activity. IC50 value represents the sample concentration at which 50% of the metal chelation occurred.21,22


