Thermal and refining processes, not fermentation, tend to reduce lipotropic capacity of plant-based foods†
Anthony
Fardet
*ab,
Jean-François
Martin
ab and
Jean-Michel
Chardigny
ab
aINRA, UMR 1019, UNH, CRNH Auvergne, F-63000, Clermont-Ferrand, France
bClermont Université, Université d'Auvergne, Unité de Nutrition Humaine, BP 10448, F-63000, Clermont-Ferrand, France
First published on 15th August 2011
Abstract
Plant-based foods (PBF) are relevant and diversified sources of lipotropes, which are compounds preventing excess hepatic fat deposits. In a first study, we defined the lipotropic capacity (LC, %) of raw PBF as the means of 8 lipotrope densities (LD, mg/100 kcal), each expressed relative to that of a reference food ranking the highest considering its mean 8 LD ranks (LCraw asparagus = 100%) (A. Fardet, J.-F. Martin and J. M. Chardigny, J. Food Comp. Anal., 2011, DOI: 10.1016/j.jfca.2011.1003.1013). We showed that vegetables appeared as the best source of lipotropes on a 100 kcal-basis compared to legumes, cereals, fruits and nuts. The main objective of this second study was to quantify the effect of processing on LD and LC of raw PBF based on lipotrope contents collected in a USDA (United State Department of Agriculture) database and the literature, i.e. betaine, choline, myo-inositol, methionine, magnesium, niacin, pantothenic acid and folate contents. Choline and betaine densities were not significantly affected by processing while methionine and lipotropic micronutrient densities were significantly decreased, especially for magnesium, pantothenate and folates. Myo-inositol density decreases were insignificant due to lower product number resulting from limited literature data. Lipotropic micronutrient densities were more affected by processing than other densities. Fermentations increased betaine (median change of +32%) and choline (+34%) densities. Canning and boiling vegetables increased choline densities (+26%). Globally, processing significantly reduced LC by ∼20%, fermentations being less drastic (median change of −5%) than refining (−33%) and thermal treatments (−16%). More specifically, canning increased LC of beetroot (536 vs 390%) and common bean (40 vs 36%) as fermentation towards LC grape (14 vs 7% for wine). Results were then mainly discussed based on percentages of lipotrope content changes on a dry-weight basis. Results of this study also showed that the LC is quite a relevant index to estimate effect of processing on lipotropic potential of PBF.
Introduction
Increased consumption of whole-grain cereals, legumes, fruits and vegetables may be protective against the development of age-related and/or chronic diseases that are, for the most significant in terms of public health, cardiovascular diseases, diabetes, cancers and obesity, the most conclusive results being observed in humans consuming whole-grain cereals.2 Among mechanisms involved, the most studied have been the antioxidant, anticarcinogenic and hypolipidemic effects of phyto-micronutrients, and the role of fibre-type compounds on digestive physiology and carbohydrate and lipid metabolisms. The ability of phytochemicals of numerous plant-based foods (PBF) to limit excess hepatic fat deposits has been largely less studied and emphasized, especially in humans. Yet, hepatic steatosis or fatty liver may be diagnosed in situations of alcoholism, overweight, obesity, hyperlipidemia, non-insulinodependent type 2 diabetes and malnutrition3–6 and is the first step that may lead to more severe pathologies like steatohepatitis, fibrosis and cirrhosis.7 Moreover, patients with hepatic steatosis present an increased risk of developing cardiovascular diseases,8 those with non-alcoholic steatohepatitis-related cirrhosis an increased risk of developing liver cancer9 and type 1 diabetic subjects with non-alcoholic fatty liver disease (NAFLD) have a higher prevalence of chronic kidney diseases and retinopathy.10 Like obesity, fatty liver may be therefore the onset for the development of a cascade of numerous other chronic diseases. Accordingly, it has been shown to be an early predictor of other metabolic disorders.11The prevalence of NAFLD seems to largely depend on the diagnostic method used: Bloomgarden reported that “the prevalence of NAFLD varies from 3 to 20% of the population based on elevated transaminase and from 16 to 19% based on ultrasound screening” adding that “autopsy series suggest that 11–36% of the population has NAFLD”.7 More striking, 95% of obese and 50–70% of type 2 diabetic people are affected by NAFLD.7 In year 2000, it was estimated that hepatic steatosis would affect around 30 millions American obese adults and from 10 to 24% of the general population in some countries.4 These percentages are relatively high. It is therefore quite surprising that the potential positive effect of plant-based food bioactive compounds for preventing excess hepatic fat deposits in humans has been rather neglected till today.
Such compounds have been very early called lipotropes.12 Lipotropes act on lipid metabolism by hastening removal and/or preventing excessive lipid deposits,2 mainly triglycerides.13 First and main recognized PBF lipotropes are betaine, choline, myo-inositol, methionine and carnitine.2 They are generally completed by magnesium and some B vitamins that are niacin, pantothenic acid and folates that may indirectly sustain the physiological action of main lipotropes.2 However, since then, many other phytochemicals have been reported to decrease hepatic triglyceride or total lipid content in various animal models of fatty livers and, although they have been, to our knowledge, never named lipotropes as such, may be considered as contributing to the overall lipotropic effect of PBF.2 They are organosulfur compounds (e.g. s-allyl-cysteine), unsaturated FA (probably mainly n-3 PUFAs such as α-linolenic and/or n-9 MUFA such as oleic acid), phosphatidylinositol, acetic acid, melatonin, deoxynojirimycin, phytic acid, soluble and insoluble fiber (e.g. lignins, pectin and guar gum), oligofructose (e.g. fructans like inulin), resistant starch (RS), phenolic acids, flavonoids (e.g. epigallocatechin gallate), lignans (e.g. sesamin), stilbenes, curcumin, coumarin, caffeine, γ-oryzanol and saponins, as well as plant protein extracts or isolates (e.g. from soybean or lupin), as having a lipotropic effect.2
What is the most striking and appears as quite paradoxical in a first view is the scarcity of human intervention studies that aim at unravelling lipotropic effect of PBF compounds or products2 whereas steatosis (non-alcoholic type) is highly prevalent among Western populations.4 Thus, up today, putting apart some quite old clinical reports made on isolated individuals with hepatic dysfunctions or troubles (e.g. as result of alcoholism),2 and that were improved in some cases via administration of choline chloride,14 relevant interventional studies have been lead with either commercial lipotropic complex15,16 and tablets17 or with isolated compounds like n-3 polyunsaturated fatty acids,18–24 betaine,25–27 carnitine,28 tea pigment capsules29 and silymarin.30 All showed reduced degree of liver stetaosis and/or improvement in liver functions following lipotrope administration. Other studies have been mainly lead in animal models of hepatic steatosis.2
Observational studies are also quite rare. The few results obtained showed that high-coffee consumption in patients with advanced hepatitis C-related liver disease was associated with less severe steatosis (p for trend = 0.047),31 that non-alcoholic fatty liver disease (NAFLD) patients appear to consume excessive sugar-sweetened soft drinks (>0.5 L per day)32 and more fructose than controls,33 that high alcohol consumption was positively and significantly correlated with diffuse liver steatosis in non-cirrhotic patients34 and that moderate alcohol consumption was associated with lower steatohepatitis prevalence in NAFLD patients.35 Reported average total caloric, fat, saturated fat and simple carbohydrate intake over 10 days was also significantly and positively associated with liver fat scores in obese and sedentary men.36 Finally, obese subjects on a high-fat diet during 3 weeks significantly increased their intra-hepatic lipid content by 17%.37 It is therefore not surprising that nutritional strategies like calorie restriction and/or weight loss have lead to significant improvement of liver steatosis.38,39
In a previous study, we showed that raw PBF, compared to animal-based foods (ABF), are more diversified sources of lipotropes but that both may be complementary: while ABFs seem to be richer sources of choline, methionine and niacin on a 100 kcal-basis, PBFs are richer sources of betaine, myo-inositol, magnesium, pantothenic acid and folates. We also unravelled that vegetables, compared to cereals, legumes, fruits, nuts and seeds, have the highest lipotrope densities (LD, in mg lipotropes/100 kcal), especially for B vitamins.1 High vegetables LD was mainly due to the combination of both relatively high content in lipotropes and low caloric content, generally below 70 kcal/100 g of food. Cereals, legumes, nuts and seeds, although having higher lipotrope contents than vegetables, have lower LD due to their high caloric content.1
In this previous study, the objective was also to simply define and calculate the lipotropic potential of PBF by considering equally each lipotrope in a single index.1 The only sum of LD was not possible since depreciating the importance of lipotropes with very low density as folates (within range 0–0.747 mg/100 kcal) compared to choline (within range 1–369 mg/100 kcal). So, we have defined the lipotropic capacity (LC) based on 8 LD that are betaine, choline, methionine, myo-inositol, magnesium, niacin, pantothenic acid and folate densities. The 8 compounds were chosen based on scientific evidence and available data for lipotrope contents LC.1,2 On a mathematical basis, LC is the average LD of these 8 LD expressed as a percentage of the LD of a reference PBF. The reference food was raw asparagus and its LC was set to 100%. It was chosen within our database (Supplementary Table 1†) as the raw PBF ranking the highest for the mean rank of the 8 LD (Supplementary Table 3).1 Based on this new nutritional index, we have then shown that most raw vegetables, i.e. raw spinach (LC of 672% relative to asparagus), beetroot (390%), lettuce (92%), broccoli (90%), algae (84%), celery (76%), cucumber (74%), tomato (70%), sweet pepper (66%), cabbage (65%) and radish (63%), had a high LC. Citrus fruits have also a relatively high LC within the range 41–51%, notably due to their high free myo-inositol content. Other fruits reach more heterogeneous values with 107% for blackberry and 7% for grape when taking extremes. Concerning other food groups, whole-grain quinoa and amaranth (pseudo-cereals) have LC of respectively 155 and 42%, common bean and soybean seed of respectively 36 and 33%, and sesame seed, peanut and almond of respectively 26, 20 and 14%. Interestingly, PBF of different botanical origins may have similar LD profiles, e.g. blackberry vs sweet pepper, common bean vs sesame seed, peanut vs peach, almond vs banana and onion vs plum as we showed through hierarchical classification analysis.1 In the end, we proposed that LC may be a new valuable index – and complementary to other nutritional indices – to evaluate potential health benefits of PBF, notably for guiding food choices in case of mild steatosis or high potential risk.1
However, although some PBF may be eaten raw like tomato, lettuce or sweet bell pepper, most of them are processed, either minimally like boiled vegetables and dried fruits or highly as white bread and ketchup. An important issue is therefore the evolution of PBF LD and LC upon processing. Scientific literature dealing with the effect of processing upon phytonutrient contents of PBF is rather abundant. It is not our objective to describe it herein. Briefly, one may say that the level to which the PBF phytonutrient content is affected varies greatly according to the processing conditions used in terms of pressure, degree of refining, temperature and/or water content, but also to the conditions of storage as it had been shown with micronutrient contents of fresh, frozen and canned fruits and vegetables.40,41 Concerning the 8 selected lipotrope, betaine content was shown to importantly decrease in spinach, pasta, frozen peas and silverbeet upon boiling.42 However, to our knowledge, there is no study dealing with choline and free myo-inositol content evolution upon processing. Data for methionine, B vitamins and magnesium are largely more numerous. An interesting way to reach a first estimation of the effect of processing upon PBF LD and LC may be based on data collected from various databases or articles for lipotrope contents of raw and processed PBF.
Based on the same 8 lipotropes as in our first study, our main objective was therefore to estimate effect of various technological processes upon PBF LD and LC, either considering them as a whole (i.e. raw vs processed PBF) or by considering each process separately (e.g. fermentation, boiling, refining, etc.). Otherwise, among other lipotropic compounds, literature data for total phenolic compound (TPC) and myo-inositol phosphate (IP) contents are the most available for a large number of PBF.1 Effect of processing on TPC and IP densities was therefore also considered.1 Raw and processed vegetable, fruit, cereal, legume, nut and seed, and beverage products were extracted from a database of 132 PBF (Supplementary Table 1†) whose implementation was previously described in details.1 Our database allowed reaching relevant representativeness of PBF botanical diversity and of processes applied. Among processed PBF, only edible products were considered.
Materials and methods
Selection and classification of raw and processed food products
Extraction of raw and processed PBF was realised from a list of 132 products mainly selected from USDA Choline & Betaine database.43 Selection has been previously described in detail.1 The effect of processing was analysed according to two perspectives: (1) the overall effect of processing (overall un-specific processing, OP) by comparing mean and median LD/LC and mean ranking of all raw vs edible processed PBF; and (2) the effect of specific processes (SP) by comparing mean and median LD/LC of 41 pairs of raw vs processed product (e.g. raw common bean vs boiled/canned common bean; grape vs grape juice, raisin and wine or barley malted flour vs beer).To study effect of OP on PBF LD, the 121 PBF were clustered into 2 groups: group 1 corresponded to raw products (edible or not) and included 6 cereals, 2 legumes, 19 vegetables, 10 nuts and seeds and 19 fruits (n = 56 raw PBF); and group 2 corresponded to edible processed products and included 11 cereals, 3 legumes, 24 vegetables, 5 nuts and seeds, 7 fruits and 15 beverages (n = 65 edible processed PBF) (Supplementary Table 1†). The 11 non-selected products corresponded to non-edible processed products: they were intermediary products of cereal milling (i.e. wheat bran, wheat germ, white wheat flour, raw white rice, maize and oat brans), malted barley flour, roasted buckwheat groats, whole-grain masa, dry pasta and defatted soybean flour. Dry instant coffee and lemonade concentrate have been considered as edible products since they are diluted with water only for consumption. Although having been submitted to milling, whole-grain cereal flours (i.e. from maize, oat, rye and wheat) have been considered as raw products assuming that these products, as cereal flours of very low-grade extraction, were minimally-processed cereals that remain designed as whole grains (dark rye flour being the same as whole-grain rye flour).
The effect of processing was also considered by food group. Thus, from the 121 products were constituted 5 food groups that were cereals (C group), legumes (L group), vegetables (V group), nuts and seeds (N group) and fruits (F group).1 Beverages, being all processed products from various botanical origins, were dispatched within the 5 other groups as follows: beer within the processed cereals, soybean milk within the processed legumes, canned condensed tomato soup within the processed vegetables, coconut milk within the processed nuts, and fruit juices, wines, carbonated orange soda and lemonade concentrate within the processed fruits. Brewed tea and coffee were not incorporated into a food group due to their very low caloric content (respectively 1.2 and 0.7 kcal/100 g of food) that yielded very high LD and that would have led to importantly overestimate mean LD of processed food groups; dry instant coffee and carbonated cola were not included as well within a particular processed food group since being botanically too distinct and distant from the 5 food groups.
Effect of processing was finally considered by process type (i.e. SP). Products were clustered within 3 process groups that are thermal, fermentative and refining processes. Thermal processes included toasting, baking, drying, canning and boiling. Fermentation included baking, acidic fermentation and alcoholic beverage production. Refining processes were all technological treatments that lead to ingredient losses, mainly fiber fraction, and they included cereal milling, tomato and potato processing, soybean flour defatting, and transformation of fruits into juices or soda and of peanut into butter. Fiber losses for all these products were checked from USDA databases.44 Thus, cooked roasted buckwheat groats, cooked white rice, cooked pasta, boiled common beans, boiled asparagus, boiled broccoli, boiled cabbage, boiled carrot, boiled spinach, baked potato with skin, canned common beans, canned beetroot, canned pear, canned peach, dried grapes (raisins), dried plums (prunes), toasted wheat germ and toasted French/Vienna bread together with the corresponding raw/unprocessed products were included within the Thermal group; beer, wine, whole and white wheat breads, cucumber pickles and sauerkraut together with the corresponding raw products were included in the Fermentation group; and white wheat flour, cooked white rice, defatted soybean flour, oven-heated French fries, potato chips, canned tomato sauce, canned tomato paste, ketchup, canned condensed tomato soup, peanut butter, fruit juices (apple, grape and orange juices) and carbonated orange soda together with the corresponding raw/unprocessed products were included within the Refining group.
In addition, some products that have been selected for measuring effect of OP were not selected for studying the effect of SP since they have no counterpart as processed or raw product, e.g. raw celery; and products initially removed as non-edible processed products, i.e. barley malted flour, roasted buckwheat groats, white rice, white wheat flour, wheat germ, dry pasta and defatted soybean flour, were now considered since having a raw or an edible processed paired product, i.e. respectively beers, cooked roasted buckwheat groats, cooked white rice, boiled pasta, toasted wheat germ, white wheat bread and raw soybean seed. This corresponded to 28 raw and 40 processed products with white wheat flour (C16) being both raw (vs white wheat bread: effect of baking) and processed (vs whole-grain wheat flour: effect of milling) product, whole-grain wheat flour (C15) being compared to both whole wheat bread (effect of baking) and white flour (effect of milling), and cooked white rice (C12) being compared to both raw white rice (effect of cooking) and cooked brown rice (effect of polishing). Besides, several processed products were derived from the same raw products that are common bean, soybean, cabbage, potato, tomato, grape and orange. We finally reached 41 pairs of raw vs processed PBF.
Calculation of the average rank and of the lipotropic density and capacity
The LD was the [lipotrope content (mg/100 g of food) × 100/caloric content (kcal/100 g of food)] ratio and was expressed in mg lipotrope/100 kcal.Processed PBF were first ranked according to each lipotrope, TPC, IP and sums of lipotrope densities. Mean ranks were then calculated for each processed PBF by averaging the rank obtained for each LD, as follows:
| Mean rankPBF = (∑rankPBF)/number of lipotrope (n = 7 or 8) |
| Mean rankfood group = (∑rankfood group)/number of lipotrope (n = 7 or 8) |
Plant-based food LC was calculated as previously described1 with raw asparagus as the reference food product (LC = 100%):
| LCfood (%) = (∑[(LDfood/LDraw asparagus) × 100])/n |
Statistical analyses
Due to non-Gaussian distribution of densities for each of the 8 or sum of lipotropes, bilateral non-parametric Mann-Whitney's test was applied to measure the effect of OP upon LD and LC for all products without food group distinction and for the 5 food groups (LD only). Since brewed coffee and tea LD were very high – due to their very low caloric content of ∼1 kcal/100 mL – the effect of OP was estimated without these products. The effect of SP on LD was also measured, firstly without process type distinction and secondly by considering Thermal, Refining and Fermentation groups. Among Thermal treatments, distinction was also made between Thermal treatments with (boiling and canning) and without (toasting, baking and drying) water. Effect of SP was measured with the Wilcoxon matched-pairs signed-ranks test. A level of p < 0.05 was considered significant for both tests.Lipotrope density distributions for raw and processed products were otherwise characterized by means ± standard deviation, mean rank ± standard deviation, range (min- and max-values), median (50% quartile) and 25%- and 75%-quartiles. Similarities and differences for LD profiles (n = 8) of raw vs processed PBF was analysed and visualized through principal component analysis (PCA) and hierarchical classification (HC) using Euclidian distance and Ward aggregation. All statistical analyses have been realised on a PC computer with the XLSTAT software (Addinsoft Editors, Paris, France).
Results
Ranking of processed PBFs according to their LD and sum of LDs were described in details in supplementary material 1 (see Expanded results, i.e. comments to Supplementary Tables 2–6).†Effect of processing without food group distinction
| Lipotropes | Effect of overall processing: unspecifica | Effect of specific processesb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Means ± SD | Mean rankc ± SD | Range [min.–max.] | Range (food codes)d | Quartilese | Means ± SD | Mean rankc ± SD | Range [min.–max.] | Range (food codes)d | Quartilese | |
| a All raw and processed plant-based foods were considered: however, due to their very low caloric content, brewed coffee and tea were excluded from processed products to avoid an overestimation of density values; results are expressed in mg/100 kcal. b Only pairs of raw vs processed plant-based foods are considered, e.g. raw common bean vs boiled, raw common bean vs canned common bean, raw white rice vs cooked white rice, etc.; results are expressed in mg/100 kcal. c Mean rank corresponds to the means of all the ranks for either raw or processed PBF relative to the totality of PBF (e.g. n = 119 for the effect of unspecific overall processing and n = 82 products for the effect of specific processes for betaine, choline, methionine, magnesium, niacin, pantothenic acid, folate and total B vitamin densities); the mean rank has therefore to be always considered in relation with the sum of all raw and processed PBF for the lipotrope density considered. d Raw and processed plant-based foods description by food code are to be found in Supplementary Table 1, ESI.† e Values for the 25, 50 (median) and 75% quartiles correspond to the lipotrope density intervals in which 25, 50 and 75% of the raw or processed plant-based foods are included. f IP: myo-inositol moities derived from myo-inositol phosphates (from IP6 to IP1): for effect of the overall processing, raw = 45 products and processed = 24 products; for effect of specific processes, raw = 16 products and processed = 16 products. g PAI: Potentially available myo-inositol fraction; BeChIMe: sum of betaine, choline, myo-inositol (PAI) and methionine; total 8 lipotropes: betaine, choline, myo-inositol (PAI), methionine, magnesium, niacin, pantothenic acid and folates; for effect of the overall processing upon PAI, BeChIMe and total 8 lipotrope densities, raw = 38 products and processed = 20 products; for effect of specific processes upon PAI, BeChIMe and total 8 lipotrope densities, raw = 11 products and processed = 11 products. h TPC: Total phenolic compounds as determined by the Folin Ciocalteu's colorimetric method; for effect of the overall processing, raw = 55 products and processed = 42 products; for effect of specific processes, raw = 27 products and processed = 27 products. i For effect of the overall processing, raw = 37 products and processed = 16 products; for effect of specific processes, raw = 11 products and processed = 11 products. j The effect of processing was significant at the level of p < 0.05: for testing effect of overall unspecific processing, non-parametric bilateral Mann-Whitney's test was used (left-hand part of Table 1); for testing effect of specific processes, Wilcoxon matched-pairs signed-ranks test was used, i.e. n = 41 pairs of raw vs processed products except for IP (n = 16 pairs), PAI (n = 11), BeChIMe (n = 11), total 8 lipotropes (n = 11), TPC (n = 27) and total 8 + TPC (n = 11) (right-hand part of Table 1). k p = 0.094. l p = 0.057. | ||||||||||
| Betaine | ||||||||||
| Raw | 44.3 ± 166.9 | 60.8 ± 35.0 | [0–887.1] | [V34–V56] | 0.2–0.6–2.5 | 43.0 ± 147.4 | 40.0 ± 23.8 | [0.1–775.5] | [L28–V69] | 0.3–0.5–3.5 |
| Processed | 23.0 ± 91.6 | 59.2 ± 34.2 | [0–674.2] | [B120–V42] | 0.2–0.5–3.6 | 29.6 ± 111.8 | 42.5 ± 23.8 | [0–674.2] | [V55–V42] | 0.2–0.5–4.5 |
| Choline | ||||||||||
| Raw | 27.4 ± 24.5 | 56.2 ± 29.0 | [4.1–95.1] | [N89–V36] | 9.8–18.1–34.2 | 27.7 ± 23.3 | 38.1 ± 20.4 | [2.9–95.1] | [C16–V36] | 9.9–19.9–43.1 |
| Processed | 31.4 ± 42.1 | 63.4 ± 38.7 | [1.0–232.3] | [B125–V52] | 5.7–16.6–38.9 | 28.8 ± 34.5 | 44.4 ± 26.8 | [1.2–140.4] | [B128–V44] | 4.4–18.9–39.5 |
| Methionine | ||||||||||
| Raw | 61.1 ± 52.1 | 55.8 ± 32.6 | [2.1–206.8] | [F92–V39] | 22.0–47.4–82.1 | 60.3 ± 40.6 | 37.9 ± 21.4 | [2.1–179.9] | [F92–V43] | 41.8–49.5–72.7 |
| Processed | 51.8 ± 49.0 | 63.7 ± 35.9 | [0–224.3] | [B128–V70] | 10.8–39.7–70.5 | 55.8 ± 56.4 | 44.6 ± 25.8j | [0–224.4] | [B119/128–L32] | 19.1–43.3–66.4 |
| Magnesium | ||||||||||
| Raw | 63.4 ± 70.3 | 51.5 ± 31.4 | [9.7–465.2] | [F100–V39] | 25.5–49.1–77.6 | 51.2 ± 45.5 | 36.7 ± 22.9 | [6.4–267.3] | [C16–V69] | 24.3–41.7–70.7 |
| Processed | 45.3 ± 50.1 | 67.6 ± 35.6j | [0–311.7] | [B121–V70] | 14.6–33.5–53.3 | 42.5 ± 52.2 | 45.8 ± 24.2j | [2.0–311.7] | [B128–V70] | 12.2–26.0–53.1 |
| Niacin | ||||||||||
| Raw | 1.79 ± 2.68 | 55.9 ± 34.0 | [0.04–19.00] | [N78–V59] | 0.55–0.98–2.18 | 1.54 ± 1.31 | 37.0 ± 23.8 | [0.19–5.81] | [F92–V36] | 0.40–1.48–2.04 |
| Processed | 1.36 ± 1.62 | 63.6 ± 34.8 | [0–10.81] | [B121/128–B123] | 0.33–1.07–1.74 | 1.14 ± 1.10 | 45.5 ± 23.4j | [0–5.44] | [B128–V37] | 0.34–0.84–1.64 |
| Pantothenic acid | ||||||||||
| Raw | 0.71 ± 0.92 | 49.9 ± 32.7 | [0.03–5.93] | [N78–V59] | 0.24–0.41–0.97 | 0.48 ± 0.51 | 36.7 ± 21.6 | [0.07–2.61] | [C16–V54] | 0.19–0.37–0.57 |
| Processed | 0.40 ± 0.51 | 69.0 ± 33.7j | [0–3.03] | [B121/128–V52] | 0.09–0.23–0.46 | 0.38 ± 0.42 | 45.8 ± 25.4j | [0–2.16] | [B128–V44] | 0.09–0.22–0.44 |
| Folates | ||||||||||
| Raw | 0.096 ± 0.132 | 50.7 ± 30.7 | [0.003–0.572] | [F100–V57] | 0.013–0.040–0.097 | 0.086 ± 0.108 | 36.0 ± 22.4 | [0.002–0.476] | [C11–V69] | 0.011–0.425–0.096 |
| Processed | 0.063 ± 0.124 | 68.1 ± 35.6j | [0–0.747] | [B118/121/123/128/131–V37] | 0.004–0.014–0.067 | 0.070 ± 0.145 | 46.4 ± 24.3j | [0–0.747] | [B118/128/131–V37] | 0.005–0.015–0.070 |
| Total B vitamins | ||||||||||
| Raw | 2.600 ± 3.531 | 54.4 ± 33.7 | [0.074–25.048] | [N78–V59] | 0.961–1.656–3.138 | 2.115 ± 1.665 | 36.3 ± 24.0 | [0.331–7.748] | [F92–V36] | 0.683–1.825–2.658 |
| Processed | 1.827 ± 1.869 | 65.0 ± 34.7k | [0–10.845] | [B121/128–B123] | 0.443–1.422–2.419 | 1.586 ± 1.447 | 46.2 ± 22.9j | [0–7.312] | [B128–V37] | 0.487–1.291–2.090 |
| IPf | ||||||||||
| Raw | 42.4 ± 39.2 | 33.5 ± 19.6 | [0–179.3] | [V57/F103–V59] | 10.6–36.8–60.6 | 47.0 ± 40.2 | 15.6 ± 10.2 | [1.3–105.5] | [V73–L28] | 12.8–39.0–81.3 |
| Processed | 34.7 ± 35.9 | 37.8 ± 20.8 | [0–112.7] | [B128–V68] | 8.2–15.4–58.2 | 47.3 ± 52.0 | 16.9 ± 10.4 | [1.7–192.5] | [V76–L32] | 10.9–21.5–75.3 |
| PAIg | ||||||||||
| Raw | 105.8 ± 134.6 | 26.2 ± 15.3 | [0–668.4] | [V53/N77/F94 - F95] | 14.4–52.5–139.9 | 144.9 ± 137.0 | 9.5 ± 6.0 | [21.9–316.5] | [F100–F107] | 22.6–85.3–304.0 |
| Processed | 62.8 ± 103.0 | 34.5 ± 16.8l | [0–329.3] | [C20–22/N80/B121–B127] | 1.5–12.3–87.7 | 65.9 ± 94.5 | 13.0 ± 6.8 | [2.0–329.3] | [B128–B127] | 12.3–27.3–88.3 |
| BeChIMeg | ||||||||||
| Raw | 232.4 ± 196.7 | 25.6 ± 15.3 | [20.3–965.1] | [F94–V69] | 93.2–160.7–318.8 | 248.4 ± 179.8 | 9.3 ± 5.9 | [42.2–561.4] | [F100–V41] | 68.6–295.0–373.8 |
| Processed | 147.9 ± 188.7 | 37.0 ± 17.7j | [3.2–752.5] | [B121–V42] | 31.6–80.0–171.1 | 171.8 ± 220.7 | 13.3 ± 6.6 | [3.4–752.5] | [B128–V42] | 45.2–94.9–206.8 |
| Total 8 lipotropesg | ||||||||||
| Raw | 294.5 ± 233.7 | 25.0 ± 14.9 | [52.2–1235.4] | [F100–V69] | 125.6–225.7–384.1 | 290.4 ± 203.0 | 9.0 ± 6.0 | [52.2–629.2] | [F100–V41] | 79.1–381.0–426.4 |
| Processed | 174.5 ± 210.5 | 38.0 ± 17.5j | [3.2–817.2] | [B121–V42] | 47.5–95.9–227.2 | 198.5 ± 236.8 | 13.5 ± 6.3 | [5.4–817.2] | [B128–V42] | 62.8–102.7–262.5 |
| TPCh | ||||||||||
| Raw | 473.6 ± 526.3 | 43.9 ± 26.8 | [8.2–2514.5] | [N87–F95] | 117.3–290.5–566.6 | 359.4 ± 260.2 | 22.5 ± 15.0 | [34.8–967.0] | [C15–F113] | 140.6–290.5–553.9 |
| Processed | 403.0 ± 833.8 | 55.6 ± 28.8j | [0–5113.7] | [B128–B123] | 63.1–145.3–459.7 | 219.9 ± 228.3 | 32.1 ± 15.4j | [0–797.4] | [B128–V37] | 64.8–122.7–397.8 |
| Total 8 + TPCi | ||||||||||
| Raw | 799.1 ± 644.0 | 22.8 ± 14.2 | [142.5–3316.4] | [N77–F95] | 354.9–723.9–997.1 | 686.9 ± 321.0 | 7.8 ± 4.6 | [277.1–1135.6] | [F100–V41] | 277.1–758.1–1301.7 |
| Processed | 367.1 ± 339.6 | 36.7 ± 14.0j | [5.4–1301.7] | [B128–V42] | 164.3–232.3–547.3 | 367.2 ± 356.7 | 14.7 ± 6.4j | [5.4–1301.7] | [B128–V42] | 176.4–233.5–452.3 |
Then, raw and processed products were compared for LD profile similarities and differences through PCA and HC. The loading plot showed that raw products as a whole (additional variable) tended to be characterized by higher betaine, choline, magnesium and B vitamin densities (active variables) while the effect appeared less marked between raw and processed products for PAI and methionine densities (Fig. 1A). Analysis of PCA score plot based on loading plot allowed deducing that products on the right-hand part of the plot had higher densities in choline, betaine, magnesium and B vitamins while those on left-hand part had lower densities for these lipotropes. In addition, products in the upper right part of the plot had higher PAI density while those at the bottom right had higher methionine density (Fig. 1B). Thus, highly refined and/or processed products, energy-dense foods and fruits (except blackberry) exhibited an LD profile less well than most vegetables, legumes and brewed tea. Among processed products, boiled and canned vegetables and legume, and brewed tea exhibited better LD profile than processed cereals and beverages, especially carbonated sodas. Raw and canned beetroot, raw and boiled cabbage, and raw and canned common bean had relatively closed LD profiles. Blackberry had an exceptionally high PAI density and algae a high methionine density. Otherwise, citrus fruits and their corresponding juices had closed LD profiles in the upper-left part of the plot.
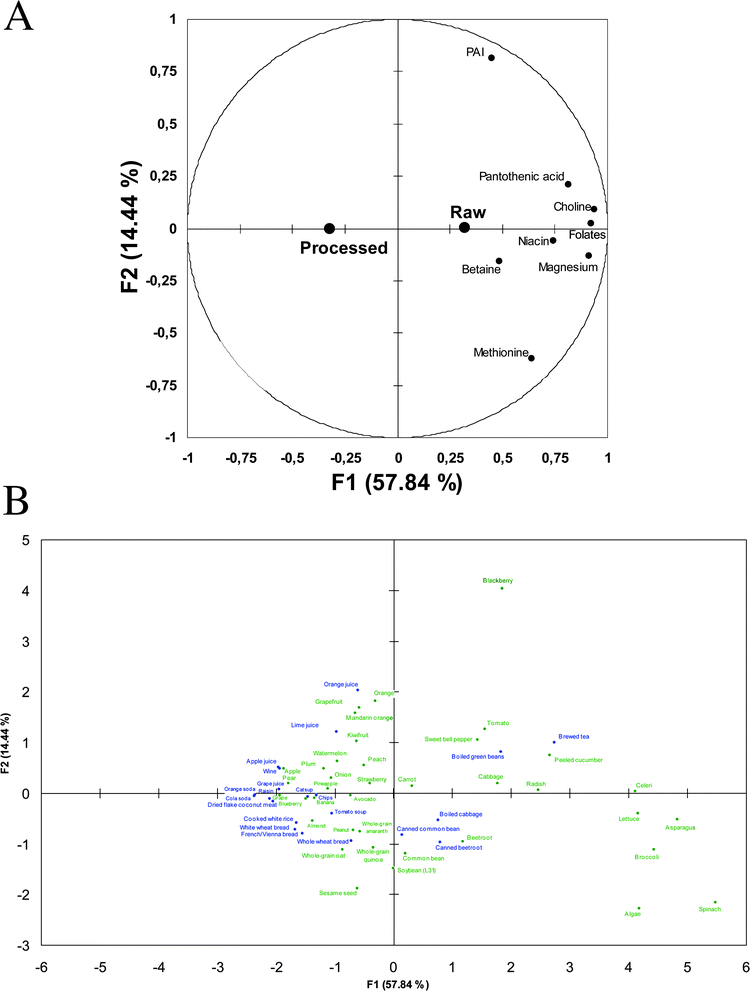 | ||
| Fig. 1 A–B: Principal component analysis loading (A) and score (B) plots derived from the “59 (food items, 38 raw and 21 processed) × 8 (lipotrope densities)” matrix (PC1 × PC2 plan represents 72% of total variance). On the loading plot are shown both active (8 lipotrope densities, •) and supplementary (raw and processed products, ●) variables. Green and blue colours on the scores plot respectively correspond to raw and processed plant-based foods. Food codes can be found in Supplementary Table 1 in the ESI.† | ||
To summarize, processed products tended to be clustered into the following groups: (1) brewed tea and boiled green beans; (2) canned beetroot and common bean and boiled cabbage; (3) whole wheat bread; (4) citrus fruit juices; (5) highly refined and/or energy-dense products (Fig. 1B); while raw products tended to be clustered as follows: (1) vegetables with high LD; (2) vegetables with intermediate LD; (3) blackberry; (4) cereals, legumes, nuts and seeds; (5) citrus fruits; and (6) other fruits, onion, avocado and carrot (Fig. 1B).
Hierarchical classification supplied supplementary information about LD profiles. Products were early clustered within 4 classes (Fig. 2) with their corresponding mean LD profiles (Table 2). Except C4 composed of only brewed tea, other classes (C1–C3) contained both raw and processed products. Class C2 was characterized by the highest density in betaine and included whole-grain quinoa, raw and canned beetroot and spinach, and in total 8 lipotrope and B vitamin densities as well. Class C1 had intermediate mean LD and was composed of raw and boiled vegetables and legume products, of blackberry, of whole-grain amaranth and sesame seed. Brewed tea from C4 had a LD profile very distinct from those of other classes. Finally, C3 was characterized by the lowest densities in total 8 lipotrope and B vitamins and was composed of refined, highly processed and energy-dense vegetables, fruits and their derived products, almond, peanut, processed and refined cereal products, whole-grain oat, wines and sodas (Table 2). The latest products were clustered, the more they had similar LD profile (Fig. 2). Thus, some unexpected products, irrespective of being processed or not, had relatively closed LD profiles as e.g. avocado vs chips or raw cabbage vs boiled green beans (Table 3 and Fig. 2). Between early and late clustering, intermediate clusters allowed classification of products within more specific food groups than the 4 pre-defined classes, such as e.g. (1) citrus fruits and juices, and watermelon; (2) tomato soup, ketchup, avocado, potato chips and pineapple; (3) raisins, sodas, blueberry, dried flaked coconut meat, grape, pear, grape juice and banana; (4) plum, peach, kiwifruit and onion; (5) peanut, processed cereals and whole-grain oat; (6) apple, apple juice and wines; (7) raw radish, celery, algae/seaweed and boiled cabbage; (8) whole-grain amaranth and sesame seed; or (9) raw soybean and common bean.
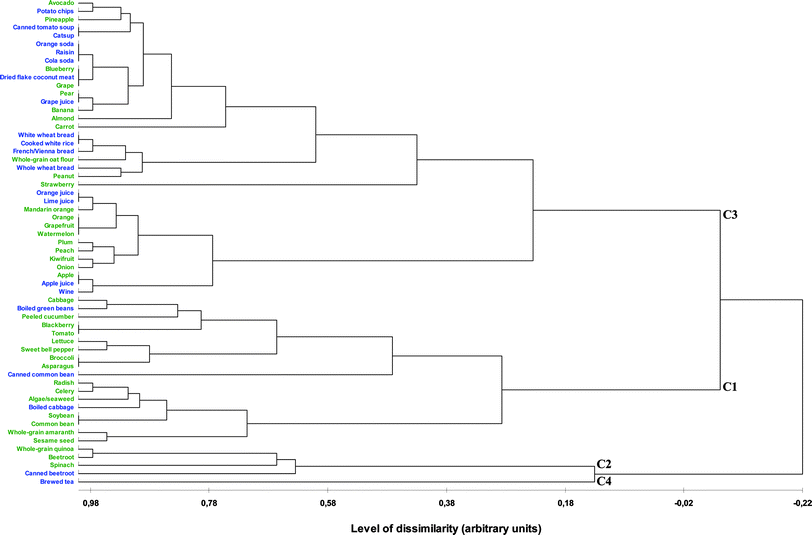 | ||
| Fig. 2 Plot of the hierarchical classification derived from the “59 (food items, 38 raw and 21 processed) × 8 (lipotrope density)” matrix. The highest the level of dissimilarity, the most plant-based foods have different lipotrope density profile. Classes C1, C2, C3, C4 and C5 cluster raw (green) and processed (blue) plant-based foods based on the level of similarity for their lipotrope density profile. | ||
| Food product codesb | Betaine | Choline | PAIc | Methionine | Magnesium | Niacin | Vit. B5d | Folates | ∑B vitaminse | Totalf | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Results are expressed in mg/100 kcal; n = 38 raw and 21 processed plant-based food products. b C, L, V, N, F and B respectively correspond to Cereal, Legume, Vegetable, Nut and seed, Fruit and Beverage groups (food description according to its code can be found in Supplementary Table 1, ESI†). c PAI is potentially available myo-inositol fraction. d Vit. B5 is pantothenic acid. e B vitamins are the sum of niacin, pantothenic acid and folate densities. f Total is the sum of the 8 lipotrope densities. g C is the class as defined by hierarchical classification. | |||||||||||
| C1g | Raw: V34-V36-V43-V46-V49-V53-V54-V57-V67-V73 | ||||||||||
| N89 | |||||||||||
| F95 | |||||||||||
| L28-L31 | |||||||||||
| C1 | 2.8 | 52.5 | 140.0 | 95.5 | 86.7 | 2.13 | 0.98 | 0.183 | 3.29 | 380.92 | |
| Processed: V40-V47 | |||||||||||
| L30 | |||||||||||
| C2 | Raw: V41-V69 | ||||||||||
| C10 | 522.8 | 36.4 | 15.5 | 64.2 | 113.8 | 1.11 | 0.44 | 0.229 | 7.78 | 754.53 | |
| Processed: V42 | |||||||||||
| C3 | Raw: V38-V50-V60 | ||||||||||
| C8 | |||||||||||
| N77-N84 | |||||||||||
| F92-F94-F96-F100-F102-F103-F104-F107-F108-F110-F112-F113-F116-F117 | |||||||||||
| Processed: V65-V76 | 2.4 | 11.1 | 74.8 | 22.7 | 21.4 | 0.76 | 0.31 | 0.024 | 1.10 | 133.55 | |
| C12-C20-C21-C22 | |||||||||||
| N80 | |||||||||||
| F101 | |||||||||||
| B118-B121-B124-B126-B127-B128-B131-B132 | |||||||||||
| C4 | Processed: B130 | 143.4 | 31.1 | 210.3 | 0 | 252.3 | 0 | 0.93 | 0.421 | 1.35 | 638.44 |
| Class C1b | Class C1 | Class C1 | Class C1 | Class C2 | Class C3 | Class C3 | Class C3 | Class C3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw cabbage V46c | Boiled green beans V40 | Raw tomato V73 | Blackberry F95 | Lettuce V57 | Sweet pepper V49 | Whole-grain Amaranth; C1 | Sesame seed; N89 | Whole-grain quinoa C10 | Raw beetroot V41 | Avocado V38 | Chips, V65 | Dried flaked coconut meat, N80 | Blueberry, F96 | Whole wheat bread, C20 | Raw peanut, N84 | Kiwifruit, F103 | Raw onion, F96 | |
| a Results are expressed in mg lipotrope/100 kcal. b Classes are those defined by hierarchical classification (see Table 2). c Food code as defined in Supplementary Table 1, ESI.† d PAI is potentially available myo-inositol. | ||||||||||||||||||
| Betaine | 3.2 | 0.4 | 0.4 | 1.2 | 1.0 | 0.5 | 19.8 | 0.1 | 174.7 | 466.9 | 0.85 | 0.04 | 0.3 | 0.4 | 26.3 | 0.1 | 0.9 | 0.2 |
| Choline | 64.6 | 66.5 | 43.1 | 33.8 | 76.8 | 28.3 | 20.4 | 4.1 | 20.4 | 19.4 | 9.2 | 2.2 | 4.0 | 11.3 | 11.6 | 9.2 | 14.3 | 16.7 |
| PAId | 154.4 | 322.2 | 304.0 | 668.4 | 161.6 | 273.3 | 25.2 | 8.8 | 9.2 | 23.3 | 5.1 | 6.5 | 0 | 26.1 | 0 | 4.4 | 255.4 | 93.5 |
| Methionine | 72.7 | 85.6 | 45.0 | 16.4 | 138.1 | 58.2 | 65.7 | 139.0 | 72.7 | 51.6 | 24.1 | 20.2 | 12.8 | 22.7 | 66.4 | 55.0 | 44.1 | 26.0 |
| Magnesium | 87.8 | 114.1 | 70.7 | 78.5 | 95.3 | 55.7 | 77.3 | 54.5 | 58.2 | 66.1 | 18.9 | 12.8 | 10.1 | 11.3 | 36.8 | 29.1 | 31.3 | 27.4 |
| Niacin | 1.51 | 1.82 | 3.82 | 2.54 | 2.31 | 3.69 | 0.37 | 0.92 | 0.81 | 0.96 | 1.24 | 0.77 | 0.06 | 0.79 | 1.64 | 2.09 | 0.63 | 0.31 |
| Pantothenat | 0.70 | 0.23 | 0.57 | 1.08 | 1.09 | 1.05 | 0.30 | 0.05 | 0.29 | 0.45 | 0.95 | 0.80 | 0.15 | 0.23 | 0.24 | 0.31 | 0.34 | 0.32 |
| eFolates | 0.210 | 0.109 | 0.096 | 0.098 | 0.572 | 0.076 | 0.014 | 0.018 | 0.014 | 0.313 | 0.058 | 0.014 | 0.002 | 0.011 | 0.021 | 0.042 | 0.046 | 0.061 |
Multivariate analyses therefore revealed that LD profiles of processed products were not so importantly distinct from the corresponding raw products. Otherwise, except grain products (cereals, legumes and oleaginous seeds) that tended to exhibit homogeneity of LD profiles, other clusters did not fully match up with the 5 previously defined food groups (C, L, V, N and F groups) and may assemble PBF of different botanical origins.
Lipotrope density variations following processing resulted from both changes in caloric and/or lipotrope contents. Thus, for a more thorough interpretation of LD variations, lipotrope loss and gain percentages have been also evaluated on dry weight basis (dwb) for each process and each compound. An important point to underline is that data compared in this study arose of different sources for both raw and processed PBF, i.e. general database and/or specific database and/or original scientific papers. When data for both raw and processed PBF were collected within USDA database, the product defined as raw may not be exactly from the same source/lot as the raw product used for processing, e.g. raw common bean vs boiled and canned common bean. Conversely, in original scientific papers, raw and boiled products generally arise of same lots. In this latter case, the evolution of the lipotrope content upon processing was therefore more precise. In our study, there is undoubtedly an initial variability between raw products that were used for the products defined as “raw” and “processed”. Thus, it would be hazardous to draw conclusions from only a 10% difference in LD and/or lipotrope content following processing. Therefore, we chose to consider only lipotrope content variations above +10% or below −10%, the threshold that we estimated to be at least that of lipotrope density/content variability for raw PBF. A difference between −10 and +10% was therefore not considered as a meaningful processing effect. Results by process type were as follows (Table 4; see also Expanded Results and Supplementary Tables 7–9 in the ESI† for a detailed description of the influence of processing on lipotrope densities and contents product by product – TPC and IP contents being included):
| Thermal treatments | ||||||
|---|---|---|---|---|---|---|
| With and without water (boiling, canning, toasting, baking and drying) (n = 18 initial pairs) | With water (boiling and canning) (n = 13 initial pairs) | Without water (toasting, baking and drying) (n = 5 initial pairs) | ||||
| Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | |
| Betaine | −24m [−89/+45]b | −31 [−91/+53] | −25n [−89/+45] | −36 [−91/+53] | −1 [−86/0] | −26 [−84/−1] |
| Choline | +6 [−93/+81] | +6 [−94/+84] | +26 [−93/+81] | +17 [−94/+84] | −5 [−63/+10] | −5 [−64/+11] |
| Methionine | −8 [−62/+136] | −1 [−63/+115] | −4 [−32/+136] | +3 [−29/+115] | −12 [−62/+20] | −12 [−63/+19] |
| Bet-Chol-Metc | −13 [−55/+33] | −10 [−53/+30] | −13 [−55/+33] | −6 [−53/+30] | −34 [−42/+3] | −33 [−51/+3] |
| Magnesium | −9 [−73/+41] | −11 [−70/+43] | −18n [−73/+41] | −19 [−70/+43] | +4n [ + 2/+28] | +5 [ + 2/+26] |
| Niacin | −19m [−62/+13] | −25 [−59/+28] | −27m [−62/+13] | −31 [−59/+28] | −9 [−22/+9] | −10 [−23/+10] |
| Pantothenate | −32m [−83/+29] | −28 [−95/+38] | −29n [−83/+29] | −25 [−95/+38] | −40 [−57/+2] | −42 [−58/+3] |
| Folates | −29 [−93/+81] | −21 [−88/+163] | −10 [−93/+150] | −14 [−88/+163] | −33 [−86/+16] | −28 [−85/+18] |
| ∑B vitaminsd | −25m [−70/+35] | −24 [−81/+44] | −20m [−70/+35] | −23 [−81/+44] | −33 [−49/+9] | −25 [−48/+10] |
| ∑7 lipotropese | −11 [−55/+7] | −11 [−57/+11] | −6 [−55/+7] | −13 [−57/+11] | −20 [−35/−10] | −10 [−41/−7] |
| PAIf | −54 [−82/+314] | −52 [−81/+305] | −33 [−82/+314] | −28 [−81/+305] | −75g | −75g |
| Median1h | −22 | −23 | −22 | −22 | −11 | −19 |
| Median2i | −19 | −21 | −18 | −19 | −9 | −12 |
| TPCj | −43m [−88/+60] | −46 [−86/+74] | −47n [−88/+60] | −52 [−86/+74] | −38 [−43/−33] | −40 [−46/−33] |
| IPk | −5 [−25/+78] | −7 [−21/+89] | −5 [−25/+78] | −7 [−21/+89] | —l | —l |
| Refining treatments (n = 14 pairs) | Fermentative treatments (n = 6 pairs) | All treatments (n = 41 pairs) | ||||
|---|---|---|---|---|---|---|
| Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | Density change (%, mg/100 kcal) | Content change (%, dry weight basis) | |
| a For all thermal treatments, numbers of paired products are 4, 4, 4, 11, 4 and 5 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; For thermal treatment with water, numbers of paired products are 3, 3, 3, 9, 3 and 5 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; For thermal treatment without water, numbers of paired products are 1, 1, 1, 2, 1 and 0 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; For refining treatments, numbers of paired products are 6, 6, 6, 10, 6 and 7 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; for fermentative treatments, numbers of paired products are 1, 1, 1, 3, 1 and 2 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; For all treatments, numbers of paired products are 11, 11, 11, 27, 11 and 17 for respectively PAI, BeChIMe, ∑lipotropes, TPC, Total and IP; All treatments also include whole-grain masa, tofu and soybean milk not initially classified with thermal, refining and fermentative groups. b Numbers are median followed by [min/max] values. c Bet-Chol-Met is the sum of betaine, choline and methionine; myo-inositol was excluded due to lower number of data. d ∑B vitamins is the sum of niacin, pantothenate and folates. e ∑7 lipotropes is the sum of betaine, choline, methionine, magnesium and B vitamins; myo-inositol was excluded due to lower number of data. f PAI: potentially available myo-Inositol. g Only one pair of raw vs processed product could have been considered. h Median1 corresponds to the median of the 8 medians obtained for each lipotrope densities or contents. i Median2 corresponds to the median of the 7 medians obtained for betaine, choline, methionine, magnesium and B vitamin densities or contents (due to lower number of paired raw vs processed products for which PAI content could have been found, i.e. n = 11, compared to other lipotrope contents, i.e. n = 41). j TPC: total phenolic compounds. k IP: myo-inositol phosphate. l No data available. m The effect of processing on LD was significant (p < 0.05, Wilcoxon matched-pairs signed-ranks test; see also Table 1); the significativity of process effect was tested only on densities not on contents. n p = 0.063 for the effect of thermal treatments without water on magnesium density; p = 0.080, p = 0.068, p = 0.068 and p = 0.074 for the effect of thermal treatments with water on respectively betaine, magnesium, pantothenic acid and TPC density; p = 0.068 for the effect of refining on choline density. | ||||||
| Betaine | 0 [−98/+1650] | +24 [−98/+1790] | +32 [−100/+2320] | +35 [−100/+2516] | 0 [−100/+2320] | −18 [−100/+2516] |
| Choline | −33n [−94/+134] | −34 [−92/+58] | +34 [−62/+114] | +32 [−76/+215] | +2 [−94/+134] | +3 [−94/+215] |
| Methionine | −33m [−100/+87] | −30 [−100/+27] | −22 [−100/+17] | −50 [−100/+5] | −11m [−100/+136] | −17 [−100/+115] |
| Bet-Chol-Metc | −9m [−80/+578] | +3 [−86/+577] | +24 [−68/+805] | +36 [−80/+871] | −6m [−80/+805] | −5 [−86/+871] |
| Magnesium | −46m [−92/+50] | −33 [−90/+75] | +9 [−46/+44] | −12 [−64/+130] | −18m [−92/+50] | −19 [−90/+130] |
| Niacin | −31m [−100/+130] | −20 [−100/+71] | −21m [−90/−3] | +6 [−94/+30] | −19m [−100/+130] | −13 [−100/+71] |
| Pantothenate | −38 [−100/+274] | −15 [−100/+205] | −27 [−86/+27] | −17 [−91/+14] | −31m [−100/+447] | −25 [−100/+397] |
| Folates | −59m [−100/+17] | −54 [−100/+90] | +18 [−95/+55] | −1 [−97/+122] | −43m [−100/+150] | −33 [−100/+163] |
| ∑ B vitaminsd | −33m [−100/+140] | −24 [−100/+62] | −1 [−90/+12] | +9 [−94/+40] | −25m [−100/+142] | −16 [−100/+122] |
| ∑ 7 lipotropese | −25m [−84/+204] | −10 [−93/+207] | +15 [−74/+356] | +24 [−84/+384] | −9m [−84/+356] | −10 [−93/+384] |
| PAIf | −43 [−99/+16] | −32 [−99/+23] | +573g | +573g | −33 [−99/+314] | −28 [−99/+573] |
| Median1h | −35 | −31 | +13 | +3 | −19 | −19 |
| Median2i | −33 | −30 | +9 | −1 | −18 | −18 |
| TPCj | −44 [−100/+97] | −50 [−100/+98] | +81 [−44/+169] | +83 [−41/+297] | −43m [−100/+169] | −41 [−100/+297] |
| IPk | +14 [−100/+137] | +32 [−100/+217] | −30 [−36/−24] | −27 [−35/−18] | −7 [−100/+137] | −7 [−100/+217] |
Thermal treatments. Considering the 13 pairs of boiled/canned raw vs processed products as a whole, thermal treatments with water tended to decrease betaine, PAI and micronutrient contents (median changes ≤ −14%), to increase choline content while having no marked effect on methionine content. Sums of betaine-choline-methionine, of B vitamin and of 7 lipotrope (PAI excluded due to lower number of raw vs processed pairs) contents were all decreased, effect being more marked for sum of B vitamin contents (median change = −23%). Considering the 8 lipotropes, boiling/canning decreased content by ∼−20% (median of the 8 medians was −22%, and −19% when excluding PAI content).
Considering the 5 pairs of toasted/baked/dried raw vs processed products as a whole, thermal treatments without water tended to decrease betaine, methionine, PAI, pantothenic acid and folates content (median changes ≤ −12%) while having no marked effect on choline, magnesium and niacin contents. Considering the 8 lipotropes, toasting/baking/drying as a whole decreased contents, PAI excluded (Median2 change = −12%) or not (Median1 change = −19%).
Considering now the 18 pairs of products as a whole, thermal treatments ± water tended to decrease betaine, PAI, micronutrient and sums of lipotrope contents (median changes ≤ −10%) while having no marked effect on choline and methionine contents. Densities (mg/100 kcal) tended to change in a similar way to the contents on a dwb. Considering the 8 lipotropes, thermal treatments decreased content by ∼−22% (Median1 change = −23% and Median2 change = −21%).
Refining treatments. Considering the 14 pairs of products as a whole, refining tended to decrease choline, methionine, PAI, micronutrient, sum of B vitamin and sum of 7 lipotrope contents (median changes ≤ −10%) while increasing betaine content (median change = +24%) and having no marked effect on sum of betaine-choline-methionine content (median change = +3%). Except betaine, densities changed in a similar way to contents. Considering the 8 lipotropes, refining processes decreased content by ∼−30% (Median1 change = −31% and Median2 change = −30%).
Fermentations. Considering the 6 pairs of products as a whole, fermentation tended to decrease methionine, magnesium and pantothenic acid contents (median changes ≤ −12), to increased betaine, choline, PAI, sum of betaine-choline-methionine and sum of 7 lipotrope contents (median changes ≥ +24%) while having no marked effect on niacin, folate and sum of B vitamin contents. Except niacin and folates, densities changed in a similar way to contents. Considering the 8 lipotropes, fermentation had no effect on content (Median1 change = +3% and Median2 change = −1%).
All processes. Specific processes tended to decrease betaine, methionine, PAI and micronutrient contents (median changes ≤ −13%) while having no marked effect on choline content. Sums of lipotrope contents tended to be slightly decreased: −5% for betaine-choline-methionine, −16% for B vitamins and −10% for the 7 lipotropes. Except betaine, densities globally changed in a way similar to contents. Significativity was reached for all LDs except those of betaine and choline, and of PAI as well but the number of paired products was lower. Considering the 8 lipotropes, SP decreased content and densities by 18–19%.
Effect of overall un-specific processing by food group
Processing tended to increase mean rank of PBF groups, indicating that processed PBF groups were generally less well ranked than raw PBF groups towards LD (Table 5). Thus, putting aside L group due to the very low number of products, increased percentage of mean rank was: for magnesium, C = N (+35%; p < 0.05) > F (+29%; p < 0.05) > V (+15; NS); for total B vitamin, F (+36%; p < 0.05) > V (+20%; p < 0.05) > N (+13%; p < 0.05) > C (+8%; NS); for PAI, C (+31%; p = 0.057) > N (+25%; n = 1 processed PBF) > V (+13%; NS) > F (+10%; NS); for BeChIMe: C (+50%; p = 0.057) = N (+50%; n = 1 processed PBF) > F (+14%; NS) > V (+13%; NS); and for sum of 8 LD: C (+50%; p = 0.057) = N (+50%; n = 1 processed PBF) > F (+20; NS) > V (+15%; NS).The influence of OP upon TPC (Table 5) and IP (Supplementary Table 10) densities by food group is described in Expanded Results (see ESI†).
| Cereals | Legumes | Vegetables | Nuts and seeds | Fruits | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rawb | Processedc | Raw | Processed | Raw | Processed | Raw | Processed | Raw | Processed | |
| a Results are expressed in mg/100 kcal; beverages, being all processed PBF, have been dispatched among the 5 solid/semi-solid processed food groups. b Both edible and non-edible raw products are considered. c Only edible processed products are considered. d Values for the 25, 50 (median) and 75% quartiles correspond to the lipotrope density intervals in which 25, 50 and 75% of the raw or processed plant-based foods are included. e ∑B vitamins is the sum of niacin, pantothenic acid and folate densities. f No value since number of products is <3. g Number of products considered for calculating statistical descriptors. h PAI is the potentially available myo-insoitol fraction. i Sum of betaine, choline, potentially available myo-inositol (PAI) and methionine densities. j ∑8 lipotropes is the sum of betaine, choline, myo-inositol (PAI), methionine, magnesium, niacin, pantothenic acid and folate densities. k TPC is total phenolic compounds. l Significant effect of processing at p < 0.05 (non-parametric Mann-Whitney's test). m p = 0.057 (non-parametric Mann-Whitney's test). | ||||||||||
| Magnesium | ||||||||||
| Means | 60 ± 22 | 31 ± 25 | 64 ± 6 | 47 ± 2 | 108 ± 104 | 73 ± 65 | 44 ± 21 | 23 ± 12 | 29 ± 20 | 14 ± 9 |
| Mean rank | 5.3 ± 3.3 | 11.6 ± 5.0l | 1.5 ± 0.7 | 4.5 ± 1.3 | 18.8 ± 12.4 | 25.3 ± 12.7 | 6.4 ± 4.2 | 12.0 ± 6.0l | 12.9 ± 8.5 | 22.6 ± 8.5l |
| Range | [37–94] | [9–91] | [60–69] | [45–50] | [19–465] | [13–312] | [17–85] | [10–45] | [10–79] | [2–29] |
| Product code | [C5–C14] | [C21–C19] | [L28–L31] | [L30–B129] | [V38–V39] | [V65–V70] | [N86–N81] | [N78–N79] | [F100–F95] | [B128–F99] |
| Quartilesd | 46–54–73 | 11–25–43 | 62–64–66 | 46–47–48 | 56–88–107 | 34–54–75 | 28–42–55 | 17–22–25 | 18–24–31 | 7–14–19 |
| ∑B vitaminse | ||||||||||
| Means | 1.37 ± 0.74 | 1.32 ± 0.84 | 0.90 ± 0.30 | 0.99 ± 0.456 | 5.04 ± 5.19 | 2.88 ± 1.52 | 0.89 ± 0.77 | 0.70 ± 0.85 | 1.63 ± 0.98 | 0.59 ± 0.41 |
| Mean rank | 8.5 ± 4.8 | 10.0 ± 5.7 | 3.5 ± 2.1 | 3.5 ± 2.1 | 17.6 ± 12.6 | 26.2 ± 12.0l | 7.7 ± 5.0 | 9.8 ± 4.4 | 11.9 ± 8.5 | 23.9 ± 6.5l |
| Range | [0.67–2.37] | [0.35–2.93] | [0.68–1.11] | [0.62–1.66] | [0.69–25.05] | [0.42–7.31] | [0.07–2.44] | [0.21–2.44] | [0.33–3.72] | [0–1.37] |
| Product code | [C8–C15] | [C26–C27] | [L31–L28] | [L33–B129] | [V60–V59] | [V55–V37] | [N78–N84] | [N78–N85] | [F92–F95] | [B128–F93] |
| Quartiles | 0.80–1.15–1.94 | 0.48–1.43–1.91 | —f | 0.78–0.84–1.06 | 2.72–3.70–5.45 | 1.80–2.42–3.62 | 0.34–0.71–0.96 | 0.36–0.39–0.43 | 1.10–1.32–2.26 | 0.29–0.47–0.92 |
| n | 6 | 12 | 2 | 4 | 19 | 25 | 10 | 6 | 19 | 14 |
| PAIh | ||||||||||
| Means | 12.3 ± 11.7 | 1.0 ± 1.9 | 18.4 ± 8.7 | 101.8 | 99.7 ± 94.0 | 68.9 ± 124.5 | 4.4 ± 4.4 | 0 | 162.4 ± 174.3 | 105.3 ± 120.0 |
| Mean rank | 2.3 ± 1.5 | 4.5 ± 1.0m | 2.5 ± 0.7 | 1 | 10.2 ± 5.4 | 13.0 ± 8.1 | 2.0 ± 1.0 | 3 | 10.8 ± 6.0 | 13.0 ± 7.5 |
| Range | [2.4–25.2] | [0–3.9] | [12.3–24.6] | — | [0–304.0] | 6.5–322.2 | [0–8.8] | — | [0–668.4] | 2.0–329.3 |
| Product code | [C8–C1] | [C20/C22–C12] | [L31–L28] | L30 | [V53–V73] | [V65–V40] | [N77–N89] | N80 | [F94–F95] | [B128–B127] |
| Quartiles | 5.8–9.2–17.2 | 0–0–1.0 | — | — | 19.5–93.5–136.4 | 10.6–21.5–31.6 | 2.2–4.4–6.6 | — | 44.8–113.8–254.9 | 15.4–87.1–143.9 |
| BeChIMei | ||||||||||
| Means | 172 ± 92 | 80 ± 21 | 161 ± 2 | 260 | 321 ± 226 | 256 ± 294 | 88 ± 57 | 17 | 195 ± 183 | 117 ± 129 |
| Mean rank | 2.0 ± 1.0 | 5.5 ± 1.3m | 2.5 ± 0.7 | 1 | 10.2 ± 5.4 | 13.0 ± 8.1 | 2.0 ± 1.0 | 4 | 10.5 ± 6.0 | 13.6 ± 7.5 |
| Range | [108–277] | [56–104] | [159–162] | — | [39–171] | [29–753] | [42–152] | — | [20–720] | [3–356] |
| Product code | [C8–C10] | [C12–C20] | [L28–L31] | L30 | [V38–V69] | [V65–V42] | [N77–N89] | N80 | [F94–F95] | [B128–B127] |
| Quartiles | 120–131–204 | 68–80–92 | 160–161–161 | — | 171–306–372 | 60–111–395 | 55–69–110 | — | 69–145–299 | 24–95–159 |
| ∑8 lipotropesj | ||||||||||
| Means | 234.9 ± 91.3 | 97.6 ± 33.1 | 225.7 ± 7.7 | 305.8 | 418.8 ± 274.2 | 306.6 ± 321.6 | 132.8 ± 64.8 | 27.4 | 223.8 ± 196.1 | 131.9 ± 136.5 |
| Mean rank | 2.0 ± 1.0 | 5.5 ± 1.3m | 2.5 ± 0.7 | 1 | 10.1 ± 5.3 | 13.2 ± 8.1 | 2.0 ± 1.0 | 4 | 10.1 ± 5.8 | 14.4 ± 7.3 |
| Range | [159–336] | [67–143] | [220–231] | — | [60–1235] | [43–817] | [91–208] | — | [53–802] | [5–381] |
| Product code | [C8–C10] | [C12–C20] | [L28–L31] | L30 | [V38–V69] | [V65–V42] | [N77–N89] | N80 | [F100–F95] | [B128–B127] |
| Quartiles | 184–209–273 | 78–90–110 | — | — | 274–421–472 | 81–156–498 | 96–100–154 | — | 89–163–326 | 38–103–180 |
| n | 3 | 4 | 2 | 1 | 15 | 6 | 3 | 1 | 15 | 7 |
| TPCk | ||||||||||
| Means | 46 ± 26 | 69 ± 43 | 225 ± 143 | 67 ± 5 | 556 ± 411 | 538 ± 495 | 126 ± 100 | 42 ± 39 | 717 ± 677 | 206 ± 166 |
| Mean rank | 6.5 ± 2.9 | 5.4 ± 4.0 | 1.5 ± 0.7 | 4.0 ± 1.0 | 17.5 ± 9.9 | 18.6 ± 10.9 | 5.9 ± 3.8 | 9.5 ± 3.1 | 11.8 ± 7.4 | 23.8 ± 7.6l |
| Range | [23–94] | [20–123] | [124–326] | [63–73] | [3–1701] | [19–2058] | [8–281] | [5–89] | [160–2515] | [112–553] |
| Product code | [C8–C10] | [C13–B119] | [L31–L28] | [B129–L30] | [V62–V49] | [B131–V35] | [N87–N90] | [N83–N85] | [F106–F95] | [B128–F114] |
| Quartiles | 29–39–50 | 30–82–88 | — | 64–66–69 | 276–506–623 | 220–480–626 | 52–101–215 | 12–37–67 | 258–437–898 | 112–148–274 |
| n | 6 | 5 | 2 | 3 | 19 | 16 | 9 | 4 | 19 | 12 |
| ∑8 lipotropesj + TPCk | ||||||||||
| Means | 291.7 ± 126.7 | 180.0 ± 72.3 | 450.7 ± 135.5 | 378.4 | 963.4 ± 568.7 | 623.2 ± 461.3 | 156.1 ± 19.2 | — | 868.4 ± 757.5 | 285.1 ± 227.6 |
| Mean rank | 2.3 ± 1.5 | 4.0 ± 1.4 | 2.0 ± 1.4 | 2 | 9.6 ± 5.6 | 13.2 ± 6.6 | 1.5 ± 0.7 | — | 8.8 ± 5.4 | 17.3 ± 4.6l |
| Range | [183–431] | [129–231] | [355–547] | — | [159–2136] | [112–1302] | [143–170] | — | [229–3316] | [5–663] |
| Product code | [C8–C10] | [C22–C20] | [L31–L28] | L30 | [V38–V69] | [B131–V42] | [N77–N84] | — | [F94–F95] | [B128–B126] |
| Quartiles | 222–262–346 | — | — | — | 679–950–1109 | 304–611–788 | — | — | 421–733–949 | 176–215–380 |
| n | 3 | 2 | 2 | 1 | 15 | 5 | 2 | 0 | 15 | 7 |
Effect of processing on lipotrope capacity
| LCa (%) | LC (%) | ||
|---|---|---|---|
| a LC is lipotropic capacity based on 8 lipotrope densities except for LC of cereal brans (from maize, oat and wheat) that are based on 7 lipotrope densities (PAI density being excluded) and for LC of wheat germ that is based on either 7 or 8 lipotrope densities (LD). b Due to absence of PAI data for 62 raw and processed PBF products among the initial 121, LC could have been calculated for only 38 raw and 21 processed PBF products. c Food codes with corresponding food description can be found in Supplementary Table 1, ESI.† d C1–C4 correspond to the 4 classes as defined by hierarchical classification. e LD is lipotrope density (mg/100 kcal). f The effect of processing was significant (p = 0.015, non-parametric bilateral Mann-Whitney's test). | |||
| Raw asparagus (reference) | 100 | Raw asparagus (reference) | 100 |
| Raw (edible or not): n = 38b | Processed (edible): n = 21 | ||
| V69c: Spinach (C2)d | 672 | V42: Canned beetroot (C2) | 536 |
| V41: Beetroot (C2) | 390 | B130: Brewed tea (C4) | 196 |
| C10: Quinoa, whole-grain (C2) | 155 | V40: Boiled green beans (C1) | 79 |
| F95: Blackberry (C1) | 107 | B127: Orange juice (C3) | 49 |
| V36: Asparagus (C1) | 100 | L30: Canned common bean (C1) | 40 |
| V57: Lettuce (C1) | 92 | C20: Whole wheat bread (C3) | 39 |
| V43: Broccoli (C1) | 90 | V47: Boiled cabbage (C1) | 38 |
| V34: Algae (C1) | 84 | B126: Lime juice (C3) | 34 |
| V53: Celery (C1) | 76 | C22: French/Vienna bread (C3) | 27 |
| V54: Cucumber, peeled (C1) | 74 | B131: Tomato soup (C3) | 20 |
| V73: Tomato (C1) | 70 | C21: White wheat bread (C3) | 18 |
| V49: Sweet bell pepper (C1) | 66 | B132: Wine (C3) | 14 |
| V46: Cabbage (C1) | 65 | V76: Ketchup (C3) | 13 |
| V67: Radish (C1) | 63 | V65: Chips (C3) | 13 |
| F107: Orange (C3) | 51 | B118: Apple juice (C3) | 13 |
| F102: Grapefruit (C3) | 46 | C12: Cooked white rice (C3) | 10 |
| F103: Kiwifruit (C3) | 44 | B124: Grape juice (C3) | 8 |
| C1: Amaranth, whole-grain (C1) | 42 | N80: Dried flaked coconut meat (C3) | 5 |
| F104: Mandarin orange (C3) | 41 | F101: Raisins (C3) | 4 |
| L28: Common bean (C1) | 36 | B128: Carbonated orange juice (C3) | 1 |
| F108: Peach (C3) | 33 | B121: Carbonated cola (C3) | 1 |
| V50: Carrot (C3) | 33 | ||
| L31: Soybean seed (C1) | 33 | Means processed ± SD | 55 ± 118 |
| F116: Strawberry (C3) | 28 | Median | 18 |
| F117: Watermelon (C3) | 28 | Mean rank | 37.0 ± 17.9f |
| C8: Oat flour, whole-grain (C3) | 28 | ||
| N89: Sesame seed (C1) | 26 | ||
| V60: Onion (C3) | 24 | ||
| F113: Plum (C3) | 23 | ||
| N84: Peanut (C3) | 20 | Cereal brans: | |
| V38: Avocado (C3) | 20 | C17: Wheat bran | 748 |
| F112: Pineapple (C3) | 20 | C6: Maize bran | 72 |
| N77: Almond (C5) | 14 | C9: Oat bran | 37 |
| F92: Apple with skin (C3) | 14 | ||
| F96: Blueberry (C3) | 12 | C18: Wheat germ 2 (n = 7 LDe) | 348 |
| F94: Banana (C3) | 12 | C18: Wheat germ 1 (n = 8 LD) | 308 |
| F110: Pear (C3) | 11 | ||
| F100: Grapes (C3) | 7 | ||
| Means raw ± SD | 72 ± 119 | ||
| Median | 38 | ||
| Mean rank | 25.6 ± 15.2 | ||
Canned beetroot and canned common bean had higher LC than corresponding raw products. Concerning grape, fermentation into wine increased by 2-fold the LC (from 7 to 14%) while transformation into juice had no effect and drying decreased LC by almost 2-fold (LC raisins = 4%): however, grape and all its derived products had low LC below 15. Transformation of grape, apple and orange into juices had no effect on LC. Boiling cabbage decreased LC from 65 to 38%, i.e. almost by 2-fold. Transformation of raw tomato into ketchup and canned condensed tomato soup was drastic towards LC that was reduced from 70 to respectively 20 and 13%. Compared to raw orange (LC = 51%), carbonated orange soda had a LC of 1%. Finally, whole wheat bread had a LC ∼2-fold higher than white wheat bread.
Compared to multivariate analyses, LC ranking globally matched the 4 classes given by HC, i.e. PBF products clustered within classes C2, C1 and C3 had respectively high, intermediate and low LC (Tables 2 and 6). In addition, median difference of −20% between LC of raw and processed PBF (Table 6) was closed to median of −19% obtained when considering the 8 medians of each LD change for all treatments (n = 8 lipotropes and n = 41 pairs of raw vs processed products, Table 4).
| Raw | LC1 (%)a | Processed | LC2 (%) | Change (%)b |
|---|---|---|---|---|
| Barley malt flour | 38 | Beer (F)c | 28 | −28 |
| Roasted buckwheat groats | 27 | Cooked roasted buckwheat groats (T + W) | 26 | −4 |
| Whole-grain maize cornmeal | 20 | Whole-grain masa | 17 | −12 |
| White rice | 16 | Cooked white rice (T + W) | 11 | −28 |
| Wheat germ | 348 | Toasted wheat germ (T-W) | 234 | −33 |
| Cooked brown rice | 21 | Cooked white rice (R) | 11 | −47 |
| Whole-grain wheat flour | 45 | White wheat flour (R) | 10 | −79 |
| Whole-grain wheat flour | 45 | Whole wheat bread (F) | 45 | 0 |
| White wheat flour | 10 | White wheat bread (F) | 21 | +115 |
| French/Vienna bread | 30 | Toasted French/Vienna bread (T-W) | 30 | −3 |
| Dry pasta | 44 | Boiled pasta (T + W) | 35 | −21 |
| Raw common bean | 38 | Boiled bean (T + W) | 36 | −4 |
| Raw common bean | 38 | Canned bean (T + W) | 33 | −11 |
| Raw soybean | 36 | Defatted soybean flour (R) | 67 | +83 |
| Raw soybean | 36 | Tofu | 33 | −9 |
| Raw soybean | 36 | Soybean milk | 38 | +3 |
| Raw asparagus | 100 | Boiled asparagus (T + W) | 107 | +7 |
| Raw beetroot | 442 | Canned beetroot (T + W) | 609 | +38 |
| Raw broccoli | 90 | Boiled broccoli (T + W) | 89 | −1 |
| Raw cabbage | 55 | Boiled cabbage (T + W) | 40 | −27 |
| Raw cabbage | 55 | Sauerkraut (F) | 71 | +30 |
| Raw carrot | 34 | Boiled carrot (T + W) | 31 | −7 |
| Peeled cucumber | 70 | Pickles (F) | 24 | −66 |
| Raw potato | 24 | Baked potato (T-W) | 23 | −4 |
| Raw potato | 24 | Oven-heated French fries (R) | 15 | −39 |
| Raw potato | 24 | Chips (R) | 14 | −40 |
| Raw spinach | 765 | Boiled spinach (T + W) | 280 | −63 |
| Raw tomato | 43 | Canned tomato sauce (R) | 36 | −15 |
| Raw tomato | 43 | Canned tomato paste (R) | 31 | −27 |
| Raw tomato | 43 | Ketchup (R) | 10 | −76 |
| Raw tomato | 43 | Tomato soup (R) | 21 | −50 |
| Raw peanut | 22 | Peanut butter (R) | 19 | −13 |
| Apple | 5 | Apple juice (R) | 4 | −26 |
| Grape | 6 | Raisin (T-W) | 4 | −29 |
| Grape | 6 | Grape juice (R) | 6 | +2 |
| Grape | 6 | Wine (F) | 5 | −10 |
| Orange | 20 | Orange juice (R) | 16 | −19 |
| Orange | 20 | Carbonated orange juice (R) | 1 | −97 |
| Peach | 20 | Canned peach (T + W) | 7 | −64 |
| Pear | 7 | Canned pear (T + W) | 3 | −56 |
| Plum | 13 | Prunes (T-W) | 9 | −31 |
| Means ±SD | 68 ± 35 | Means ±SD | 52 ± 47 | −19 ± 39 |
| Mean rank ±SD | 37 ± 23 | Mean rank ±SD | 46 ± 24 | +11f |
| Median [min/max] | 36 [5/765] | Median [min/max] | 24 [1/609] | −19 [−97/+115] |
| Process type | LC1 (%) | Process type | LC2 (%) | Change (%) |
|---|---|---|---|---|
| a LC is the Lipotrope capacity as defined in the Materials and methods section. b Change was calculated as [(LC1 − LC2) × 100/LC1]. c F, T + W, T-W and R correspond respectively to Fermentations, Thermal treatments +water, Thermal treatments − Water (dry) and Refining processes. d Correspond to the number of paired of raw vs processed products considered to calculate medians, min- and max-values. e Values are median [min/max]. f The effect of processing was significant for all process (n = 41 pairs, p = 0.0004, Wilcoxon matched-pairs signed-ranks test), Thermal treatments (n = 18 pairs, p = 0.012) and Refining processes (n = 14 pairs, p = 0.011), but not for Fermentations (p > 0.05). g p = 0.080 for Thermal treatments with water and p = 0.063 for Thermal treatments without water. | ||||
| Thermal treatments (n = 18 pairs)d | 36 [6/765]e | Thermal treatments (n = 18 pairs) | 32 [3/609]e | −16f [−64/+38] |
| With water (n = 13 pairs) | 38 [7/765] | With water (n = 13 pairs) | 35 [3/609] | −11g [−64/+38] |
| Without water (n = 5 pairs) | 24 [6/348] | Without water (n = 5 pairs) | 23 [4/234] | −29g [−33/−3] |
| Refining processes (n = 14 pairs) | 24 [5/45] | Refining processes (n = 14 pairs) | 14 [1/67] | −33f [−97/+83] |
| Fermentations (n = 6 pairs) | 42 [6/70] | Fermentations (n = 6 pairs) | 26 [5/71] | −5 [−66/+115] |
When looking at each process separately, except white wheat bread (+115%), defatted soybean flour (+83%), canned beetroot (+38%) and sauerkraut (+30%), all other process either reduced LC (<−10%) or had no marked effect (−10% ≤ % LC change ≤ +10%). Of all food groups, L group (raw common bean and soybean) appeared as the less affected by processing, especially for soybean and its derived products, with −11% ≤ % LC change ≤ +83% (median = −4%), then C group (−79% ≤ % LC change ≤ +115%; median = −21%), V group (−76% ≤ % LC change ≤ +38%; median = −27%) and F group (−97% ≤ % LC change ≤ +2%; median = −29%) (result not shown).
Discussion
The LD of processed PBFs alone, i.e. not compared to raw PBFs, has been discussed in the Expanded Discussion (ESI†). Here, only effect of processing was discussed.Effect of processing on lipotrope densities and contents
Due to the limited number of both products and data collected for PAI content, some conclusions have to be considered cautiously and should be rather regarded as tendencies. In addition, due to non-Gaussian distributions of LD and lipotrope content of processed and raw products, the effect of processing cannot be evaluated based on means, but should be considered first via the medians, min- and max-values and mean ranking.Considering all the 8 LD, both OP (n = 56 raw vs 63 processed products) and SP (n = 41 pairs of raw vs processed products) increased processed PBF mean ranking by ∼+10%. Median changes for all lipotrope densities and contents following SP were respectively −19 and −19%, and −18 and −18% when excluding PAI density and content.
Considering each lipotrope separately, mean choline and betaine densities were not significantly affected by both OP and SP while methionine and lipotropic micronutrient densities were decreased, especially magnesium, pantothenic acid and folate densities (p < 0.05 for both OP and SP). Considering PAI density, decreases were not significant, but this has to be attributed to lower number of products. Otherwise, lipotropic micronutrient densities (i.e. magnesium and B vitamins) tended to be more affected by processing (mean ranking increase of ∼+12.1%) than main lipotrope densities (i.e. BeChIMe, mean ranking increase of ∼+7.7%). Considering products by food groups, F group appeared as the most affected by OP, especially towards magnesium and total B vitamin densities (p < 0.05). Except sauerkraut and wine, the way LD changed upon processing was generally similar to the way lipotrope contents (dwb) changed.
Of all treatments, fermentation appeared less drastic towards LD (median change of +13%) than refining (−35%) and thermal treatments (−22%), and it tended to increase betaine (+32%) and choline (+34%) densities. Canning (except fruits) and boiling vegetables also tended to increase choline densities (median change of +26%). Refining is undoubtedly the most drastic treatment for LD and lipotrope contents (median changes of respectively −35 and −31%). Among B vitamins, folate density appeared as the most affected (median change of −43%) compared to niacin (−19%) and pantothenic acid (−31%).
In the following parts, discussion and interpretation of results was mainly based on content changes (dwb). It is also important to mention that of all 8 lipotropes, only methionine may not be water soluble, notably when included within structured protein networks. All others are recognized as hydrosoluble compounds likely to be released into water. Besides, the rarity of literature data about the effects of processing upon the main lipotrope contents of PBF was limiting for explaining all calculated changes. The effect of processing on magnesium, B vitamin, TPC and IP densities has been discussed in the Expanded Discussion (see ESI†). Since their content may increase upon processing, the lipotropic potential of acetate and resistant starch (RS) has been also considered and discussed in Expanded discussion.
Betaine. Overall un-specific processing (OP) did not significantly modify betaine density. Despite a median content change of −18%, SP did not also significantly affect betaine density (median = 0). However, this apparent status quo masked heterogeneous effects on betaine densities and contents depending on process considered.
Literature data about effect of processing on PBF betaine content are rather scarce. The only study reported severe losses upon boiling for peas (43%), spinach (70%), silverbeet (73%) and pasta (76–84%), but it is not indicated if losses were expressed on dry or fresh weight-basis.42 Based on our data, we estimated 23 and 78% losses in respectively boiled pasta and spinach. More generally, boiling led to betaine losses ranging from −18 (common bean) to −91% (broccoli) (median change of −70%) confirming results of de Zwart et al.42 and confirming relevant betaine release into boiling water. Such release was supported by increased betaine content in soybean milk (+493%) and fruit juices (from +43 to +177%) and decreased content in masa or nixtamalized whole-grain cornmeal (−84%) compared to raw soybean seed, fruits and whole-grain maize flour, respectively. Nixtamalisation is a traditional process involving soaking, cooking of maize grain within alkaline solution, usually limewater, and dehulling. Masa is nixtamalized maize dough and betaine loss was very likely to result from release into alkaline solution.
Contrary to boiling, canning enhanced betaine content from +3% (peach) to +53% (beetroot). This effect was also supported by increased betaine content in canned refined tomato products that are tomato sauce (+478%), paste (+20%) and condensed soup (+1790%). Since betaine can not be synthesized upon canning, one plausible explanation might be that it exists within these food matrices as a fraction of trapped betaine that would have initially escaped analysis and that was freed by canning under high pressure and temperatures above 100 °C in presence of water.
Betaine losses upon thermal treatments without water (i.e. toasting, baking and drying) appeared quite paradoxical compared to previous results and difficult to explain. Indeed, our results suggested that betaine would be sensitive to and degraded by increased temperatures. However, this was not supported by increased betaine contents following canning that involves very high temperatures.
Cereal refining was a drastic process towards betaine content since, following bran and germ removing, wheat flour lost 98% of betaine. Recently, average betaine contents of 22.9 and 103.0 mg/100 g have been reported for respectively white wheat and whole-grain wheat flours,45i.e. a decrease of 78% in agreement with our results, again clearly showing that betaine is concentrated in bran fraction,46 more particularly in the aleurone layer.47 Similarly to wheat, when comparing cooked brown and cooked white rice (i.e. polished rice), 49% loss were estimated on dwb. These results also agreed with those of Bruce et al. that measured betaine contents of 0.9 and 0.5 mg/100 g for respectively brown and white rice.45 Explanation for the betaine content increase of defatted soybean flour compared to raw soybean seed (+107%) remains uncertain, but defatting may have concentrated betaine within flour.
Except acidic fermentation of cucumber into pickles and cabbage into sauerkraut, all other fermentative processes increased betaine content from +21% for whole wheat bread to +2516% for white wheat bread. First, this may suggest various contributions of the fermentative microbial flora to betaine content in beer, wine and breads. For example, based on baker's yeast betaine content of 3.6 mg/100 g43 and on common white bread recipe (10 g salt, 10 g yeast and 500 g white wheat flour), we roughly estimated that yeast might increased initial betaine content of white wheat flour by ∼+44% (this involves that bacteria do not consume betaine). Secondly, besides microflora, concerning alcoholic beverages, the betaine content of wine may also partly originates from the beet sugar added to increase alcohol content of cheap wines;48 and that of beer probably originates from barley malted flour that contains 66 mg betaine/100 g.43
Choline. As for betaine, both OP and SP did not significantly modify choline densities; and medians for both density and content changes were near 0 for the 41 pairs of raw vs processed products. However, considering each process type, some tendencies were revealed regarding content changes.
While baking potato and toasting cereals had no effect, drying importantly decreased choline content to an extent greater than for betaine. We have no explanation for this. However, contrary to betaine, boiling vegetables, cereals – except white rice – and common beans increased choline contents (median change for boiling was +17%, result not shown). Effect of canning resembled that observed with betaine concerning beetroot and common bean with increased contents but not for peach and pear (decrease of ∼−70%). Such results do not agree with hydrosolubility of choline; but they may be partly explained by heterogeneity of compounds from which choline moieties are derived for calculating total choline content, i.e. free choline, glycerophosphocholine, phosphocholine, phosphatidylcholine and sphingomyelin, which is not the case for betaine.43
For refining, despite tendency to a decreased choline content (median = −34%), there was no clear homogeneity of changes except for cereal products. Thus, white wheat flour had its choline content reduced by −67% when compared to whole-grain wheat flour: indeed, similarly to betaine, choline is concentrated within bran and germ fractions of wheat.46,47 This was confirmed by results of Bruce et al. for whole-grain vs white wheat flour (means of 10.6 vs 5.5 mg/100 g) and bread (means of 16.1 vs 11.9 mg/100 g) choline contents.45 High choline content difference was also calculated when comparing cooked brown vs cooked white rice (−81%). Difference between raw brown (3 mg/100 g) and white (2.3 mg/100 g) rice was less marked in the study of Bruce et al..45 However, cooking might have accentuated differences. Decreased choline contents in canned tomato sauce and condensed soup, ketchup, oven-heated French fries, potato chips, and apple and grape juices suggested that choline would be more bound to fibre and/or skin fraction than betaine, which could explain why boiling vegetables, common beans and some cereal products have concentrated choline within the food matrix.
Choline content changes following fermentation were similar to those of betaine: decreased content following acidic fermentation for pickles and sauerkraut and increased content for wine, beer and breads. In natural lactic acid fermented cornmeal, choline content was first shown to decrease by 38% after 1 day of fermentation, then to return to its normal level after 4 days.49 These observations tended to support the hypothesis that acidic fermentation may degrade choline. For breads, based on choline content of baker's yeast given by USDA (32 mg/100 g)43 and on common bread recipe, we roughly estimated that yeast might increased initial choline content of white and whole-grain wheat flours by respectively ∼+61 and ∼+20%. Values of +95 and +25% were calculated for respectively white and whole wheat breads.
Methionine. While OP had no significant effect on methionine density, SP significantly reduced methionine density (median change of −11%).
Concerning thermal treatments, except spinach and wheat germ, they led to either no change or to decreased content. As for choline, drying surprisingly led to content reductions whereas toasting and baking had no marked effects: we found no relevant explanation for this. For thermal treatments in the presence of water, the increased methionine content in spinach following boiling also remains unexplained compared to other boiled vegetables. However, results tended to reveal that canning degrades methionine in beetroot, peach and pear but not in common bean. This effect was supported by reductions also observed for canned tomato products. Maillard browning reaction, i.e. reduction of a sugar with methionine, is the most cited explanation for methionine degradation,50 but methionine may be also simply degraded by thermal treatments or oxidized by hydroperoxides to its sulphoxides.50 More generally, the use of food model systems showed that free methionine losses during elevated temperature processing were influenced by protein, sugar and water activity, suggesting a food matrix-dependent effect.51 Interactions among food components may therefore explain why methionine in cereal products and common bean appeared more resistant to thermal treatments than in vegetables and fruits, maybe since included within a more structured and insoluble protein network than in fruits and vegetables.
Concerning refining, median for content change was of −30%. Except defatted soybean flour and apple juice, there was somewhat tendency to content reduction that probably resulted from thermal treatments accompanying refining process in oven-heated French fries, potato chips, tomato-derived products and fruit juice packaging.
Concerning fermentations, median for content change was of −50%. Except breads (no changes), alcoholic (−94% for wine and −100% for beer) and acidic (−63% for pickles and −36% for sauerkraut) fermentations importantly degraded methionine. Even by taking maximum value found in literature for wine methionine content, i.e. ∼0.54 mg/100 mL, calculated from Barrado et al.52 for Spanish Rueda-type wine (n = 10 values), methionine reduction remains high ∼−91%. While microflora may be responsible for methionine degradation into beer and wine, acidic fermentation may have partly oxidized methionine. Otherwise, increased free methionine contents were measured in the water soluble fraction of the batter of black gram and rice blend (idli) after 20 h fermentation.53 Similarly to idli water soluble fraction, release of free methionine may have also occurred in sauerkraut and pickle juices upon fermentation leading to reduced content within the food matrix. However, sorghum flour fermented for producing bread (Sudanese kisra) led to methionine enrichment;54 and in Cheonggukjang, a traditional Korean fermented soy food, methionine content first decreased up to 20 h fermentation by ∼−80% then increased after 50 h fermentation by ∼+70% compared to the 0 hour-time.55 In this latter case, methionine would be produced by Bacillus subtilis following nutrient degradation.55
Based on our estimations for methionine content changes, we hypothesized that the effect of processing on methionine contents tended to differ according to food group more than with process type even if tendencies were observed for canning and refining. Accordingly, we calculated medians of +5, +8, −27 and −63% for respectively C, L, V and F groups (results not shown). Cereal and legume methionine contents appeared therefore to be not markedly affected by processing contrary to fruits and vegetables with one exception for each food group that were beer (−100%, C group), soybean milk (−44%, L group), boiled spinach (+115%, V group) and apple juice (+20%, F group).
Myo-inositol. Myo-inositol belongs to the cyclitol family and is one of 9 isomers of inositol, the only form up today with a demonstrated lipotropic effect.56 It is present in PBF as free form or conjugated with phosphate (e.g. phytate) or glycosyl (e.g. galactinol and di-glycosylated myo-inositol) groups. Since it is potentially readily available within the digestive tract, only myo-inositol moieties derived from soluble free myo-inositol and glycosylated myo-inositol were considered in what we have called the PAI fraction. This fraction may also include myo-inositol moieties derived from phosphatidylinositol (i.e. lipid-bound myo-inositol) when PAI content was deduced from total and IP contents. For other cases, since data about PBF lipid-bound myo-inositol content are almost non-existent, PAI content was probably under-estimated.
Due to the way PAI contents were estimated (i.e. by subtracting IP to total myo-inositol content in most cases)1 and due to the low number of products for statistical analyses, effect of processing on PAI density was more difficult to grasp than for other lipotropes and results must be therefore considered cautiously. The low number of product indeed explain why, despite the tendency to reduced PAI densities following processing, effect was not significant for SP (median = −33%, n = 11 pairs of products). However, effect of OP was closed to significativity (p = 0.057, 30 raw vs 20 processed products). Results have to be therefore interpreted product by product.
Concerning thermal treatments without water, as for choline (−55%) and methionine (−46%), PAI loss of −75% for grape upon drying remains difficult to explain. One hypothesis may be linked to the way grapes were dried, i.e. in an oven and not with natural sun temperature. This would mean a high sensitivity of myo-inositol to increased temperature: other calculated changes would tend not to contradict this hypothesis. Otherwise, total myo-inositol content value used for calculating PAI content may have been under-estimated due to the fact that raisins used in Clements and Darnell database57 may have higher water content than that given for raisins in USDA database, i.e. 15.4%;44 however, by considering, for example, a 25% water content for raisins of Clements and Darnell database instead of 15.4%, content changes forwarded from −75% to only −67%. Another plausible explanation may be found in PAI content variability according to grape variety, e.g. according to color: thus, from literature data, we estimated that white wines might have higher mean myo-inositol content (means of 95 mg/100 mL, n = 8 wines) than red wines (means of 73 mg/100 mL, n = 4 wines) and we found a high range of ∼10–248 mg myo-inositol/100 mL (calculated from references in Supplementary Material 21). Heterogeneity of PAI content changes following canning of beetroot (−28%) and common bean (+305%), and boiling of cabbage (−81%) suggested both degradation of IP fraction into free myo-inositol, then leaching of it into boiling water. In canned bean, IP degradation would be sufficiently important to overcome PAI losses. This explanation was partly supported by the data we have collected for IP6 (phytate)-IP1 content of raw and canned common beans (see Supplementary Table 10): indeed, IP6, IP5, IP4 and IP3 percentages forwarded from respectively 80.4, 10.7, 1.8, 0.2 to 71.6, 20.0, 5.7 and 1.4% following canning unravelling IP6 degradation. Canning was already reported to reduce phytate content in beans.58 In beetroot and cabbage, initial IP content is largely lower (respectively ∼8 and ∼45 mg/100 g on dwb) than in common bean (∼325 mg/100 g) and initial PAI contents (respectively ∼65 and ∼398 mg/100 g) were higher than IP content, contrary to common bean whose PAI content is 76 mg/100 g (calculated from Supplementary Table 1). Our hypothesis was therefore that in beetroot and cabbage, PAI losses in water have exceeded IP degradation.
Concerning refining treatments, transformation of orange into carbonated soda and of tomato into ketchup and canned condensed tomato soup importantly reduced PAI content (<−85%), PAI being possibly removed with the fiber fraction. Finally, all fruit juices have PAI content ∼23% higher than corresponding raw fruits: however, this result was artefactual and remains to be confirmed since raw fruit PAI content have been deduced from juice PAI content59 assuming that producing fruit juices mainly removes fibre fraction and considering that PAI fraction was released into juice.1
Concerning fermentations, only one change could have been estimated for wines (+573%). Higher PAI content of wine has been already reported.60 However, such a high increase may be not representative of reality: indeed, for grape, PAI content was derived from only one Spanish grape variety purchased at local market,59 while for wines, numerous data have been collected to calculate the aggregated value of 73 mg/100 mL derived from both red and white wines and from several brands, i.e. French, Spanish and German.1 The initial range was 10.2–248.3 mg/100 mL which will respectively give −7% and +2714% PAI change on dwb. One may also not excluded that myo-inositol would be produced upon grape fermentation via, for example, IP6 degradation.
Effect of processing on plant-based food lipotropic capacity
Considering each lipotrope separately for each process is not an easy way to study the effect of processing on overall PBF lipotropic potential. In a previous study, we have shown that the only sum of lipotrope densities is not nutritionally relevant, and that the LC (as defined in the Materials and methods section), that takes into account at equal weight each LD whatever its absolute value, be it 0.01 or 10 mg/100 kcal, may be an interesting and simple nutritional index for expressing overall PBF lipotropic potential,1 and consequently evaluating effect of processing.Processed PBF mean LC was 24% significantly lower than raw PBF mean LC following OP with 19% higher mean ranking and around 2-fold higher median. Based on the 41 pairs of raw vs processed products and on all LD except that of PAI, LC median and mean change was of −19% (p = 0.0004). Lipotrope capacity change following fermentations (−5%, effect not significant) was less marked (median change of −5%) than following refining (−33%, p = 0.011), then thermal treatments (−16%, p = 0.013), either with (−11%, p = 0.080) or without water (−29%, p = 0.063). Only white wheat flour, soybean flour, beetroot and cabbage had their LC markedly increased following respectively baking (+115%), defatting (+83%), canning (+38%) and fermentation (+30%). Finally, among the 5 food groups, legumes (raw common bean and soybean) appeared to be the least affected by processing (median = −4%).
Interestingly, canned beetroot and canned common bean had a higher LC than raw products, emphasizing that canning should not always be considered as a negative process towards nutritional value of PBF. A similar conclusion has been previously reached with canned fruits and vegetables for their vitamins C and B and phenolic compound contents.40 On the contrary, boiling appeared more drastic towards lipotropic potential of vegetables as regards with the almost 2-fold lower LC of boiled cabbage compared to raw cabbage (38 vs 75%).
Refining undoubtedly reduced lipotropic potential of PBF: for example, processing tomato decreased its LC from 70 (raw tomato) to 20 (tomato soup) and 13 (ketchup). Highly refined PBF that are orange soda, chips and dried flaked coconut meat exhibited very low LC (≤13%). Otherwise, whole wheat bread (LC = 39%) had a higher LC than white breads, either common (LC = 18%) or French/Vienna (LC = 27%), which should incite to avoid refining wheat flour too importantly. Conversely, transformation of fruits into juice appeared to have no negative effect on the overall lipotropic potential.
An important issue was the fermentation of wheat flours into breads. Although we have no data for the PAI content of whole-grain and white wheat flours, we may reasonably suppose that it was not very high. In addition, those of breads were closed to 0. Thus, excluding PAI density, LC of whole-grain and white wheat flours were respectively of 45 and 10%, and those of corresponding breads were respectively 45 and 21%. These results emphasized both the necessity of using less refined wheat flours, and that despite refining white wheat flour baking increases its LC by ∼2-fold. It was probably due to baker's yeast microflora (i.e. Saccharomyces cerevisiae) during the fermentative process that may increase phytonutrient content as has been shown with fermentation of wholemeal rye flour61 and tempeh (made from fermented cooked soya beans),62 or more specifically with riboflavin content in white bread vs white flour.63 Both our results and those of literature therefore suggest that fermentations may enhance lipotropic potential of initial raw PBF. One exception was the important decreased of LC when comparing raw peeled cucumber with pickles (from 70 to 24% based on 7 LD). The low lipotropic potential of pickles might be simply due to lower degree of maturity relative to cucumbers used for pickling, i.e. an initial lower content of lipotropes than in raw peeled mature cucumbers.
Our results showed that canned beetroot, brewed tea, boiled green beans, orange juice, canned common bean, whole wheat bread and boiled cabbage are relevant processed PBF for their LC. In addition, considering coffee PAI density ∼0 (sugar content given by USDA database is 0), coffee LC would be of ∼470% (results not shown), more than 2-fold that of tea. However, due to their very low caloric content of around 1 kcal/100 mL, their high lipotropic potential should be especially considered for regular or high tea and/or coffee drinkers. Accordingly, baseline high-coffee consumption (≥3 cups/day) was recently shown to be associated with less severe steatosis on biopsy in patients with advanced hepatitis C-related liver disease (19.1% patients with stetaosis grade 0 for high-coffee drinkers vs 12.2% for non-coffee drinkers, p for trend = 0.047).31 Conversely, the low LC of some fruit-derived beverages like wine (LC = 14), beers (LC = 28%), sodas (LC = 1) and non citrus fruit juices (LC = 13 for apple and 8 for grape) indirectly support results from the few observational studies showing: (1) that excess sugar-sweetened soft drinks (notably Coca Cola and fruit juices containing caramel) consumption (>500 cm3/day) was largely more prevalent in patients diagnosed with fatty liver (80%) than in healthy controls (17%);32 and also (2) that high alcohol consumption was directly correlated with diffuse steatosis in non-cirrhotic patients.34 Conversely, moderate alcohol consumption in NAFLD patients was associated with “lower odds of diagnostic features for nonalcoholic steatohepatitis” compared to non-alcohol drinkers.35 However, steatosis development based on regular high alcohol or high refined sugar consumption is based on different impaired physiological mechanisms.2 Yet, beers and wines are probably among alcoholic beverages that have the highest contents in phytochemicals suggesting very low LC for other alcohols that generally contain more energy and less phytonutrients.
These few observational studies might give a first rough indication about LC thresholds above which processed PBF may have a significant lipotropic effect in humans and below which processed PBF may be steatogen when regularly and importantly consumed. Thus, energy-dense beverages (soft drinks and alcoholic beverages) with LC below 30% should not be consumed in excess; while beverages with LC above 100% like coffee would be rather protective in regular and/or high drinkers. The example of alcohol would also tend to show that not only the LC value is important but also amount of product consumed, what might be called in fine the lipotropic load.
Conclusions and perspectives
Processing globally reduced the lipotropic potential of plant-based foods by ∼20%
Very globally, without process distinction, processing would decrease lipotropic potential of PBF by ∼−20%. From a process perspective, our results first clearly showed that refined products have a low lipotropic potential, as regards with either LD or LC. Secondly, processing tended to degrade or release micronutrient lipotropes (magnesium and B vitamins) more than other 4 main lipotropes, especially compared to betaine, choline and methionine. And among B vitamin, folate was more frequently negatively affected than pantothenic acid, then niacin. Among food groups, legume LC was the least affected by processing. Practically, high degrees of PBF refining should be therefore avoided.Fermentation may increase lipotropic potential of plant-based foods
Otherwise, results also showed that fermentative processes may be favourable to LD or at least have no effect on it as shown with breads, sauerkraut and wine. Indeed, fermentation was previously shown to increase density in some phytonutrients, resulting in a potential enhanced bioactivity of cereals.61,64,65 More generally, the literature tends to show that fermentative processes are above all able to release bound compounds into their free form – as, for example, with TPC,66 niacin67 and methionine68 – which can be of interest to increase their bioavailability and, in the end, their lipotropic effect since several PBF lipotropes are present in both bound and free forms. Therefore, fermentative processes appear not sufficiently valorized and applied to PBF in Western countries.Canning may increase content in main lipotropes
By considering LC changes, canned beetroot and common bean had higher LC than raw products. Canning is therefore not so drastic a process as it appears at first view: it increased betaine content in common bean, beetroot and pear, choline content in common bean and beetroot, and PAI content in common bean; and in products with initial high phytate content, it may degrade myo-inositol phosphates in free myo-inositol. Our results would therefore tend to emphasize that canning is quite favourable to main LD and would reinforce the conclusion drawn by Rickman et al. that canned foods should not be always regarded “as less nutritious than fresh or frozen products”.40 However, further studies are needed to confirm or not the potential nutritional benefit of PBF canning.Other processes
Some specific processed PBF were emphasized as having their lipotropic potential only little affected following processing. Thus, defatted soybean flour that ranked high for total LD among PBF,1 had 7 LD increased compared to raw soybean, making this product nutritionally relevant for its lipotropic potential: indeed, excluding PAI density, its LC was almost 2-fold that of raw soybean. To go further, considering or not PAI density, transformation of soybean into tofu and soybean milk, of grape into juice, of raw peanut into butter, nixtamalization of whole-grain cornmeal into masa, cooking roasted buckwheat groats, toasting French/Vienna bread, boiling common bean, boiling asparagus, broccoli and carrot, and baking potato with skin almost did not affect LC (−10% ≤ % LC change ≤ +10%).Special attention has also to be paid to the brewed beverages of tea and coffee for their high LC of respectively 196 and 469% when based on 8 LD, notably as regards with the recent study of Freedman et al. showing less severe steatosis in high-coffee – but not high-tea – drinkers.31 However, as discussed by the authors, the question remains which specific coffee compounds are involved in this beneficial effect. Since both brewed coffee and tea are both rich in TPC, especially on a dwb with respectively 14.9 and 35.0 g/100 g (Supplementary Table 1), but also in caffeine (>6 g/100 g dwb, USDA database), the lipotropic effect may be therefore ascribed to other compounds.
Finally, besides these particular processes, one may also optimize processing to release free myo-inositol from phytate in cereal products like barley.69 Notably, extrusion process is known to have a significant effect upon phytate dephosphorylation.70 Besides, germination is also a natural process able to increase phytonutrient density as shown with TPC content of 12 germinated edible seed species71 and with free amino acids, α-tocopherol, γ-oryzanol, thiamine, niacin and pyridoxine in germinated rough rice.72
Perspectives
Finally, we must bear in mind that processed PBF may also contain significant amounts – when summed – of other phytonutrients with a potential lipotropic effect such as organosulfur compounds, n-3 polyunsaturated fatty acids, acetic acid, carnitine, lignans, flavonoids, stilbenes, curcumin, saponins, fibre (lignins included), oligosaccharides and/or resistant starch.2 As one goes along with completion of databases for these compounds, LC definition will have to include them and perhaps a new reference food other than raw asparagus would have to be re-defined. While waiting for this, results of this study showed that this simple index was quite useful to estimate effect of processing on lipotropic potential of PBF. More particularly, it can be used as a simple tool to rapidly characterize the lipotropic potential of foods as, for example, those produced by agro-food industry, and our results provide some early and interesting information to optimize through processes the PBF LC. Indeed, this will be helpful to produce lipotrope-dense PBF, notably for subjects with mild steatosis (5–33% of the liver) or prone to develop steatosis (in preventive nutrition), as this is done for low-glycaemic index foods recommend for type 2 diabetic subjects.It however remains that human intervention studies are strongly needed to correlate LC values of processed PBF with lipotropic effects in humans, and also to define the LC threshold above which significant physiological effect may be reached to prevent steatosis development in its initial stage, notably among the obese, diabetic and/or alcoholic population. In the end, based on each food LC, one may calculate the diet LC, which appears still more relevant since chronic disease development is related to the way of eating, not solely to consumption of isolated foods.
Abbreviations
| ABF | Animal-based foods |
| BeChIMe | Sum of betaine, choline, myo-inositol and methionine |
| dwb | Dry-weight basis |
| HC | Hierarchical classification |
| IP | Myo-inositol phosphate |
| IP6 | Phytate or myo-inositol hexakisphosphate |
| LC | Lipotrope capacity |
| LD | Lipotrope density |
| OP | Overall un-specific processing |
| PBF | Plant-based foods |
| PCA | Principal component analysis |
| PAI | Potentially available myo-inositol (included myo-inositol moieties derived from soluble free myo-inositol and glycosylated myo-inositol) |
| RS | Resistant starch |
| SP | Specific processes |
| TPC | Total phenolic compounds |
| USDA | United State Department of Agriculture |
Acknowledgements
Sabine Rossi and Françoise Barré are gratefully acknowledged for their precious assistance in collecting references (INRA-SDAR, F-63122 Saint Genès Champanelle, France).References
- A. Fardet, J.-F. Martin and J. M. Chardigny, J. Food Comp. Anal., 2011 DOI:10.1016/j.jfca.2011.1003.1013.
- A. Fardet and J. M. Chardigny, Crit. Rev. Food Sci. Nutr., 2011 DOI:10.1080/10408398.10402010.10549596.
- J. F. Silverman, W. J. Pories and J. F. Caro, Pathology Annual, 1989, 24, 275–302 Search PubMed.
- P. Angulo, N. Engl. J. Med., 2002, 346, 1221–1231 CrossRef CAS.
- C. S. Lieber, Clin. Chim. Acta, 1997, 257, 59–84 CrossRef CAS.
- J. F. Doherty, E. J. Adam, G. E. Griffin and M. H. Golden, Arch. Dis. Child., 1992, 67, 1348–1352 CrossRef CAS.
- Z. T. Bloomgarden, Diabetes Care, 2005, 28, 1518–1523 CrossRef.
- G. Targher and G. Arcaro, Atherosclerosis, 2007, 191, 235–240 CrossRef CAS.
- M. S. Ascha, I. A. Hanouneh, R. Lopez, T. A. R. Tamimi, A. F. Feldstein and N. N. Zein, Hepatology, 2010, 51, 1972–1978 CrossRef.
- G. Targher, L. Bertolini, M. Chonchol, S. Rodella, G. Zoppini, G. Lippi, L. Zenari and E. Bonora, Diabetologia, 2010, 53, 1341–1348 CrossRef CAS.
- H. J. Kim, H. J. Kim, K. E. Lee, D. J. Kim, S. K. Kim, C. W. Ahn, S.-K. Lim, K. R. Kim, H. C. Lee, K. B. Huh and B. S. Cha, Arch. Intern. Med., 2004, 164, 2169–2175 CrossRef.
- C. H. Best, Nature, 1935, 135, 821–822 CrossRef CAS.
- L. A. Adams, P. Angulo and K. D. Lindor, Can. Med. Assoc. J., 2005, 172, 899–905 CrossRef.
- A. H. Russakoff and H. Blumberg, Ann. Intern. Med., 1944, 21, 848–862 CAS.
- J. A. Colson and C. Gallay, Toulouse Méd., 1964, 65, 207–229 Search PubMed.
- H. Warembourg and M. Bertrand, Lille Méd., 1964, 37, 285–289 Search PubMed.
- G. Nadeau, Y. Rouleau and J. Delage, Laval Méd., 1954, 19, 52–58 Search PubMed.
- L. Spadaro, O. Magliocco, D. Spampinato, S. Piro, C. Oliveri, C. Alagona, G. Papa, A. M. Rabuazzo and F. Purrello, Dig. Liver Dis., 2008, 40, 194–199 CrossRef CAS.
- F. Zhu, S. Liu, X. Chen, Z. Huang and D. Zhang, World J. Gastroenterol., 2008, 14, 6395–6400 Search PubMed.
- A. J. Cussons, G. F. Watts, T. A. Mori and B. G. A. Stuckey, J. Clin. Endocrinol. Metab., 2009, 94, 3842–3848 CrossRef CAS.
- M. Capanni, F. Calella, M. R. Biagini, S. Genise, L. Raimondi, G. Bedogni, G. Svegliati-Baroni, F. Sofi, S. Milani, R. Abbate, C. Surrenti and A. Casini, Aliment. Pharmacol. Ther., 2006, 23, 1143–1151 CrossRef CAS.
- F. Sofi, I. Giangrandi, F. Cesari, I. Corsani, R. Abbate, G. F. Gensini and A. Casini, Int. J. Food Sci. Nutr., 2010, 61, 792–802 CrossRef CAS.
- N. Tanaka, K. Sano, A. Horiuchi, E. Tanaka, K. Kiyosawa and T. Aoyama, J. Clin. Gastroenterol., 2008, 42, 413–418 CrossRef CAS.
- A. Hatzitolios, C. Savopoulos, G. Lazaraki, I. Sidiropoulos, P. Haritanti, A. Lefkopoulos, G. Karagiannopoulou, V. Tzioufa and K. Dimitrios, Indian J. Gastroenterol., 2004, 23, 131–134 Search PubMed.
- M. F. Abdelmalek, P. Angulo, R. A. Jorgensen, P. B. Sylvestre and K. D. Lindor, Am. J. Gastroenterol., 2001, 96, 2711–2717 CrossRef CAS.
- S. Mukherjee, T. Bernard, D. Schafer, A. Barak, M. Sorrell and D. Tuma, J. Hepatol., 2005, 42, 610 CrossRef.
- H. Shapiro, M. Tehilla, J. Attal-Singer, R. Bruck, R. Luzzatti and P. Singer, Clin. Nutr., 2011, 30, 6–19 CrossRef CAS.
- M. Malaguarnera, M. P. Gargante, C. Russo, T. Antic, M. Vacante, M. Malaguarnera, T. Avitabile, G. Li Volti and F. Galvano, Am. J. Gastroenterol., 2010, 105, 1338–1345 CrossRef CAS.
- D. Mu, J. X. Yu and D. Li, J. Norman Bethune Univ. Med. Sci., 1997, 23, 528–530 Search PubMed.
- L. Abenavoli, G. Aviello, R. Capasso, N. Milic and F. Capasso, Hepatitis Monthly, 2011, 11, 173–177 Search PubMed.
- N. D. Freedman, J. E. Everhart, K. L. Lindsay, M. G. Ghany, T. M. Curto, M. L. Shiffman, W. M. Lee, A. S. Lok, A. M. Di Bisceglie, H. L. Bonkovsky, J. C. Hoefs, J. L. Dienstag, C. Morishima, C. C. Abnet, R. Sinha and H.-C. T. Grp, Hepatology, 2009, 50, 1360–1369 CrossRef CAS.
- A. Abid, O. Taha, W. Nseir, R. Farah, M. Grosovski and N. Assy, J. Hepatol., 2009, 51, 918–924 CrossRef CAS.
- S. Thuy, R. Ladurner, V. Volynets, S. Wagner, S. Strahl, A. Konigsrainer, K. P. Maier, S. C. Bischoff and I. Bergheim, J. Nutr., 2008, 138, 1452–1455 CAS.
- L. A. Kondili, G. Taliani, G. Cerga, M. E. Tosti, A. Babameto and B. Resuli, Eur. J. Gastroenterol. Hepatol., 2005, 17, 155–159 CrossRef CAS.
- W. Dunn, E. M. Brunt, A. J. Sanyal, A. J. McCullough, A. Unalp, J. Tonascia and J. B. Schwimmer, J. Hepatol., 2009, 50, 89 CrossRef.
- J. L. Kuk, L. E. Davidson, R. Hudson, K. Kilpatrick, K. Bacskai and R. Ross, Appl. Physiol., Nutr., Metab., 2008, 33, 239–245 CrossRef CAS.
- N. A. van Herpen, V. B. Schrauwen-Hinderling, G. Schaart, R. P. Mensink and P. Schrauwen, J. Clin. Endocrinol. Metab., 2011, 96, E691–E695 CrossRef CAS.
- J. D. Browning, J. A. Baker, T. Rogers, J. Davis, S. Satapati and S. C. Burgess, Am. J. Clin. Nutr., 2011 Search PubMed.
- M. Lazo, S. F. Solga, A. Horska, S. Bonekamp, A. M. Diehl, F. L. Brancati, L. E. Wagenknecht, F. X. Pi-Sunyer, S. E. Kahn and J. M. Clark, Diabetes Care, 2010, 33, 2156–2163 CrossRef.
- J. C. Rickman, D. M. Barrett and C. M. Bruhn, J. Sci. Food Agric., 2007, 87, 930–944 CrossRef CAS.
- J. C. Rickman, C. M. Bruhn and D. M. Barrett, J. Sci. Food Agric., 2007, 87, 1185–1196 CrossRef CAS.
- F. J. de Zwart, S. Slow, R. J. Payne, M. Lever, P. M. George, J. A. Gerrard and S. T. Chambers, Food Chem., 2003, 83, 197–204 CrossRef CAS.
- K. Y. Patterson, S. A. Bhagwat, J. R. Williams, J. C. Howe, J. M. Holden, in collaboration with S. H. Zeisel, K. A. Dacosta and M.-H. Mar, USDA Database for the Choline Content of Common Foods, U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory, 2008, http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choln02.pdf Search PubMed.
- U.S. Department of Agriculture, Agricultural Research Service, Nutrient Data Laboratory, USDA National Nutrient Database for Standard Reference, 2005, http://www.nal.usda.gov/fnic/foodcomp/Data/SR18/reports/sr18page.htm Search PubMed.
- S. J. Bruce, P. A. Guy, S. Rezzi and A. B. Ross, J. Agric. Food Chem., 2010, 58, 2055–2061 CrossRef CAS.
- R. Likes, R. L. Madl, S. H. Zeisel and S. A. S. Craig, J. Cereal Sci., 2007, 46, 93–95 CrossRef CAS.
- S. F. Graham, J. H. Hollis, M. Migaud and R. A. Browne, J. Agric. Food Chem., 2009, 57, 1948–1951 CrossRef CAS.
- M.-H. Mar and S. H. Zeisel, Med. Hypotheses, 1999, 53, 383–385 CrossRef CAS.
- F. A. Murdock and M. L. Fields, J. Food Sci., 1984, 49, 373–375 CrossRef CAS.
- J. Mauron, J. Nutr. Sci. Vitaminol., 1990, 36, S57–69 Search PubMed.
- J. C. Wolf, D. R. Thompson, J. J. Warthesen and G. A. Reineccius, J. Food Sci., 1981, 46, 1074–1078 CrossRef CAS.
- E. Barrado, J. A. Rodriguez and Y. Castrillejo, Talanta, 2009, 78, 672–675 CrossRef CAS.
- V. W. Padhye and D. K. Salunkhe, J. Food Biochem., 1978, 2, 327–347 CrossRef.
- A. H. E. Tinay, A. M. A. Gadir and M. E. Hidai, J. Sci. Food Agric., 1979, 30, 859–863 CrossRef.
- M. K. Park, I. H. Cho, S. Lee, H.-K. Choi, D.-Y. Kwon and Y.-S. Kim, Food Chem., 2010, 122, 1313–1319 CrossRef CAS.
- Y. Okazaki, T. Setoguchi and T. Katayama, Biosci., Biotechnol., Biochem., 2006, 70, 2766–2770 CrossRef CAS.
- R. Clements Jr and B. Darnell, Am. J. Clin. Nutr., 1980, 33, 1954–1967 CAS.
- M. M. Tabekhia and B. S. Luh, J. Food Sci., 1980, 45, 406–408 CrossRef CAS.
- M. L. Sanz, M. Villamiel and I. Martinez-Castro, Food Chem., 2004, 87, 325–328 CrossRef CAS.
- A. Ournac, Ann. Nutr. Alim., 1970, 24 Search PubMed.
- K. Katina, K.-H. Liukkonen, A. Kaukovirta-Norja, H. Adlercreutz, S.-M. Heinonen, A.-M. Lampi, J.-M. Pihlava and K. Poutanen, J. Cereal Sci., 2007, 46, 348–355 CrossRef CAS.
- R. Djurtoft and J. P. Nielsen, J. Plant Foods, 1984, 5, 135–141 Search PubMed.
- F. Batifoulier, M.-A. Verny, E. Chanliaud, C. Rémésy and C. Demigné, Eur. J. Agron., 2006, 25, 163–169 CrossRef CAS.
- K. Katina, A. Laitila, R. Juvonen, K.-H. Liukkonen, S. Kariluoto, V. Piironen, R. Landberg, P. Åman and K. Poutanen, Food Microbiol., 2007, 24, 175–186 CrossRef CAS.
- J. K. Chavan and S. S. Kadam, Crit. Rev. Food Sci. Nutr., 1989, 28, 349–400 CrossRef CAS.
- T. M. Dordevic, S. S. Siler-Marinkovic and S. I. Dimitrijevic-Brankovic, Food Chem., 2010, 119, 957–963 CrossRef CAS.
- N. Dhankher and B. M. Chauhan, Int. J. Food Sci. Technol., 1987, 22, 173–176 CAS.
- C. G. Rizzello, L. Nionelli, R. Coda, M. De Angelis and M. Gobbetti, Food Chem., 2010, 119, 1079–1089 CrossRef CAS.
- E.-L. Bergman, K. Fredlund, P. Reinikainen and A.-S. Sandberg, J. Cereal Sci., 1999, 29, 261–272 CrossRef CAS.
- A. Proskova, Czech J. Food Sci., 1998, 16, 215–220 Search PubMed.
- B. A. Cevallos-Casals and L. Cisneros-Zevallos, Food Chem., 2010, 119, 1485–1490 CrossRef CAS.
- A. Moongngarm and N. Saetung, Food Chem., 2010, 122, 782–788 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1fo10041f |
| This journal is © The Royal Society of Chemistry 2011 |
