Protective actions of microalgae against endogenous and exogenous advanced glycation endproducts (AGEs) in human retinal pigment epithelial cells
Zheng
Sun
a,
Jin
Liu
a,
Xiaohui
Zeng
a,
Jieqiong
Huangfu
a,
Yue
Jiang
b,
Mingfu
Wang
*a and
Feng
Chen
*ac
aSchool of Biological Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China. E-mail: mfwang@hku.hk; Fax: +852 22990340; Tel: +852 22990338
bDepartment of Biology, Hong Kong Baptist University, Kowloon Tong, Hong Kong, P. R. China
cInstitute for Food & Bioresource Engineering, College of Engineering, Peking University, Beijing, P. R. China. E-mail: sfchen@hku.hk; Fax: +852 22990311; Tel: +852 22990309
First published on 21st April 2011
Abstract
The formation and accumulation of advanced glycation endproducts (AGEs) is a key pathophysiological process involved in various diabetic complications such as diabetic retinopathy. In the present study, for the first time, protective effects of three microalgal strains, including their extracts and active compounds, against both endogenous and exogenous AGEs in cell-based models were investigated. Results showed that in cultured human-derived retinal pigment epithelial ARPE-19 cells, the extract of Chlorella zofingiensis and its nutritional ingredient astaxanthin exhibited significant inhibitory effects on the formation of endogenous Nε-carboxymethyllysine (CML), a key AGE representative, through the suppression of intracellular oxidative stress. On the other hand, extracts of Chlorella zofingiensis, Chlorella protothecoides and Nitzschia laevis as well as their nutritional ingredients, namely astaxanthin, lutein and eicosapentaenoic acid (EPA), attenuated the deleterious effects induced by exogenous AGEs, such as cell proliferation and mRNA upregulation of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP)-2, which are critical steps involved in the pathogenesis of diabetic retinopathy. These results suggested the positive roles of astaxanthin, lutein and EPA in controlling the development of diabetes. These microalgae, therefore, might be regarded as beneficial foods and preventive agent choices for patients with diabetic retinopathy.
1. Introduction
Diabetes mellitus is a significant public health threat that can be found in almost every population in the world. Individuals affected by diabetes are prone to many long-term complications such as retinopathy, cataract, neuropathy and nephropathy.1 Diabetic retinopathy is the most serious diabetic eye disease and a leading cause of adult blindness in the Western countries. The prevalence rate of diabetic retinopathy is as high as 80% for patients with insulin-dependent diabetes of 10 years' duration.2 Under hyperglycemic conditions, the formation and accumulation of advanced glycation endproducts (AGEs) is believed to be an important factor associated with diabetic retinopathy. AGEs are generated from the non-enzymatic glycation (or Maillard reaction) between reducing sugars and amino groups of proteins.3 When the reaction occurs in living organisms, these adducts can generate cross-links between key molecules, leading to their structural modifications and functional impairments.4 Numerous studies have reported the increased concentrations of AGE residues at sites of diabetic retinopathy. Levels of AGEs in serum,5,6 skin7 or cornea8 were found to be correlated with the onset or clinical grade of diabetic retinopathy. In addition to those endogenously formed, AGEs can be also introduced from exogenous sources, acting as mediators to induce pathophysiological events of diabetic retinopathy. A growing body of evidence has shown that in many retinal cell types, the experimental exposure to AGE-modified proteins or AGEs precursors, e.g. methylglyoxal, may result in morphological changes and tissue damages, due to the disruption of key signaling pathways involved in cellular functions.9,10,11To solve these problems, in recent years a number of herbal medicines and plant extracts have been evaluated for their inhibitory effects on the glycation process. Such research may provide a novel preventive and therapeutic approach for diabetic retinopathy, because natural products may have few side effects and are safe for human consumption compared with synthetic compounds.12 Previous work from our laboratory has demonstrated the antiglycative capacities of some microalgal samples in cell-free systems. The ethyl acetate extract of green alga Chlorella zofingiensis significantly suppressed the glycation cascade in vitro, whilst astaxanthin, a red ketocarotenoid possessing potent anti-oxidative capacity was found to be a major effective ingredient.13 A few other microalgal strains, such as the green alga Chlorella protothecoides and the diatom Nitzschia laevis also exhibited similar effects, where the major effective ingredients were lutein and eicosapentaenoic acid (EPA), respectively.14 In the present study, we used a cell-based model to further investigate the intracellular antiglycoxidative actions of these microalgal extracts and nutritional components. The aims of the present study were to examine whether (1) the C. zofingiensis extract and astaxanthin could block the formation of endogenous AGEs; and (2) extracts of C. zofingiensis, C. protothecoides and N. laevis as well as their nutritional compounds, namely astaxanthin, lutein and EPA could intervene against the deleterious effects induced by exogenous AGEs.
2. Materials and methods
2.1 Reagents and chemicals
Dulbecco's modified Eagle medium/nutrient mixture F-12 (DMEM/F-12) (containing 5.6 mM glucose), Trizol reagent, penicillin-streptomycin solution (100X), Trypsin-EDTA (0.05%), SuperScript III First-Strand Synthesis System and SYBR Green PCR Master Mix were all purchased from Invitrogen Corporation (Carlsbad, CA, USA). Fetal bovine serum (FBS) was purchased from the American Type Culture Collection (Rockville, MD, USA). Limulus amebocyte lysate (LAL) endotoxin test kit was purchased from Genscript Corporation (Piscataway, NJ, USA). Nε-Carboxymethyllysine (CML) antibody and rabbit IgG secondary antibody were purchased from Abcam Company (Cambridge, UK). Lutein was purchased from Winherb Medical Science Company (Shanghai, China). Astaxanthin, EPA, BSA, sodium azide thiobarbituric acid, trichloroacetic acid, naphthyl-ethylenediamine dihydrochloride, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and p-nitrophenyl phosphate substrate solution were purchased from Sigma-Aldrich Company (St. Louis, MO, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was purchased from USB Corporation (Cleveland, OH, USA). All analytical and HPLC grade solvents used were obtained from BDH Laboratory Supplies (Pool, UK).2.2 Algal strains and sample preparations
Green algae C. protothecoides (CSIRO 41) and C. zofingiensis (ATCC 30412), and diatom N. laevis (UTEX 2047) were cultured in our laboratory and algal cells were harvested at the stationary growth phase. After freeze-drying for 24 h, the algal biomass (0.1 g) was extracted with ethyl acetate (8 mL) at room temperature. The tube containing extracts was centrifuged at 4500 g for 10 min and the supernatant was recovered. The extraction was repeated and two supernatants were combined. Samples were purged to dryness using nitrogen and stored at 0 °C prior to use.2.3 Carotenoids analysis
Carotenoid contents of C. zofingiensis and C. protothecoides extracts were analyzed and compared using the HPLC system as described by Baroli et al. with minor modification.15 The HPLC system was equipped with a Waters 2695 separations module and a Waters 2996 photodiode array detector. Briefly, the dried algal biomass was re-dissolved in 1 mL of acetone. After the filtration through a 0.22 μm Millipore organic membrane, 20 μL of samples were separated by HPLC on a Waters Spherisorb 5 μm ODS2 4.6 × 250 mm analytical column (Waters, Milford, MA, USA). The flow rate was 1.2 mL min−1. Solvent A was acetonitrile : methanol : 0.1 M Tris-HCl, pH 8.0 (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14). Solvent B was methanol : ethyl acetate (68
14). Solvent B was methanol : ethyl acetate (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32). A linear gradient was performed from 100% solvent A to 100% solvent B for 15 min, followed by 20 min of solvent B. The absorption spectra were 300–700 nm. Peaks were measured at 450 nm. The carotenoids were identified and quantified using standard curves.
32). A linear gradient was performed from 100% solvent A to 100% solvent B for 15 min, followed by 20 min of solvent B. The absorption spectra were 300–700 nm. Peaks were measured at 450 nm. The carotenoids were identified and quantified using standard curves.
2.4 Fatty acid analysis
Fatty acid contents of the N. laevis extract were analyzed and compared using gas chromatography (GC) as described by Chen et al. with minor modification.16 The GC system (Hewlett-Packard, HP-6890) was equipped with an HP7673 injector, a flame-ionization detector and an HP-INNOWax™ capillary column (HP 19091N-133, 30 m × 0.25 mm × 0.25 μm). Briefly, the dried algal biomass was re-dissolved in 1 mL of toluene. After direction transmethylation with sulfuric acid in methanol, 20 μL of samples were injected. High purity nitrogen gas was used as the carrier gas. The inlet and detector temperatures were kept at 250 °C and 270 °C, respectively, and the oven temperature was programmed from 170 °C to 230 °C increasing at 1 °C min−1. The fatty acids were tentatively identified by comparing retention times against known standards. Quantities were determined by comparing peak areas with that of the internal standard (C17: 0).2.5 Cell cultures
The human retinal pigment epithelial cell line (ARPE-19) was kindly provided by Dr Amy Lo (Eye Institute, The University of Hong Kong). Cells were cultured in DMEM/F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin. The medium was changed every other day. Cultures were maintained at 37 °C in a humidified incubator saturated with 5% CO2 and 95% air. Cells were trypsinized and expanded when they reached 80% confluence. Passages 4–6 were used for all experiments. Stock solutions of the algal extracts and pure compounds (astaxanthin, lutein and EPA) were prepared fresh in DMSO. The final concentration of DMSO added to the cells was 0.5% (v/v), exhibiting no significant cytotoxicity.2.6 Measurement of reactive oxygen species (ROS)
The accumulation of intracellular ROS after exposure to high glucose was determined fluorometrically using DCFH-DA. Retinal pigment epithelial (RPE) cells were seeded at 2 × 108 cells L−1 in 6-well plates. After attachment, cells were pre-treated with different concentrations of the C. zofingiensis extract or astaxanthin for 30 min and subsequently exposed to high glucose (30 mM) for 24 h. After treatments, the medium was removed and cells were washed twice with PBS. DCFH-DA (25 μM) was added to each well and incubated for 30 min at 37 °C. The formation of DCF was determined by a fluorescence micro-plate reader (Beckman Coulter, California, CA, USA). The excitation filter was set at 485 nm and the emission filter was set at 535 nm.2.7 Measurement of nitric oxide (NO) levels
Levels of NO in RPE cells were determined by assessing the nitrite (NO2−) concentration according to the Griess assay.In brief, after the treatment with high glucose (30 mM) in the presence or absence of C. zofingiensis extract/astaxanthin for 24 h, cells were trypsinized and washed with PBS. 100 μL aliquots of cell supernatants from each well were incubated with an equal volume of the Griess reagent (1% sulfanilamide and 0.1% naphthyl-ethylenediamine dihydrochloride in 2.5% phosphoric acid). After an incubation of 10 min at room temperature, the absorbance at 540 nm was determined by a micro-plate reader (TECAN, Sunrise, Crailsheim, Germany).
2.8 Determination of lipid peroxidation
The extension of lipid peroxidation was determined according to the thiobarbituric acid reactive substances (TBARS) assay. After the treatment with high glucose (30 mM) in the presence or absence of C.zofingiensis extract/astaxanthin for 24 h, cells were rinsed with PBS and collected. Cells were lysed by freeze-thaw three times on dry-ice followed by sonication for three cycles of 30 s duration. 1 mL of the culture supernatant was subsequently incubated with 1 mL of 10% trichloroacetic acid and 2 mL of 0.67% thiobarbituric acid in boiling water for 15–30 min. After reaching room temperature, the absorbance of solutions was measured at 535 nm.2.9 Determination of the extension of CML protein adducts
The intracellular formation of CML-modified proteins in RPE cells was quantified using enzyme-linked immunosorbent assay (ELISA) as described by Sun et al. with some modification.14 In brief, after the treatment with high glucose (30 mM) in the presence or absence of C. zofingiensis extract/astaxanthin for 4 days, cells were rinsed with PBS and collected. Cells were lysed by freeze-thaw three times on dry-ice followed by sonication for three cycles of 30 s duration. Total protein concentrations were normalized using the protein assay of Bradford. Antigen was diluted to 10 μg mL−1 in 50 mM sodium carbonate buffer, pH 9.5–9.7 and loaded in a 96-well polystyrene plate 200 μL per well). The plate was coated overnight at 4 °C. After coating, wells were washed three times with PBS and blocked with gelatin for 2 h at 37 °C. Rabbit anti-CML antibody was diluted at a titer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in antibody diluent and loaded in each well. After the overnight incubation at 4 °C, wells were washed three times with NP-40. An alkaline phosphatase-conjugated antibody to rabbit IgG was added as the secondary antibody at a titer of 1
500 in antibody diluent and loaded in each well. After the overnight incubation at 4 °C, wells were washed three times with NP-40. An alkaline phosphatase-conjugated antibody to rabbit IgG was added as the secondary antibody at a titer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in antibody diluent, incubated for 2 h at 37 °C and washed with NP-40 again. Wells were developed with p-nitrophenyl phosphate substrate solution (pH 10.4). The reaction was terminated by adding 2 M sodium hydroxide. The absorbance at 405 nm was determined by a micro-plate reader (TECAN Sunrise, Crailsheim, Germany).
1000 in antibody diluent, incubated for 2 h at 37 °C and washed with NP-40 again. Wells were developed with p-nitrophenyl phosphate substrate solution (pH 10.4). The reaction was terminated by adding 2 M sodium hydroxide. The absorbance at 405 nm was determined by a micro-plate reader (TECAN Sunrise, Crailsheim, Germany).
2.10 Preparation of exogenous AGE-modified BSA
BSA (50 mg mL−1) was incubated with 0.8 M glucose in phosphate buffered saline (PBS, 0.2 M, pH 7.4) containing 0.2 g L−1 sodium azide at 37 °C for 6 weeks under sterile conditions. After the incubation, unincorporated sugars were removed by dialysis against PBS at 4 °C. Non-glycated BSA was prepared under the same condition without the addition of glucose. These preparations contained no endotoxin, as determined by LAL endotoxin test kit. The formation of AGEs was estimated by their characteristic fluorescence (excitation spectra: 330 nm; emission spectra: 410 nm) using a Hitachi F-2500 fluorescent spectrometer (Hitachi Corporation, Tokyo, Japan). Both AGE-BSA and non-glycated BSA were filtered through a sterile 0.2 μm filter before addition to cell cultures.2.11 Assessment of cell proliferation
The cell proliferation induced by exogenous AGEs was assessed using MTT. 200 μL of 5 × 107 cells L−1 were plated in 96-well plates. After cell attachment, the medium was removed and cells were serum-starved for an additional 24 h to attain quiescence. Quiescent cells were pre-incubated with algal extracts (C. zofingiensis, C. protothecoides and N. laevis) or pure compounds (astaxanthin, lutein and EPA) for 30 min. Cells were subsequently exposed to different concentrations of AGE-BSA or non-glycated BSA for 24 h and 48 h, respectively. After the treatment, a 20 μL aliquot of MTT solution (5 mg mL−1) was added to each well and incubated for 4 h at 37 °C. At the end of incubation, the medium containing MTT was removed. The incorporated formazan crystals were dissolved in 150 μL DMSO, and the absorbance at 540 nm of each well was determined by a micro-plate reader (TECAN, Sunrise, Crailsheim, Germany).2.12 Evaluation of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP)-2 mRNA expressions
Quantitative real-time RT-PCR was performed to evaluate the expression of VEGF and MMP-2 genes. 1 mL of 5 × 108 cells L−1 was plated in 6-well plates. After attachment, the medium was removed and cells were serum-starved for an additional 24 h to attain quiescence. Quiescent cells were pre-incubated with algal extracts or pure compounds for 2 h. Cells were subsequently exposed to AGE-BSA or non-glycated BSA for 24 h. After treatments, total RNA was extracted from cells using Trizol reagent according to the manufacturer's instructions. The quantification and purity of RNA was determined spectrophotometrically at 260 nm. Total RNA (1 μg) extracted from different samples was reverse transcribed to cDNA using a SuperScript III First-Strand Synthesis System primed with oligo (dT) according to the manufacturer's instructions. Quantitative real time RT-PCR was performed in a BIO-RAD iCycler IQ Multi-Color RealTime PCR Detection System (Bio-Rad, Hercules, CA, USA) in the presence of a SYBR Green PCR Master Mix. The relative levels of the amplified mRNA were evaluated according to the 2−ΔΔCT method using actin gene for normalization.17 The cDNA sequence of the primers for PCR and length of predicted products are shown in Table 1.| mRNA | Forward primer (5′ → 3′) | Reverse primer (5′ → 3′) | Product |
|---|---|---|---|
| VEGF 165 | GGACATCTTCCAGGAGTA | TGCAACGCGAGTCTGTGT | 341 bp |
| MMP-2 | TTGACGGTAAGGACGGACTC | ACTTGCAGTACTCCCCATCG | 153 bp |
2.13 Statistical analysis
All experiments were determined in triplicate and repeated three times to ensure reproducibility. Experimental results were expressed as mean value ± SD. Statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). Paired-samples T-test was applied. The statistical significances were achieved when P < 0.05.3. Results
3.1 Carotenoid and fatty acid analyses
Microalgae C. zofingiensis, C. protothecoides and N. laevis have been highlighted by many studies for their high contents of astaxanthin, lutein and EPA, respectively.18,19,20 Astaxanthin (Fig. 1A) and lutein (Fig. 1B) belong to carotenoids, whilst EPA (Fig. 1C) is a member of omega-3 polyunsaturated fatty acids. In the present work, through HPLC and GC analyses, contents of these nutritional compounds in algal extracts were identified. Astaxanthin: 0.85 ± 0.02 mg g−1 in C. zofingiensis; lutein: 1.95 ± 0.10 mg g−1 in C. protothecoides; and EPA: 4.22 ± 0.75 mg g−1 in N. laevis.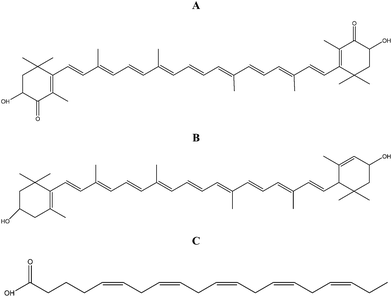 | ||
| Fig. 1 Chemical structures of astaxanthin (A), lutein (B) and EPA (C). | ||
3.2 Intracellular CML levels after exposure to high glucose
The intracellular levels of CML-modified proteins were determined using ELISA. As shown in Fig. 2, in RPE cells, the treatment with continuous high glucose (30 mM) for 4 days led to a significant increase of CML formation compared to normal glucose (5.6 mM) (157.56% of normal glucose, P < 0.01). The addition of the C. zofingiensis extract dose-dependently inhibited the formation of intracellular CML. The inhibitory rate was 17.07% (P < 0.01) at a concentration of 500 μg mL−1. Astaxanthin showed similar effects. At a concentration of 2.5 μg mL−1, no significant inhibitory effect was observed. However, after treatments with 5 and 10 μg mL−1 of astaxanthin, the contents of CML were significantly reduced by 14.89% (P < 0.05) and 24.05% (P < 0.01), respectively.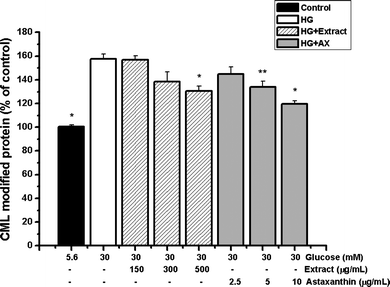 | ||
| Fig. 2 Effects of the C. zofingiensis extract (150–500 μg mL−1) and astaxanthin (2.5–10 μg mL−1) on the formation of intracellular CML-modified proteins. Each value represents the mean ± SD (n = 3). Significant differences were marked with * (P < 0.01) or ** (P < 0.05) compared with cells exposed to high glucose. Control: cells exposed to normal glucose (5.6 mM); HG: high glucose (30 mM); AX: astaxanthin. | ||
3.3 Intracellular oxidative stress after exposure to high glucose
The intracellular anti-oxidative actions of the C. zofingiensis extract and astaxanthin were evaluated. After the exposure to high glucose for 24 h, remarkable increases of intracellular ROS production (Fig. 3A), NO2− generation (Fig. 3B) and TBARS concentration (Fig. 3C) were observed, compared to normal glucose (192.36%, 213.40% and 172.08% of normal glucose, P < 0.01). Such high glucose-induced oxidative stresses were significantly prevented by the pre-treatment with the C. zofingiensis extract or astaxanthin in a dose-dependent manner. At concentrations of 150–500 μg mL−1, the algal extract suppressed the ROS, NO2− and lipid peroxidation by 18.76%–32.54%, 29.59%–45.02% and 3.75%–23.52%, respectively. Meanwhile, at concentrations of 2.5–10 μg mL−1, inhibitory rates of astaxanthin towards these parameters were 12.76%–40.91%, 39.27%–52.09% and 19.14%–41.53%, respectively. These data demonstrated the broad and effective scavenging activities of the C. zofingiensis extract and astaxanthin.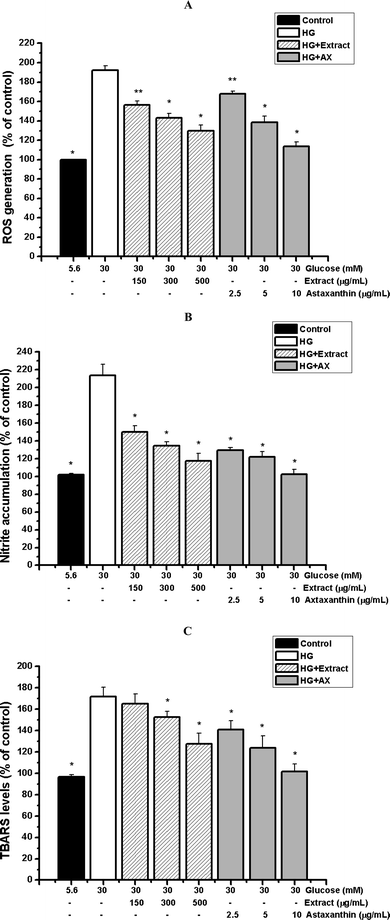 | ||
| Fig. 3 Effects of the C. zofingiensis extract (150–500 μg mL−1) and astaxanthin (2.5–10 μg mL−1) on intracellular ROS production (A), nitrite accumulation (B) and TBARS concentrations (C). Each value represents the mean ± SD (n = 3). Significant differences were marked with * (P < 0.01) or ** (P < 0.05) compared with cells exposed to high glucose. Control: cells exposed to normal glucose (5.6 mM); HG: high glucose (30 mM); AX: astaxanthin. | ||
3.4 Cell proliferation stimulated by exogenous AGEs
The cell proliferation was determined using MTT. Results showed that the treatment with exogenous AGE-BSA for 24 h dose-dependently led to the proliferation of RPE cells (Table 2). At the concentration of 10 μg mL−1, AGEs had no significant influences. However, after treatments with 50, 100 and 200 μg mL−1 AGEs for 24 h, strong increases in proliferation of 12.01%, 31.09% and 43.95% were observed, respectively. Such actions of AGEs were not in a time-dependent manner, as evidenced by the fact that the proliferation did not change significantly when the incubation was prolonged to 48 h (proliferative rate: 44.31%). Interestingly, pretreatments of RPE cells with three microalgal extracts and pure compounds for 30 min markedly reduced the AGE-stimulated proliferation, whilst the exposure to microalgal extracts/nutritional compounds alone, or non-glycated BSA, had no significant effects. As shown in Fig. 4A, at concentrations of 150–500 μg mL−1, the extracts of C. zofingiensis, C. protothecoides and N. laevis inhibited the proliferation by 4.36–19.80%, 7.72–32.33% and 7.60–23.20%, respectively. Meanwhile, at concentrations of 2.5–10 μg mL−1, the inhibitory rates of astaxanthin, lutein and EPA were 12.10–26.56%, 14.56–31.23% and 7.13–19.65%, respectively (Fig. 4B).| OD (540 nm) | % Proliferation | |
|---|---|---|
| a Each value represents the mean ± SD (n = 3). Significant differences with untreated cells (Control) were marked with * (P < 0.01) or ** (P < 0.05). | ||
| Control | 0.386 ± 0.011 | — |
| Microalgal extracts alone (500 μg mL−1) | 0.389 ± 0.015 | 0.08 ± 0.04 |
| Nutritional compounds alone (10 μg mL−1) | 0.374 ± 0.011 | −3.46 ± 0.49 |
| AGE (10 μg mL−1) | 0.379 ± 0.012 | −1.64 ± 0.78 |
| AGE (50 μg mL−1) | 0.432 ± 0.027** | 12.01 ± 2.53 |
| AGE (100 μg mL−1) | 0.506 ± 0.016* | 31.09 ± 2.45 |
| AGE (200 μg mL−1) | 0.556 ± 0.010* | 43.95 ± 3.12 |
| Non-glycated BSA (200 μg mL−1) | 0.378 ± 0.015 | −2.16 ± 0.75 |
| AGE (200 μg mL−1) for 48 h | 0.574 ± 0.013* | 44.31 ± 2.35 |
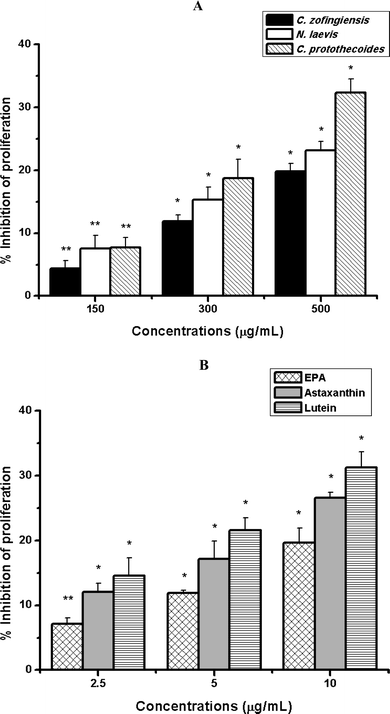 | ||
| Fig. 4 Inhibitory effects of extracts of C. zofingiensis, N. laevis and C. protothecoides (A) as well as pure compounds of astaxanthin, EPA and lutein (B) on the proliferation of RPE cells induced by AGEs. Each value represents the mean ± SD (n = 3). Significant differences were marked with * (P < 0.01) or ** (P < 0.05) compared with cells stimulated with 200 μg mL−1 AGEs. | ||
3.5 mRNA expression of VEGF and MMP-2 stimulated by exogenous AGEs
Quantitative real time RT-PCR was performed to evaluate the mRNA expression of VEGF and MMP-2 in RPE cells. Cells were pre-treated with or without microalgal extracts/pure compounds for 2 h before incubation with AGE-BSA. Results showed that different from non-glycated BSA exhibiting no influence on mRNA levels, the stimulation with 200 μg mL−1 AGE-BSA for 24 h markedly increased the expression of VEGF and MMP-2 by 245% and 98%, respectively. Such upregulation was significantly reduced by the treatments with microalgal extracts and their nutritional ingredients. For VEGF, at a concentration of 500 μg mL−1, C. protothecoides showed the highest inhibitory rate (23.32%), followed by N. laevis (20.41%) and C. zofingiensis (12.83%). Otherwise, at a concentration of 10 μg mL−1, lutein showed the highest inhibitory rate (57.35%), followed by astaxanthin (52.68%) and EPA (45.58%) (Fig. 5A). For MMP-2, at a concentration of 500 μg mL−1, N. laevis showed the highest inhibitory rate (18.69%), followed by C. protothecoides (15.30%) and C. zofingiensis (13.43%). Otherwise, at a concentration of 10 μg mL−1, lutein showed the highest inhibitory rate (39.39%), followed by astaxanthin (31.82%) and EPA (28.03%) (Fig. 5B).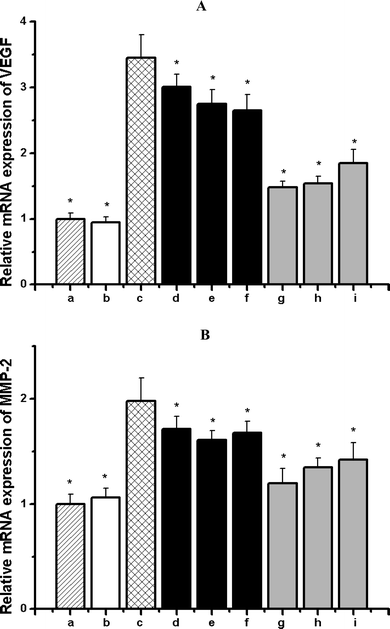 | ||
| Fig. 5 Effects of microalgal extracts as well as their nutritional ingredients on the mRNA expressions of VEGF (A) and MMP-2 (B) induced by AGEs in RPE cells. (a) Untreated cells; (b) cells treated with 200 μg mL−1 non-glycated BSA alone; (c) cells treated with 200 μg mL−1 AGE-BSA alone; (d) cells treated with 200 μg mL−1 AGE-BSA + 500 μg mL−1C. zofingiensis; (e) cells treated with 200 μg mL−1 AGE-BSA + 500 μg mL−1N. laevis; (f) cells treated with 200 μg mL−1 AGE-BSA + 500 μg mL−1C. protothecoides; (g) cells treated with 200 μg mL−1 AGE-BSA + 10 μg mL−1 lutein; (h) cells treated with 200 μg mL−1 AGE-BSA + 10 μg mL−1 astaxanthin; (i) cells treated with 200 μg mL−1 AGE-BSA + 10 μg mL−1 EPA. Each value represents the mean ± SD (n = 3). Significant differences were marked with * (P < 0.01) compared with (c) cells treated with 200 μg mL−1 AGEs-BSA. | ||
4. Discussion
AGEs can trigger the transcriptional activation of various genes, such as cell-adhesion molecules, tissue factors, chemokines and cytokines, giving rise to a number of deleterious cellular events, and therefore acting as a key pathophysiological process in the initiation and development of diabetic complications including diabetic retinopathy.21 According to our previous work, several microalgal strains rich in nutrients exhibited strong blockage activities on the glycation cascade in cell-free systems. In the present study, for the first time, the intracellular antiglycoxidative activities of extracts of these microalgal strains (C. zofingiensis, C. protothecoides and N. laevis) as well as their nutritional ingredients (astaxanthin, lutein and EPA) were investigated. Human-derived retinal pigment epithelial ARPE-19 cells, which are known to play important roles in the pathogenesis of diabetic retinopathy, were used.22,23We firstly examined the effects of the C. zofingiensis extract and astaxanthin on the formation of intracellular AGEs. Since AGEs are a group of heterogeneous molecules containing numerous products with distinct structures and functions, the level of CML, a major antigenic AGE structure was evaluated.24 CML is a non cross-linking AGE and forms no fluorescence. According to previous reports, accelerated concentrations of CML have been detected in skins and lens collagen matrix of diabetic patients.25 The high-glucose stimulation was performed in the present work because high extracellular glucose levels have been considered as a leading cause of diabetic retinopathy, which may result in pathological changes in numerous tissues including the retina.26 Observations of the present work that the treatment with continuous high glucose led to a significant increase of CML formation were fundamentally consistent with previous findings. These CML modified proteins can bind to cell-surface receptors for AGEs (RAGEs), inducing the expression of NF-κB-dependent proinflammatory genes and contributing to diabetic retinopathy.27 Results showed that the elevated accumulation of intracellular CML was dose-dependently inhibited by the C. zofingiensis extract and astaxanthin. Since CML can be generated through glycoxidation as well as lipid peroxidation,28 the anti-CML effects of C. zofingiensis extract/astaxanthin may be associated with their anti-oxidative activities. In fact, free radicals and oxidative steps have long been regarded as crucial factors in the glycoxidation process.29 To understand the underlying actions of intracellular antiglycative capacities in relation to antioxidant activities, effects of the C. zofingiensis extract and astaxanthin on the generation of oxidative stress including ROS, NO and lipid peroxidation were evaluated.
Retina is very susceptible to oxidative stress due to its higher contents of polyunsaturated fatty acids.30 A number of animal studies have revealed the elevated levels of superoxide and hydrogen peroxide in the retina of diabetic rats.31,32 More seriously, even after a good glycemic control is re-established, the remaining intracellular ROS may lead to the resistance of diabetic retinopathy to reverse.33 In addition to ROS, levels of NO and lipid peroxidation are also known to increase under diabetic conditions.34,35 Results of the present study showed that the high glucose-induced intracellular oxidative stress was significantly prevented by the C. zofingiensis extract and astaxanthin in a dose-dependent manner, indicating their broad and effective scavenging activities. Astaxanthin is known as a potent antioxidant agent, its antioxidant activity is as high as 10 times more than other carotenoids such as zeaxanthin, lutein, canthaxanthin and β-carotene, and 100 times more than α-tocopherol.36 Results of the present study confirmed its intracellular antioxidant actions in RPE cells, strongly suggesting it could be a key contributing factor to the antiglycative activities, and supporting the possibility of using natural antioxidants as intracellular glycation inhibitors.
In addition to blocking the formation of endogenous AGEs, the C. zofingiensis extract and astaxanthin, as well as another two microalgal extracts (C. protothecoides and N. laevis) and their nutritional ingredients (lutein and EPA) were further evaluated for their effects towards the cytotoxicity induced by exogenous AGEs. Results showed that the treatment with exogenous AGE-BSA led to the proliferation of RPE cells in a dose-dependent but not time-dependent manner. Such findings were in agreement with previous reports.37,38 Interestingly, the AGE-induced proliferation was markedly suppressed by three microalgal extracts and their nutritional ingredients. The abnormal proliferation is important in the onset and development of proliferative diabetic retinopathy39 and some other proliferative ocular diseases, such as age-related macular degeneration40 and proliferative vitreoretinopathy.41 Particularly in proliferative vitreoretinopathy, with the presence of growth factors, cytokines and neurotransmitter compounds, the destruction of blood-retina barrier facilitates RPE cells to re-enter the cell cycle and initiate the proliferation. RPE cells proliferate and migrate to the vitreous cavity, forming fibrocellular membranes on both surfaces of the retina, which eventually leads to the detachment of the retina and the loss of vision.42 Therefore, microalgal extracts and nutritional compounds may influence the cytokine release or cellular signaling directly to diminish the AGE-induced proliferation.
According to many studies, the development of the abnormal blood vessels in proliferative diabetic retinopathy can be stimulated by a protein called VEGF.43,44 VEGF is a potent endothelial-cell-specific angiogenic and vasopermeable factor that can be produced by a variety of cell types in the retina including RPE cells.45 Through stimulating angiogenesis and neovascularisation, VEGF plays a vital role in proliferative diabetic retinopathy. In fact, its level has been found to be significantly elevated in the vitreous and aqueous fluids in the eyes of patients with proliferative diabetic retinopathy.46 Some studies have shown the exposure to AGEs may cause the upregulation of VEGF in various retinal cells.9,37,47 In keeping with previous findings, an evaluated mRNA expression of VEGF in RPE cells was observed in the present work. Such upregulation of VEGF secretion after AGE exposure explains in addition the proliferation response of RPE cells, and suggests the possible mechanisms that how AGEs participate in the pathogenesis of diabetic retinopathy. It is now believed that the regulation of genes encoding VEGF might provide effective strategies to therapeutically control the growth of blood vessels and prevent diabetic retinopathy.44 In this study, the protective actions of microalgal extracts and nutritional compounds were ascertained. They effectively attenuated the upregulation of VEGF. Similarly, these agents decreased the mRNA expression of another signal, MMP-2, which was also induced by the exogenous AGEs at the same dose. MMPs are a family of zinc-dependent endopetidases involved in the pathogenesis of diabetic retinopathy and chorodial neovascularization. All MMPs are synthesized as proenzymes and function to degrade extracellular matrix components, which is an important event in the development of the fibrovascular tissue in proliferative diabetic retinopathy.48 Through the proteolytic degradation of basement membranes and extracellular matrix components, MMPs support the migration and proliferation of vascular endothelial cells. MMP-2 and MMP-9 (gelatinases A and B) are two major members. Their levels have been found to be elevated in both vitreous and fibrovascular tissues in eyes with proliferative diabetic retinopathy.48 MMP-2 is a type IV collagenase that specifically cleaves type IV collagen, a major structural component of basement membranes. Previous studies have reported the upregulation of MMP-2 in choroidal endothelial cells37 and cardiac fibroblasts49 after the AGE exposure. In addition to AGEs, the expression of MMP-2 may also be upregulated by some growth factors and inflammatory mediators, including VEGF.50 Since the VEGF level has been increased after the treatment of AGEs in the present study, this may in addition explain the enhanced expression of MMP-2.
In conclusion, findings of the present work offer an important insight into the actions of AGEs in vitro. In addition to being generated endogenously under the presence of high glucose, AGEs may also act as exogenous mediators to induce pathophysiological processes. Such deleterious effects were attenuated by the pre-treatment of several microalgal extracts and their nutritional ingredients. The C. zofingiensis extract and astaxanthin were found to inhibit the formation of a key AGE representative, CML in RPE cells through the suppression of intracellular oxidative stress. Furthermore, extracts of C. zofingiensis, C. protothecoides and N. laevis as well as nutritional compounds of astaxanthin, lutein and EPA were found to prevent the cell proliferation and mRNA upregulation of VEGF and MMP-2 induced by AGEs, which are critical steps in the development of proliferative diabetic retinopathy. This is the first time that the intracellular antiglycative capacities of microalgal samples have been investigated. Serving as a continuous and reliable source of natural products, microalgae contain numerous beneficial substances and many of them have biomodulatory effects. The microalgal strains used in this study, i.e. C. protothecoides, C. zofingiensis and N. laevis have been highlighted by a number of studies for their high accumulations of lutein, astaxanthin, and EPA, respectively.19,18,20 These nutritional components have strong antioxidant activities and are essential for human health, especially supplying protective benefits in ocular diseases, such as cataract and macular degeneration.51,52 Results of this study may provide some more evidence. These nutrient-rich microalgae, therefore, might be regarded as beneficial foods and preventive agent choices for patients with diabetic retinopathy. Further studies are needed to elucidate the exact molecular mechanisms underlying their actions.
References
- D. M. Nathan, N. Engl. J. Med., 1993, 328, 1676–1685 CrossRef CAS.
- T. A. Ciulla, A. G. Amador and B. Zinman, Diabetes Care, 2003, 26, 2653–2664 CrossRef.
- M. Brownlee, Nature, 2001, 414, 813–820 CrossRef CAS.
- H. Vlassara and M. R. Palace, J. Intern. Med., 2002, 251, 87–101 CrossRef CAS.
- Y. Ono, S. Aoki, K. Ohnishi, T. Yasuda, K. Kawano and Y. Tsukada, Diabetes Res. Clin. Pract., 1998, 41, 131–137 CrossRef CAS.
- Z. Wagner, I. Wittmann, I. Mazak, R. Schinzel, A. Heidland and R. Kientsch-Engel, Am. J. Kidney Dis., 2001, 38, 785–791 Search PubMed.
- D. R. Sell, A. Lapolla, P. Odetti, J. Fogarty and V. M. Monnier, Diabetes, 1992, 41, 1286–1292 CrossRef CAS.
- E. Sato, F. Mori, S. Igarashi, T. Abiko, M. Takeda and S. Ishiko, Diabetes Care, 2001, 24, 479–482 CrossRef CAS.
- M. Lu, M. Kuroki, S. Amano, M. Tolentino, K. Keough, I. Kim, R. Bucala and A. P. Adamis, J. Clin. Invest., 1998, 101, 1219–1224 CrossRef CAS.
- J. T. Handa, K. M. Reiser, H. Matsunaga and L. M. Hjelmeland, Exp. Eye Res., 1998, 66, 411–419 CrossRef CAS.
- J. Berlanga, D. Cibrian and I. Guillen, Clin. Sci., 2005, 109, 83–95 Search PubMed.
- M. S. Ahmad and N. Ahmad, J. Nutr., 2006, 136, 796–799.
- Z. Sun, J. Liu, X. H. Zeng, J. Q. Huangfu, Y. Jiang, M. F. Wang and F. Chen, Food Chem., 2011, 126, 1629–1635 CrossRef CAS.
- Z. Sun, X. F. Peng, J. Liu, K. W. Fan, M. F. Wang and F. Chen, Food Chem., 2010, 120, 261–267 CrossRef CAS.
- I. Baroli, A. D. Do, T. Yamane and K. K. Niyogi, Plant Cell, 2003, 15, 992–1008 CrossRef CAS.
- G. Q. Chen, Y. Jiang and F. Chen, Food Chem., 2007, 104, 1580–585 CrossRef CAS.
- K. J. Livak and T. D. Schmittgen, Methods, 2001, 25, 402–408 CrossRef CAS.
- P. F. Ip and F. Chen, Process Biochem., 2005, 40, 733–738 CrossRef CAS.
- X. M. Shi, F. Chen, J. P. Yuan and H. Chen, J. Appl. Phycol., 1997, 9, 445–450 Search PubMed.
- Z. Y. Wen and F. Chen, Biotechnol. Adv., 2003, 21, 273–294 CrossRef CAS.
- F. L. Zhang, H. Q. Gao and L. Shen, J. Cardiovasc. Pharmacol., 2007, 50, 434–440 CrossRef CAS.
- E. Nakashima, R. Pop-Busui and R. Towns, et al. , Antioxid. Redox Signaling, 2005, 7, 1530–1542 Search PubMed.
- R. R. Vogt, R. Unda, L. C. Yeh, E. K. Vidro, J. C. Lee and A. T. Tsin, J. Cell. Biol., 2006, 98, 1196–1202 CAS.
- V. M. Monnier, J. F. Fogarty, C. Monnier and D. R. Sell, in B. P. Yu, Methods in aging research, CRC Press, Boca Raton, Florida, 1999, pp. 657–683 Search PubMed.
- J. W. Baynes, Diabetes, 1991, 40, 405–412 CrossRef CAS.
- D. I. Kim, S. K. Lim, M. J. Park, H. J. Han, G. Y. Kim and S. H. Park, Life Sci., 2007, 80, 626–632 CrossRef CAS.
- M. Andrassy, J. Igwe and F. Autschbach, et al. , Am. J. Pathol., 2006, 169, 1223–1237 CrossRef CAS.
- E. D. Schleicher, E. Wagner and A. G. Nerlich, J. Clin. Invest., 1997, 99, 457–468 CrossRef CAS.
- J. V. Hunt, M. A. Bottoms and M. J. Mitchinson, Biochem. J., 1993, 291, 529–535 CAS.
- R. E. Anderson, L. M. Rapp and R. D. Wiegand, Curr. Eye Res., 1984, 3, 223–227 Search PubMed.
- Y. Cui, X. Xu, H. Bi and Q. Zhu, et al. , Exp. Eye Res., 2006, 83, 807–816 CrossRef CAS.
- E. A. Ellis, D. L. Guberski, M. Somogyi-Mann and M. B. Grant, Free Radical Biol. Med., 2000, 28, 91–101 CrossRef CAS.
- R. A. Kowluru, Diabetes, 2003, 52, 818–823 CrossRef CAS.
- N. Lzumi, T. Nagaoka, F. Mori and E. Sato, Jpn. J. Ophthalmol., 2006, 50, 465–468 CrossRef.
- L. Virag, E. Szabo, P. Gergely and C. Szabo, Toxicol. Lett., 2003, 140–141, 113–124 CrossRef CAS.
- W. Miki, Pure Appl. Chem., 1991, 63, 141–146 CAS.
- S. Hoffmann, U. Friedrichs, W. Eichler, A. Rosenthal and P. Wiedemann, Graefe’s Arch. Clin. Exp. Ophthalmol., 2002, 240, 996–1002 CrossRef CAS.
- J. Y. Kim, H. K. Park and J. S. Yoon, et al. , Int. J. Oncol., 2008, 33, 493–501 CAS.
- P. Hiscott, C. Sheridan, T. M. Magee and I. Grierson, Prog. Retinal Eye Res., 1999, 18, 167–190 Search PubMed.
- H. Miller, B. Miller and S. J. Ryan, Invest. Ophthalmol. Vis. Sci., 1986, 27, 1644–1652 CAS.
- P. A. Campochiaro, Arch. Ophthalmol., 1997, 115, 237–241 Search PubMed.
- C. M. Chan, J. H. Huang, H. S. Chiang, W. B. Wu, H. H. Lin, J. Y. Hong and C. F. Hung, Mol. Vision, 2010, 16, 586–595 Search PubMed.
- L. P. Aiello, T. W. Gardner and G. L. King, et al. , Diabetes Care, 1998, 21, 143–156 CAS.
- D. Ray, M. Mishra, S. Ralph, I. Read, R. Davies and P. Brenchley, Diabetes, 2004, 53, 861–864 CrossRef CAS.
- M. S. Jardeleza and J. W. Miller, Semin. Ophthalmol., 2009, 24, 87–92 CrossRef.
- L. P. Aiello, R. L. Avery and P. G. Arrigg, et al. , N. Engl. J. Med., 1994, 331, 1480–1487 CrossRef CAS.
- S. Yamagishi, S. Amano and Y. Inagaki, et al. , Biochem. Biophys. Res. Commun., 2002, 290, 973–978 CrossRef CAS.
- K. Noda, S. Ishida, M. Inoue, K. Obata, Y. Oguchi, Y. Okada and E. Ikeda, Invest. Ophthalmol. Visual Sci., 2003, 44, 2163–2170 CrossRef.
- S. Daoud, R. Schinzel, A. Neumann, C. Loske, D. Fraccarollo, C. Diez and A. Simm, Mol. Med., 2001, 7, 543–551 CAS.
- J. K. Chae, I. Kim and S. T. Lim, et al. , Arterioscler. Thromb. Vasc. Biol., 2000, 20, 2573–2578 CAS.
- O. Pulz and W. Gross, Appl. Microbiol. Biotechnol., 2004, 65, 624–632.
- S. Richer, W. Stiles and L. Statkute, et al. , Optometry, 2004, 75, 216–230 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
