Protective effects of prescription n-3 fatty acids against impairment of spatial cognitive learning ability in amyloid β-infused rats
Michio
Hashimoto
*a,
Ryuichi
Tozawa
b,
Masanori
Katakura
a,
Hossain
Shahdat
a,
Abdul Md.
Haque
a,
Yoko
Tanabe
a,
Shuji
Gamoh
a and
Osamu
Shido
a
aDepartment of Environmental Physiology, Shimane University Faculty of Medicine, Izumo, Shimane 693-8501, Japan. E-mail: michio1@med.shimane-u.ac.jp; Fax: +81 853 20 2110; Tel: +81 853 20 2112
bPharmaceutical Research Division, Takeda Pharmaceutical Company, Osaka, 532-8686, Japan
First published on 8th July 2011
Abstract
Deposition of amyloid β peptide (Aβ) into the brain causes cognitive impairment. We investigated whether prescription pre-administration of n-3 fatty acids improves cognitive learning ability in young rats and whether it protects against learning ability impairments in an animal model of Alzheimer's disease that was prepared by infusion of Aβ1–40 into the cerebral ventricles of rats. Pre-administration of TAK-085 (highly purified and concentrated n-3 fatty acids containing eicosapentaenoic acid ethyl ester and docosahexaenoic acid ethyl ester) at 300 mg kg−1 day−1 for 12 weeks significantly reduced the number of reference memory errors in an 8-arm radial maze, suggesting that long-term administration of TAK-085 improves cognitive leaning ability in rats. After pre-administration, the control group was divided into the vehicle and Aβ-infused groups, whereas the TAK-085 pre-administration group was divided into the TAK-085 and TAK-085 + Aβ groups (TAK-085-pre-administered Aβ-infused rats). Aβ1–40 or vehicle was infused into the cerebral ventricle using a mini osmotic pump. Pre-administration of TAK-085 to the Aβ-infused rats significantly suppressed the number of reference and working memory errors and decreased the levels of lipid peroxide and reactive oxygen species in the cerebral cortex and hippocampus of Aβ-infused rats, suggesting that TAK-085 increases antioxidative defenses. The present study suggests that long-term administration of TAK-085 is a possible therapeutic agent for protecting against Alzheimer's disease-induced learning deficiencies.
Introduction
Eicosapentaenoic acid [EPA; C20:5(n-3)] and docosahexaenoic acid [DHA; C22:6(n-3)] are n-3 polyunsaturated fatty acids (PUFAs) found in oily fish such as salmon and tuna. Recent evidence indicates that these fatty acids effectively reduce the risk of cardiovascular diseases, and epidemiological studies show that intake of fish oil is associated with a reduced risk of neurological and psychiatric disorders, especially Alzheimer's disease (AD). Kalmijn et al. initially reported that fish consumption was inversely related to the incidence of dementia/AD.1 Likewise, Morris et al. presented data from a food frequency questionnaire (FFQ) administered to 815 subjects in the Chicago Housing and Aging Project (CHAP) in 2003 and concluded that participants who consumed fish >1 time per week had a 60% reduced risk of AD compared to those who rarely or never ate fish.2 More recently, von Gelder et al. examined cognitive decline over a 5-year period and reported that increased fish consumption and intake of DHA + EPA were both associated with reduced cognitive decline.3 These findings suggest that increased consumption of n-3 fatty acids is associated with a reduced risk of age-related cognitive decline, dementia, and AD.Despite the above findings, some studies have failed to report an association between increased dietary intake of n-3 PUFAs and reduced risk of dementia or AD. Morris et al. re-examined their data of the CHAP study data from 2005 that included a large cohort of 3718 subjects and could not confirm the findings of the initial analysis.4 Freund-Levi et al. administered DHA + EPA to AD patients with mild cognitive impairment,5 and no clinically significant benefits were observed in these AD patients after 6 months. Therefore, it remains unclear whether n-3 PUFA can have beneficial effects on memory learning and learning ability impairment in AD.
DHA is essential for normal neurological development and for maintenance of neuronal functions.6 A decrease in the level of serum DHA correlates with cognitive impairment7 and memory impairment occurs because of reduced levels of brain DHA.8 We previously reported that young and aged male DHA-deficient rats considerably improved learning ability after intragastric administration of DHA.9,10 The beneficial effects were related to increase in the DHA level and DHA/arachidonic acid (AA) ratio in the cortico-hippocampal tissues. DHA level in the hippocampus is very low in patients with AD compared with that in brain samples from age-matched human controls.11,12AD is characterized by the formation and accumulation of neurofibrillary tangles and neuritic plaques of amyloid β peptide (Aβ), as well as by neuronal and memory loss.13 The accumulation of Aβ increases the production of free radicals, resulting in increased lipid peroxidation in the brain.14 Oxidative damage and formation of oxidized lipids and proteins have been observed in the brain of patients with AD.15 Infusion of Aβ1–40 into the rat cerebral ventricle increases the levels of lipid peroxide (LPO) and reactive oxygen species (ROS) in the cortex and hippocampus; these increments correlate with impaired reference- and working memory-related learning abilities, indicating a deficit in cognitive ability, a well-known characteristic of AD.16,17 Moreover, DHA promotes differentiation of neural stem cells.18DHA, thus, might help to restore the injured neurons in neurodegenerative diseases including AD, by controlling the fate of neuronal cell cycle.19
EPA administration increased neuronal and glial EPA content and glial DHA content, suggesting that EPA may protect against neurodegeneration by modulating synaptic plasticity.20 In addition, dietary administration of EPA increased DHA levels and DHA/AA ratio in the plasma and brain tissues in normal or Aβ-infused rats with a decrease in oxidative stress.21 In the present study, we investigated whether prescription pre-administration of n-3 fatty acids (TAK-085: highly purified and concentrated EPA and DHA ethyl esters) increases cognitive learning ability in young rats and whether it protects against impairment of learning ability in an animal model of AD in which Aβ1–40 was infused into the cerebral ventricles of rats.
Materials and methods
Animals and diet
Rats were handled and sacrificed in accordance with the procedures outlined in the Guidelines for Animal Experimentation of Shimane University (Shimane, Japan) and as instructed in the Guidelines for Animal Experimentation of the Japanese Association for Laboratory of Animal Science. Wistar rats (generation 1, G1) (Jcl: Wistar; Clea Japan Co., Tokyo, Japan) were housed in a room under controlled temperature (23 ± 2 °C), relative humidity (50 ± 10%), and light-dark cycle (light, 0800 to 2000 h; dark, 2000 to 0800 h) conditions and were provided with a fish oil-deficient pellet diet (F-1®; Funabashi Farm, Funabashi, Japan) and waterad libitum. The fatty acid composition of the F-1® is shown in Table 1.| F-1 ® diet | Profiles of TAK-085 | ||
|---|---|---|---|
| a DHA, docosahexaenoic acid; EE: ethyl ester; EPA, eicosapentaenoic acid; ND: Not detected. | |||
| Composition of the diet (%, w/w) | Eicosapentaenoic acid C20:5(n-3) (EE) (mg g−1) | 462 | |
| Water | 8.0 | Docosahexaenoic acid C22:6(n-3) (EE) (mg g−1) | 367 |
| Crude protein | 21.5 | EPA and DHA (mg g−1) | 829 |
| Fat | 4.4 | Docosapentaenoic acid C22:5(n-3) (%, w/w) | 3.3 |
| Fiber | 2.6 | Total n-3 (EE) (%, w/w) | 90 |
| Mineral | 4.9 | Arachidonic acid C20:4(n-6) (EE) (%, w/w) | 2.4 |
| Carbohydrate | 58.6 | Docosapentaenoic acid C22:5(n-6) (%, w/w) | 1.0 |
| Fatty acid composition (g kg−1) | α-Tocopherol (mg g−1) | 3.9 | |
| Myristic acid C14:0 | 0.034 | ||
| Palmitic acid C16:0 | 5.83 | ||
| Palmitoleic acid C16:1(n-7) | ND | ||
| Stearic acid C18:0 | 2.24 | ||
| Oleic acid C18:1(n-9) | 8.57 | ||
| Linoleic acid C18:2(n-6) | 21.5 | ||
| Linolenic acid C18:3(n-3) | 2.21 | ||
| Arachidonic acid C20:4(n-6) | ND | ||
| Eicosapentaenoic acid C20:5(n-3) | ND | ||
| Docosapentaenoic acid C22:5(n-3) | ND | ||
| Docosahexaenoic acid C22:6(n-3) | ND | ||
| Lignoceric acid C24:0 | 0.055 | ||
The experimental schedule is shown in Fig. 1. Inbred second-generation male rats (n = 27, 5 weeks old) were divided into 2 groups: the TAK-085 group (n = 14), which was orally administered TAK-085 (300 mg kg−1 day−1: Pronova BioPharma ASA, Oslo, Norway) containing 498 mg g−1 EPA and 403 mg g−1DHA suspended in 5% gum Arabic solution for 12 weeks; and the control group (n = 13), which was administered only 5% gum Arabic solution for 12 weeks. The profiles of TAK-085 are also shown in Table 1.
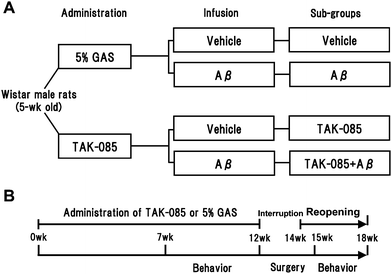 | ||
| Fig. 1 Experimental design: study grouping (A) and schedule (B). Five-week-old male Wistar rats were orally administered TAK-085 or 5% gum Arabic solution (GAS) for a total of 16 weeks. Subsequently, the rats were behaviourally tested in an 8-arm radial maze. Vehicle or amyloid β (Aβ) peptide was infused into the cerebral ventricle of the rats from the TAK-085 or 5% GAS groups, which were subsequently subdivided into the Vehicle, Aβ, TAK-085, and TAK-085 + Aβ groups. Finally, rats were behaviourally tested to assess the effects of TAK-085 on cognitive learning ability. | ||
Preparation of the AD model rats
The surgical techniques used to prepare the Aβ-infused rats were essentially the same as those described previously.16,17 In brief, each rat was anaesthetized with sodium pentobarbital (50 mg kg−1BW i.p.), the skull was exposed, and 2 holes were drilled into the skull (right and left, relative to the bregma; 0.8 mm posterior, 1.4 mm lateral) according to the atlas of Paxinos and Watson and using a stereotaxic frame (Narishige, Tokyo, Japan). A solvent comprising 35% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid (pH 2.0) was used as the vehicle for the Aβ1–40 (Peptide Inst., Osaka, Japan). AlCl3 (0.5 μg in 5 μL, 1 μL min−1) was injected through a cannula into the right ventricle, using a Hamilton syringe. Although the cause of neuritic plaques of AD is chiefly Aβ1–42, we used Aβ1–40 because it is more soluble and does not aggregate in the cannulation tube. Moreover, because a small amount of AlCl3 facilitated aggregation of Aβ1–40peptidein vitro, we used AlCl3 before implanting the osmotic pump to ensure continuous infusion of Aβ. This procedure greatly improved the reproducibility and reliability of producing this animal model of AD, i.e., rats with impaired memory. A mini osmotic pump (Alzet 2002; Durect Co., Cupertino, CA, USA) containing either Aβ1–40 solution (234 ± 13.9 μL) or vehicle alone was quickly implanted into the backs of the rats. The outlet of the pump was inserted 3.5 mm into the left ventricle and attached to the skull using screws and dental cement. The infusion rate was 0.56 μL h−1 and the total amount of Aβ1–40 infused was approximately 4.9–5.5 nmol. The entire volume in the mini-osmotic pump was completely infused after spontaneous infusion for 2 weeks. Each rat was subjected to a maze test with administration of either TAK-085 or 5% gum Arabic solution (as vehicle of TAK-085) after complete recovery from the surgery.Radial maze learning ability and TAK-085 administration
Seven weeks after the start of TAK-085 administration, the rats' learning-related behaviour was assessed by their completion of a task in an 8-arm radial maze as previously described,9,17 in which 4 reward pellets were placed randomly in 4 arms of the maze and the number of total selections resulting in 4 pellets was counted. A small solid of 45 mg (made with F-1®) was used for a reward pellet. Two parameters of memory function were examined: reference memory errors (RMEs), determined by the number of entries into the unbaited arms, and working memory errors (WMEs), estimated by the number of repeated entries into arms that had already been visited during the trial. Performance was calculated on the basis of memory-related behaviour. All rats were given an adaptation period handling and shaping for 2 weeks before which they underwent 2 daily trials 6 days a week for a total of 3 weeks (Fig. 1). After the 5-week behavior tests were completed, each of the 2 rat groups was further subdivided into 2 groups (according to the number of errors made by each rat in the last 6 trials of the preliminary behavior test) and infused with either Aβ or vehicle as follows: the control group was subdivided into the Aβ solvent-infused group (vehicle group, n = 7) and the Aβ-infused group (Aβ group, n = 6), while the TAK-085 group was subdivided into the vehicle-infused TAK-085 group (TAK-085 group, n = 6) and the Aβ-infused TAK-085 group (TAK-085 + Aβ group, n = 8). These 4 groups of rats were again orally administered either TAK-085 or 5% gum Arabic solution for a total of 4 weeks after implantation of the mini osmotic pump and behaviorally tested for a total of 3 weeks after pump implantation to assess the effect of TAK-085 pre-administration on learning ability impairment. The protocol used for the preliminary behaviour test was also followed in the final behaviour test except for the adaptation periods. The administration periods were of 16 weeks (Fig. 1).Sample preparation
After undergoing behavioral tests for 3 weeks, the rats were anaesthetised with sodium pentobarbital (65 mg kg−1BW, i.p.), blood was drawn for plasma analysis, and the hippocampus and cerebral cortex were separated as described.16 The tissues were stored at −80 °C by flash-freezing in liquid N2 until use.Measurement of fatty acid profile and oxidative status
Brain samples were immediately homogenised with ice in 1.0 mL of ice-cold 0.32 mol L−1sucrose buffer (pH 7.4) containing 2 mmol L−1ethylenediamine tetraacetic acid (EDTA), 0.5 mg L−1 leupeptin, 0.5 mg L−1 pepstatin, 0.5 mg L−1aprotinin, and 0.2 mmol L−1phenylmethylsulfonyl fluoride in a Polytron homogenizer (PCU-2-110; Kinematica GmbH, Steinhofhalde, Switzerland). The residual tissues were stored at −80 °C by flash-freezing in liquid N2 until use. The homogenates were immediately subjected to the assays described below or stored at −80 °C until use.LPO concentration was assessed by the thiobarbituric acid reactive substance assay of Ohkawa et al.22 as described16,17 and its levels were measured in nanomoles of malondialdehyde per milligram of protein. Malondialdehyde levels were calculated relative to a standard preparation of 1,1,3,3-tetraethoxypropane.
ROS was determined as previously described.16,17 ROS was quantified using a dichlorofluorescin standard curve in methanol.
The fatty acid compositions of plasma and brain tissues were determined using a modification of the one-step reaction of Lepage and Roy23 by gas chromatography (GC) as described.16 The mixture of plasma or brain tissue homogenate, augmented with 2 ml methanol containing 10 μg tricosanoic acid as an internal standard, and 200 μl acetyl chloride, was incubated at 100 °C for 60 min; then 200 μl octane and 5 ml 10% sodium chloride containing 0.5 N sodium hydroxide were added. The mixture was shaken for 10 min at room temperature and centrifuged at 1800 × g for 10 min. The octane phase, containing the fatty acid methyl esters, was subjected directly to GC on a Agilent 6850 A gas chromatograph (Agilent Technologies, Santa Clara, CA) with a flame ionization detector and an automatic sampler utilizing a 25 m × 0.25 mm i.d. fused-silica column (DB-WAX P/N 122-7032, J & W Scientific, Folsom, CA) programmed from 100 to 180 °C at 20 °C min−1, 180 to 240 °C at 2 °C min−1, 240 to 260 °C at 4 °C min−1 and at 260 °C for 5 min. The identities of the peaks were established by comparison with those of reference compounds and, in part, by GC-mass spectrometry.
Protein concentrations were estimated using the method of Lowry et al.24
Statistical analysis
Results are expressed as means ± SEM. Behavioural data (Fig. 2 and Fig. 3) were analysed by a randomized two-factor (group and block) block factorial analysis of variance (ANOVA), while all other parameters (Table 3, Table 4 and Table 5) were analysed for intergroup differences by one-way ANOVA. ANOVA was followed by Fisher's protected least significant differences test for post-hoc comparisons. Correlation was determined using simple regression analysis (Fig. 4, Table 6 and Table 7). GB-STAT™ 6.5.4 (Dynamic Microsystems, Inc., Silver Spring, MD, USA) and StatView 4.01 (MindVision Software, Abacus Concepts, Inc., Berkeley, CA, USA) were used for the statistical analyses. Statistical significance was set at P < 0.05.Results
Body weight
Final body weights did not differ among the groups (vehicle group: 430 ± 22 g; TAK-085 group: 464 ± 12 g; Aβ group: 460 ± 12 g; and TAK-085 + Aβ group: 465 ± 7 g). Findings from the brain slices prepared after 16–17 days of Aβ infusion (of the Aβ-infused rats) clearly indicated deposition of the infused Aβ1–40 in the cortico-hippocampal regions (data not shown).Effect of TAK-085 on radial-maze learning ability
The effect of long-term administration of TAK-085 on working and reference memory-related learning abilities is presented as the mean number of WMEs and RMEs for each group with data averaged over blocks of 5 trials [Fig. 2 (left) and 3 (left), respectively]. Randomized two-factor (block and group) ANOVA revealed a significant effect of both blocks of trials (P = 0.011) and groups (P < 0.0001) but without a significant block × group interaction (P = 0.9541) on the number of WMEs (Fig. 2, left). Similarly, ANOVA revealed a significant main effect of both blocks of trials (P < 0.0001) and groups (P = 0.0001) with a significant block × group interaction (P < 0.0001) on the number of RMEs (Fig. 3, left). These results indicate that TAK-085 administration improves reference memory-related learning ability in young rats.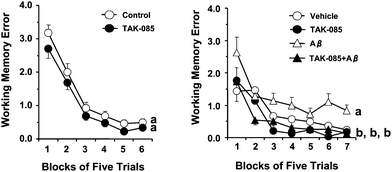 | ||
| Fig. 2 Effects of long-term administration of TAK-085 on the number of working memory errors (WMEs) (left) and the effect of the infusion of amyloid β (Aβ) peptide1–40 into the rat cerebral ventricle on the number of WMEs (right). Left: Control rats (5% gum Arabic-administered rats, n = 13), TAK-085 rats (n = 14). After completing the initial behaviour test, each of the 2 groups (Control and TAK-085) was subdivided into 2 groups: the control group was infused with either Aβ (Aβ group, n = 6) or vehicle (Vehicle group, n = 7), while the TAK-085 group was divided into a vehicle-infused TAK-085 group (TAK-085 group, n = 6) and an Aβ-infused TAK-085 group (TAK-085 + Aβ group, n = 8). The 4 groups of rats were again behaviorally tested after mini osmotic pump implantation. Each value represents the number of WMEs as the mean ± SEM in each block of 5 trials. The main effects of the blocks of trials and groups are indicated in the Results section. The significance of the differences among the 4 groups was determined by randomized two-factor (block and group) analysis of variance (ANOVA) followed by the Bonferroni post hoc test. Details of the subtest analyses between the 2 groups of the main effects of blocks of trials and groups are shown in Table 2. Groups without a common letter are significantly different at P < 0.05 in the 5 trials from final blocks. The data were analyzed by one-way ANOVA followed by Fisher's protected least significant difference test for post hoc comparisons. | ||
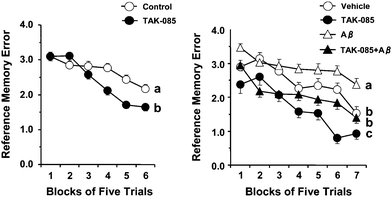 | ||
| Fig. 3 Effects of chronic administration of TAK-085 on the number of reference memory errors (RMEs) (left) and the effect of the infusion of amyloid β (Aβ) peptide1–40 into the rat cerebral ventricle on number of RMEs (right). Left: Control rats (5% gum Arabic-administered rats, n = 13), TAK-085 rats (n = 14). After completing the initial behaviour test, each of the 2 groups (Control and TAK-085) was subdivided into 2 groups: the control group was infused with either Aβ (Aβ group, n = 6) or vehicle (Vehicle group, n = 7), while the TAK-085 group was divided into a vehicle-infused TAK-085 group (TAK-085 group, n = 6) and an Aβ-infused TAK-085 group (TAK-085 + Aβ group, n = 8). The 4 groups of rats were again behaviorally tested after mini osmotic pump implantation. Each value represents the number of working memory errors (WMEs) as the mean ± SEM in each block of 5 trials. The main effects of the blocks of trials and groups are indicated in the Results section. The significance of the differences among the 4 groups was determined by randomized two-factor (block and group) analysis of variance (ANOVA) followed by a Bonferroni post hoc test. Details of the subtest analyses between the 2 groups of the main effects of the blocks of trials and groups are shown in Table 2. Groups without a common letter are significantly different at P < 0.05 in the 5 trials from the final blocks. The data were analyzed by one-way ANOVA followed by Fisher's protected least significant difference test for post hoc comparisons. | ||
The right panels in both figures show the effect of TAK-085 pre-administration to the vehicle and Aβ groups [Fig. 2 (right) and 3 (right), respectively]. Randomized two-factor (block and group) ANOVA revealed a significant main effect of both trial blocks [F(6, 234) = 38.31, P < 0.0001] and groups [F(3, 117) = 38.31, P < 0.0001] on the number of WMEs and RMEs [blocks: F(6, 234) = 48.36, P < 0.0001; groups: F(3, 117) = 34.90, P < 0.0001] with a significant block × group interaction on the number of WMEs (P < 0.0001) (Fig. 2, right) and that of RMEs (P = 0.0102) (Fig. 3, right).
Subtest analyses (Table 2) of the WMEs and RMEs showed the effect of Aβ on vehicle rats [WMEs: blocks of trials (P < 0.001) and groups (P = 0.002) with a significant block × group interaction (P = 0.050); RMEs: blocks of trials (P <0.001) and groups (P = 0.005) with the tendency of a significant block × group interaction (P = 0.071)]. These analyses demonstrated that the number of WMEs and RMEs was significantly higher in the Aβ group than in the vehicle group [Fig. 2 (right) and 3 (right), respectively], suggesting learning impairment, a well-known characteristic of AD. Similarly, subset analyses (Table 2) of the number of WMEs and RMEs showed the effect of Aβ on TAK-085 rats [WMEs: blocks of trials (P < 0.001) and groups (P < 0.001) without a significant block × group interaction (P = 0.860); RMEs: blocks of trials (P < 0.001) and groups (P < 0.001) without a significant block × group interaction (P = 0.759)]. The number of WMEs (P = 0.228), but not RMEs (P = 0.036) in the 5 trials from first block did not differ significantly between the Aβ and TAK-085 + Aβ groups, respectively, and the number of WMEs and RMEs in the 5 trials from the final block was significantly less in the TAK-085 + Aβ group than in the Aβ group (WMEs: P = 0.0013; RMEs: P = 0.0046) (Fig. 2, right and Fig. 3, right). In addition, each number of WMEs and RMEs in all trials (35 trials) was significantly less in the TAK-085 + Aβ group than in the Aβ group (WMEs: P = 0.0002, RMEs: P < 0.0001). These results demonstrated that the TAK-085 + Aβ group had lower WME and RME scores as compared with those of the Aβ group [Fig. 2 (right) and 3 (right), respectively], suggesting that pre-administration of TAK-085 prevents cognitive impairments caused by infusion of Aβ into the cerebral ventricle of rats.
| Working memory error | Reference memory error | |||
|---|---|---|---|---|
| Block | Group | Block | Group | |
| a Data are presented in Fig. 2 and Fig. 3. Aβ, amyloid β peptide. | ||||
| Vehicle vs. Aβ | <0.001 [F(6,204) = 10.11] | 0.002 [F(1,34) = 11.88] | <0.001 [F(6,204) = 16.87] | 0.005 [F(1,34) = 8.86] |
| Vehicle vs.TAK-085 | <0.001 [F(6,204) = 18.83] | <0.067 [F(1,34) = 3.58] | <0.001 [F(6,204) = 30.41] | <0.001 [F(1,34) = 22.63] |
| Vehicle vs.TAK-085 + Aβ | <0.001 [F(6,234) = 17.43] | 0.026 [F(1,39) = 5.34] | <0.001 [F(6,234) = 18.50] | 0.006 [F(1,39) = 8.35] |
| Aβ vs.TAK-085 | <0.001 [F(6,174) = 13.60] | <0.001 [F(1,29) = 25.90] | <0.001 [F(6,174) = 13.92] | <0.001 [F(1,29) = 67.99] |
| Aβ vs. TAK-085 + Aβ | <0.001 [F(6,234) = 23.63] | <0.001 [F(1,39) = 52.74] | <0.001 [F(6,234) = 16.96] | <0.001 [F(1,39) = 63.84] |
| TAK-085 vs. TAK-085 + Aβ | <0.001 [F(6,234) = 41.23] | 0.988 [F(1,39) = 0.00] | <0.001 [F(6,234) = 25.92] | 0.004 [F(1,39) = 9.65] |
Fatty acid profiles of the plasma and brain
The fatty acid composition of plasma in the rats is shown in Table 3. The plasma levels of EPA, DHA, and docosapentaenoic acid C22:5(n-3) were higher in both the TAK-085 and TAK-085 + Aβ rats than in the vehicle and Aβ rats, respectively, but those of arachidonic acid [AA; 20:4(n-6)] were significantly lower (P < 0.05) in the TAK-085 and TAK-085 + Aβ rats than in the vehicle and Aβ rats, respectively. TAK-085 administration brought about a significant decrease in the plasma n-6/n-3 molar ratio in the TAK-085 and TAK-085 + Aβ rats; however, it did not affect the plasma levels of palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, or the unsaturation index.| Vehicle (n = 7) | TAK-085 (n = 6) | Aβ (n = 6) | TAK-085 + Aβ (n = 8) | |
|---|---|---|---|---|
| a Values of fatty acids are expressed as mol % of total fatty acids. Values are means ± SEM, Means in a row with superscripts without a common letter differ, P < 0.05. Aβ, amyloid β peptide. | ||||
| Palmitic acid C16:0 | 26.76 ± 0.43 | 27.87 ± 0.34 | 27.73 ± 0.53 | 27.69 ± 0.40 |
| Stearic acid C18:0 | 12.59 ± 0.27 | 11.89 ± 0.27 | 12.49 ± 0.49 | 11.86 ± 0.43 |
| Oleic acid C18:1(n-9) | 12.50 ± 0.73 | 12.30 ± 0.63 | 13.04 ± 0.80 | 11.90 ± 0.59 |
| Linoleic acid C18:2(n-6) | 20.77 ± 0.44 | 23.34 ± 0.55 | 19.89 ± 0.51 | 23.89 ± 0.39 |
| Linolenic acid C18:3(n-3) | 0.28 ± 0.01 | 0.34 ± 0.02 | 0.29 ± 0.02 | 0.33 ± 0.02 |
| Arachidonic acid C20:4(n-6) | 23.70 ± 1.24a | 15.29 ± 0.45b | 23.10 ± 1.06a | 15.68 ± 0.56b |
| Eicosapentaenoic acid C20:5(n-3) | 0.42 ± 0.03b | 3.75 ± 0.24a | 0.47 ± 0.03b | 3.49 ± 0.20a |
| Docosapentaenoic acid C22:5(n-3) | 0.51 ± 0.04b | 1.62 ± 0.07a | 0.60 ± 0.05b | 1.51 ± 0.06a |
| Docosahexaenoic acid C22:6(n-3) | 1.67 ± 0.07b | 2.94 ± 0.07a | 1.61 ± 0.13b | 2.96 ± 0.12a |
| n-6/n-3 | 15.49 ± 0.41a | 4.50 ± 0.18b | 14.63 ± 0.74a | 4.80 ± 0.16b |
| Unsaturation index (USI) | 164.69 ± 3.90 | 165.92 ± 2.15 | 161.47 ± 3.06 | 166.42 ± 1.95 |
The major fatty acid composition in the rat cortex and hippocampus is shown in Table 4. Long-term administration of TAK-085 significantly enhanced the DHA proportion in the hippocampus of the TAK-085 and TAK-085 + Aβ rats and enhanced the EPA proportion in the cortex of TAK-085 + Aβ rats. The EPA and DHA proportion in the cortex and the DHA proportion in the hippocampus of TAK-085 + Aβ rats were significantly higher than those in Aβ rats. Administration of TAK-085 significantly decreased the proportion of AA in the cortex of the TAK-085 and TAK-085 + Aβ rats and in the hippocampus of TAK-085 + Aβ rats, causing a significant increase in the ratio of DHA/AA in both the cortex and the hippocampus. The ratios in the cortex and hippocampus were significantly higher in the TAK-085 + Aβ rats than in the Aβ rats.
| Vehicle (n = 7) | TAK-085 (n = 6) | Aβ (n = 6) | TAK-085 + Aβ (n = 8) | |
|---|---|---|---|---|
| a Values of fatty acids are expressed as mol% of total fatty acids. Values are means ± SEM, Means in a row with superscripts without a common letter differ, P < 0.05. Aβ, amyloid β peptide. | ||||
| Cerebral cortex | ||||
| Arachidonic acid C20:4(n-6) | 10.27 ± 0.17a | 9.62 ± 0.09b | 10.31 ± 0.11a | 9.67 ± 0.09b |
| Eicosapentaenoic acid C20:5(n-3) | 0.15 ± 0.01b | 0.17 ± 0.01a, b | 0.15 ± 0.01b | 0.18 ± 0.01a |
| Docosahexaenoic acid C22:6(n-3) | 15.51 ± 0.19a, b | 15.85 ± 0.18a | 15.04 ± 0.18b | 15.81 ± 0.20a |
| C22:6(n-3)/C20:4(n-6) | 1.51 ± 0.02a, b | 1.65 ± 0.03a | 1.46 ± 0.02b | 1.64 ± 0.01a |
| Hippocampus | ||||
| Arachidonic acid C20:4(n-6) | 11.84 ± 0.12a, b | 11.47 ± 0.30b, c | 12.32 ± 0.30a | 11.13 ± 0.17c |
| Eicosapentaenoic acid C20:5(n-3) | 0.27 ± 0.03 | 0.25 ± 0.03 | 0.22 ± 0.01 | 0.25 ± 0.02 |
| Docosahexaenoic acid C22:6(n-3) | 12.20 ± 0.17b | 13.08 ± 0.20a | 12.16 ± 0.11b | 13.26 ± 0.13a |
| C22:6(n-3)/C20:4(n-6) | 1.03 ± 0.01b | 1.14 ± 0.03a | 0.99 ± 0.02b | 1.19 ± 0.02a |
Highly significant positive correlations were observed between the plasma levels of EPA and DHA and both the proportion of DHA and the DHA/AA ratio in the rat cortex and hippocampus (Table 6). Significant negative correlations were also observed between the plasma levels of EPA and DHA and the proportion of AA in the rat cortex and hippocampus. Similarly, the plasma AA proportion was positively correlated with the AA proportion in the cortex and hippocampus and negatively correlated with the DHA proportion in the hippocampus and the DHA/AA ratio in the cortex and hippocampus. These results indicate that dietary administration of TAK-085 accumulates DHA, reduces AA in the cortico-hippocampal regions of the brain, and is associated with a decreased DHA/AA ratio.
Oxidative status of the plasma and brain
The levels of both LPO and ROS were significantly higher in the cerebral cortex and hippocampus of Aβ rats than in those of the vehicle, TAK-085, and TAK-085 + Aβ rats (Table 5). LPO levels were significantly lower in the cortex of the TAK-085 + Aβ rats than in the cortex of the vehicle rats (P < 0.05). The level of ROS in the cortex was significantly lower in the TAK-085 rats than in the vehicle rats. The ROS level in the hippocampus was also significantly lower in the TAK-085 + Aβ rats than in the vehicle rats. Negative correlations between the LPO and ROS levels and EPA and DHA proportions and between the DHA/AA ratios in the cortex and hippocampus were observed (Table 7); in particular, significantly negative correlations were observed between the DHA/AA ratio and LPO and ROS levels in the cortex and between the DHA/AA ratio and the ROS level in the hippocampus.| Vehicle (n = 7) | TAK-085 (n = 6) | Aβ (n = 6) | TAK-085 + Aβ (n = 8) | |
|---|---|---|---|---|
| a Values are means ± SEM, Means in a row with superscripts without a common letter differ, P < 0.05. Aβ, amyloid β peptide. Thiobarbituric acid reactive substance (TBARS) levels indicate lipid peroxide levels. | ||||
| Plasma | ||||
| TBARS (nmol mL−1) | 3.68 ± 0.42 | 3.45 ± 0.32 | 3.21 ± 0.33 | 3.16 ± 0.22 |
| Cerebral cortex | ||||
| TBARS (nmol mg protein−1) | 2.84 ± 0.17b | 2.73 ± 0.18b, c | 3.55 ± 0.11a | 2.23 ± 0.19c |
| Reactive oxygen species (pmol min−1 mg protein−1) | 0.18 ± 0.01b | 0.14 ± 0.01c | 0.27 ± 0.01a | 0.16 ± 0.02b, c |
| Hippocampus | ||||
| TBARS (nmol mg protein−1) | 2.26 ± 0.15b | 2.44 ± 0.11b | 2.93 ± 0.06a | 1.92 ± 0.11b |
| Reactive oxygen species (pmol min−1 mg protein−1) | 0.26 ± 0.01b | 0.22 ± 0.03b, c | 0.40 ± 0.03a | 0.17 ± 0.01c |
In contrast, positive correlations were observed between AA proportion in the cortex and LPO and ROS levels in the same tissue as well as between AA proportion and ROS levels in the hippocampus. These results indicate that dietary administration of TAK-085 reduces oxidative stress levels in brain tissues.
Correlations between learning ability and the proportion of fatty acids and oxidative status in the plasma and brain
Regression analyses between the number of RMEs in the final block of the radial maze task and the proportion of fatty acids and oxidative status in the plasma and brain are shown in Fig. 4. Significantly negative correlations were seen between the number of RMEs, the plasma EPA proportion, the DHA proportion, and the DHA/AA ratio in the hippocampus, whereas inversely significant positive correlations were seen between the number of RMEs, the plasma AA proportion, and the ROS levels in the cortex and hippocampus.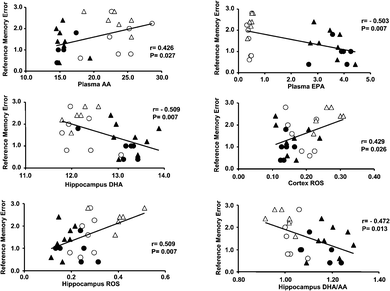 | ||
| Fig. 4 Correlations between the number of reference memory errors (RMEs) in the final blocks, the fatty acid proportion, and the levels of oxidative stress levels in plasma and brain tissues. ○ = vehicle; ● = TAK-085; △ = Aβ; and ▲ = TAK-085 + Aβ. | ||
Discussion
The present study provides evidence that dietary supplementation of n-3 PUFAs (EPA + DHA) improves reference memory-related learning ability in young rats after being fed a fish oil-deficient diet for 2 generations and protects against memory impairment in the AD model of rats infused with Aβ. This protective effect was accompanied by cortico-hippocampal increases in EPA and DHA and in DHA/AA molar ratio and by a decrease in AA in these brain tissues. Moreover, the correlations between plasma EPA or DHA and cortico-hippocampal DHA or DHA/AA ratio were highly positive, while the correlation between plasma n-3 PUFAs and brain tissue AA levels was highly negative, suggesting that plasma n-3 PUFA effectively deposits DHA and pulls off AA in the brain tissues after crossing the blood-brain barrier.We previously demonstrated an improvement in reference but not working memory-related learning ability, in the same radial maze task in both young and aged rats on dietary DHA after 3 generations were fed a fish oil-deficient diet.9,10 Pre-administration of this n-3 fatty acid prevented cognitive impairment in Aβ-infused AD model rats. It is well established that n-3 fatty acids can alter the membrane fatty acid composition of the brain. Consistent with our previous studies16,17 with purified DHA or EPA, long-term administration of TAK-085 significantly decreased AA levels in the plasma and cortex (Table 3, 4). Plasma AA proportion was positively correlated with AA levels in the cortex and hippocampus (Table 6), suggesting that TAK-085 administration-induced decreases in plasma AA levels contribute to decreased AA levels in the cortico-hippocampal region. AA levels in the cortex and hippocampus were positively correlated with the levels of ROS or LPO in these brain tissues, respectively (Table 7) but were negatively correlated with the cortico-hippocampal DHA/AA ratios that were negatively correlated with the number of RMEs (cortex: r = −0.800, P < 0.0001; hippocampus: r = −0.852, P < 0.0001). Additionally, the AA level in the cortex tended to be positively correlated with the number of RMEs (r = 0.340, P = 0.083). Therefore, the decrease in AA in the cortex and/or hippocampus may contribute to the protective effect of TAK-085 on the impairment of cognitive ability in Aβ-infused rats.
| Cortex | Hippocampus | |||||||
|---|---|---|---|---|---|---|---|---|
| AA (mol %) | EPA (mol %) | DHA (mol %) | DHA/AA | AA (mol %) | EPA (mol %) | DHA (mol %) | DHA/AA | |
| a AA, arachinonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; N.S, not significance. Results are evaluated with simple regression analysis. P values are expressed inside the parentheses. | ||||||||
| Plasma (mol %) | ||||||||
| EPA (P-value) | −0.743 (<0.0001) | +0.568 (0.002) | +0.396 (0.041) | +0.783 (<0.0001) | −0.490 (0.010) | N.S. | +0.789 (<0.0001) | +0.745 (<0.0001) |
| DHA (P-value) | −0.512 (0.006) | N.S. | +0.745 (<0.0001) | +0.732 (<0.0001) | −0.653 (0.0002) | +0.578 (0.002) | +0.566 (0.002) | +0.818 (<0.0001) |
| AA (P-value) | +0.769 (<0.0001) | N.S. | N.S. | −0.636 (0.0004) | +0.450 (0.019) | N.S. | −0.674 (0.0001) | −0.657 (0.0002) |
| Cerebral cortex | Hippocampus | |||||||
|---|---|---|---|---|---|---|---|---|
| AA | EPA | DHA | DHA/AA | AA | EPA | DHA | DHA/AA | |
| a AA, arachinonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; N.S., not significance; ROS, reactive oxygen species; TBARS, thiobarbituric acid reactive substances. Results are evaluated with simple regression analysis. P values are expressed inside the parentheses. | ||||||||
| TBARS (P-value) | +0.507 (0.007) | −0.418 (0.030) | −0.327 (0.095) | −0.561 (0.002) | N.S. | N.S. | N.S. | N.S. |
| ROS (P-value) | +0.649 (0.0003) | −0.512 (0.006) | −0.345 (0.078) | −0.672 (0.0001) | +0.512 (0.006) | N.S. | −0.571 (0.002) | −0.641 (0.0003) |
DHA deficiency is associated with a loss of discriminative learning ability.25,26DHA levels in the hippocampus are very low in patients with AD compared with those of brain samples from age-matched human controls.11,12 Thus, a change in brain DHA level may be related to behaviour impairments.27 We reported that a small increase (in mol%) in DHA content significantly contributed to limiting memory deficits in DHA-deficient rats.17 Thus, the small but significant increase in cortico-hippocampal DHA proportion in the TAK-085/TAK-085 + Aβ rats after TAK-085 administration (Table 4) seen in the present study is consistent with the findings of our earlier report.17 An increased DHA/AA ratio is associated with increased memory-related learning ability in young,9 aged10 and AD model rats16,17 with a concurrent decrease in brain LPO and/or ROS levels. In this study, the cortico-hippocampal DHA/AA ratios correlated negatively with LPO and/or ROS formation (Table 7). The DHA/AA ratios also correlated negatively with the number of RMEs (Fig. 4), suggesting a contribution to the protective effect of TAK-085 against memory-related learning ability impairments in AD model rats accompanied with increased oxidative stress.
The mechanism by which this correlation affects memory enhancement and amyloid burden is not yet clear. The free radical theory of AD pathology involves amyloid-induced oxidative stress.28 Because increasing levels of DHA in the cortex of aged or AD model rats significantly increase antioxidative status,16,17,29 we hypothesise that the DHA/AA ratio acts an indirect antioxidant indicator by inhibiting the AA level in the neuronal plasma membrane.16 Lee et al. reported that Monascus-fermented red mold rice including antioxidants ameliorated Aβ-induced impairment of memory and learning ability via repressing Aβ1–40 accumulation in the hippocampus of Aβ1–40-infused AD model rats.30 Thus, an increase in the DHA/AA ratio at least partially protects the cortico-hippocampal region from oxidative insult and provides protection against memory impairment in Aβ-infused rats. DHA inhibits the accretion of Aβ1–42 in neuronal membrane domains of the cerebral cortex31 and of Aβ-induced apoptosis-like neuronal cell death.16DHA administration reduces amyloid burden and prevent dendritic pathology in AD model mice.32,33 Dietary DHA also limits amyloid, oxidative damage and synaptic and cognitive deficits in a transgenic mouse model of AD.34 Furthermore, we recently reported that DHA significantly inhibits the in vitro fibrillation of Aβ1–4035 or Aβ1–4236 and that amyloid fibrillation-induced apoptosis is reduced by DHA in neuronal cell culture.36 Thus, the finding that TAK-085 pre-administration induces protection against memory impairment with concurrent DHA accretion in the brain is in line with our16,17 and other studies.37,38
Long-term administration of EPA increases DHA levels (and the DHA/AA ratio) in the plasma and cortico-hippocampal tissues and exerts beneficial effects on memory formation/protection in normal or Aβ-infused rats with a corresponding decrease in oxidative stress and an increase in the expression of synaptic plasticity-related proteins.21 This suggests that EPA protects against Aβ peptide-induced memory deficits in AD model rats after its transformation into DHA. However, the conversion rate from EPA to DHA through the desaturation/chain elongation system is very limited in humans and has essentially no impact on plasma DHA,39,40 thus suggesting that the lowering effect of TAK-085 on the risk of AD may be less than that of DHA alone. On the other hand, long-term administration of EPA exerts a neuroprotective effect on the modulation of rat hippocampal synaptic plasticity by not only its capacity to increase brain DHA but also its direct effects on neurons and glial cells.20 Our results are also consistent in the mice model of AD.41 Higher proportions of EPA on red blood cell membranes were also associated with better cognitive outcome.42 Additionally, potential neuroprotective effects of n-3 PUFAs have been detailed in amyloidogenesis, oxidative stress and inflammation of AD.43 There is often a discrepancy in the effect of n-3 PUFA supplementation in humans based on the source, such as pure DHA or fish oil products, including a combination of both DHA and EPA.44 Such disparate data suggest that the properties of EPA induce an increased blood flow and nutrient supply as well as increased removal of toxic metabolites and proteins from the brain that might otherwise augment AD-related degeneration. Finally, it suggests that TAK-085 is more effective than DHA or EPA alone for preventing the effects of neuronal diseases such as AD. Further studies are required to accumulate additional data on TAK-085.
Conclusion
TAK-085 protects against Aβ-induced memory deficit in AD model rats. This phenomenon is accompanied by an accumulation of DHA and EPA, a decrease in AA, and/or an increase in the DHA/AA ratio in the cortico-hippocampal tissues with a corresponding decrease in oxidative stress. The present data suggest that TAK-085 can be used as a possible therapeutic agent for protecting against AD-induced learning deficiencies. Nonetheless, further studies are needed to collect additional TAK-085 data.Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education Culture, Sports, Science and Technology, Japan (19500324, M.H.).References
- S. Kalmijn, L. J. Launer, A. Ott, J. C. Witteman, A. Hofman and M. M. Bretele, Ann. Neurol., 1997, 42, 776–782 CrossRef CAS.
- M. C. Morris, D. A. Evans, J. L. Bienias, C. C. Tangney, D. A. Bennett, R. S. Wilson, N. Aggarwal and J. Schneider, Arch. Neurol., 2003, 60, 940–946 CrossRef.
- B. M. van Gelder, M. Tijhuis, S. Kalmijn and D. Kromhout, Am. J. Clin. Nutr., 2007, 85, 1142–1147 CAS.
- M. C. Morris, D. A. Evans, C. C. Tangney, J. L. Bienias and R. S. Wilson, Arch. Neurol., 2005, 62, 1849–1853 CrossRef.
- Y. Freund-Levi, M. Eriksdotter-Jönhagen, T. Cederholm, H. Basun, G. Faxén-Irving, A. Garlind, I. Vedin, B. Vessby, L. O. Wahlund and J. Palmblad, Arch. Neurol., 2006, 63, 1402–1408 CrossRef.
- P. Green and E. Yavin, J. Neurosci. Res., 1998, 52, 129–136 CrossRef CAS.
- H. Suzuki, S. J. Park, M. Tamura and S. Ando, Mech. Ageing Dev., 1998, 101, 119–128 CrossRef CAS.
- S. Delion, S. Chalon, D. Guilloteau, J. C. Besnard and G. Durand, J. Neurochem., 1996, 66, 1582–1591 CAS.
- S. Gamoh, M. Hashimoto, K. Sugioka, M. Shahdat Hossain, N. Hata, Y. Misawa and S. Masumura, Neuroscience, 1999, 93, 237–241 CrossRef CAS.
- S. Gamoh, M. Hashimoto, S. Hossain and S. Masumura, Clin. Exp. Pharmacol. Physiol., 2001, 28, 266–270 CrossRef CAS.
- M. Söderberg, C. Edlund, K. Kristensson and G. Dallner, Lipids, 1991, 26, 421–425 CrossRef CAS.
- W. J. Lukiw, J. G. Cui, V. L. Marcheselli, M. Bodker, A. Botkjaer, K. Gotlinger, C. N. Serhan and N. G. Bazan, J. Clin. Invest., 2005, 115, 2774–2783 CrossRef CAS.
- D. J. Selkoe, Neuron, 1991, 6, 487–498 CrossRef CAS.
- R. A. Floyd and K. Hensley, Neurobiol. Aging, 2002, 23, 795–807 CrossRef CAS.
- J. Choi, A. I. Levey, S. T. Weintraub, H. D. Rees, M. Gearing, L. S. Chin and L. Li, J. Biol. Chem., 2003, 279, 13256–13264 CrossRef.
- M. Hashimoto, S. Hossain, T. Shimada, K. Sugioka, H. Yamasaki, Y. Fujii, Y. Ishibashi, J. Oka and O. Shido, J. Neurochem., 2002, 81, 1084–1091 CrossRef CAS.
- M. Hashimoto, Y. Tanabe, Y. Fujii, T. Kikuta, H. Shibata and O. Shido, J. Nutr., 2005, 135, 549–555 CAS.
- E. Kawakita, M. Hashimoto and O. Shido, Neuroscience, 2006, 139, 991–997 CrossRef CAS.
- M. Katakura, M. Hashimoto, M. S. Hossain, S. Gamoh, T. Okui, K. Matsuzaki and O. Shido, Neuroscience, 2009, 160, 651–660 CrossRef CAS.
- A. Kawashima, T. Harada, H. Kami, T. Yano, K. Imada and K. Mizuguchi, J. Nutr. Biochem., 2010, 21, 268–277 CrossRef CAS.
- M. Hashimoto, S. Hossain, Y. Tanabe, A. Kawashima, T. Harada, T. Yano, K. Mizuguchi and O. Shido, J. Nutr. Biochem., 2009, 20, 965–973 CrossRef CAS.
- H. Ohkawa, N. Ohishi and K. Yagi, Anal. Biochem., 1979, 95, 351–358 CAS.
- G. Lepage and C. C. Roy, J. Lipid Res., 1986, 27, 114–120 CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–305 CAS.
- M. Neuringer, W. E. Connor, D. S. Lin, L. Barstad and S. Luck, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 4021–4025 CAS.
- N. Yamamoto, M. Saitoh, A. Moriuchi, M. Nomura and H. Okuyama, J. Lipid Res., 1987, 28, 144–151 CAS.
- N. Salem, Jr, T. Moriguchi, R. S. Greiner, K. McBride, A. Ahmad, J. N. Catalan and B. Slotnick, J. Mol. Neurosci., 2001, 16, 299–307 Search PubMed.
- C. Behl and B. Moosmann, Free Radical Biol. Med., 2002, 33, 182–191 CrossRef CAS.
- M. S. Hossain, M. Hashimoto, S. Gamoh and S. Masumura, J. Neurochem., 2008, 72, 1133–1138 CrossRef.
- C. L. Lee, T. F. Kuo, C. L. Wu, J. J. Wang and T. M. Pan, J. Agric. Food Chem., 2010, 58, 2230–2238 CrossRef CAS.
- M. Hashimoto, S. Hossain, H. Agdul and O. Shido, Biochim. Biophys. Acta., 2005, 1738, 91–98 CAS.
- F. Calon, G. P. Lim, F. Yang, T. Morihara, B. Teter, O. Ubeda, P. Rostaing, A. Triller, N. Salem, Jr, K. H. Ashe, S. A. Frautschy and G. M. Cole, Neuron, 2004, 43, 633–645 CrossRef CAS.
- G. P. Lim, F. Calon, T. Morihara, F. Yang, B. Teter, O. Ubeda, N. Salem, Jr, S. A. Frautschy and G. M. Cole, J. Neurosci., 2005, 25, 3032–3040 CrossRef CAS.
- G. M. Cole, G. P. Lim, F. Yang, B. Teter, A. Begum, Q. Ma, M. E. Harris-White and S. A. Frautschy, Neurobiol. Aging, 2005, 26, 133–136 CrossRef.
- M. Hashimoto, H. M. Shahdat, S. Yamashita, M. Katakura, Y. Tanabe, H. Fujiwara, S. Gamoh, T. Miyazawa, H. Arai, T. Shimada and O. Shido, J. Neurochem., 2008, 107, 1634–1646 CrossRef CAS.
- S. Hossain, M. Hashimoto, M. Katakura, K. Miwa, T. Shimada and O. Shido, J. Neurochem., 2009, 111, 568–579 CrossRef CAS.
- C. Song and D. Horrobin, J. Lipid Res., 2004, 45, 1112–1121 CAS.
- S. Lim and H. Suzuki, J. Nutr., 2001, 131, 319–324 CAS.
- G. C. Burdge and P. C. Calder, Reprod., Nutr., Dev., 2005, 45, 581–597 CrossRef CAS.
- M. Plourde and S. C. Cunnane, Appl. Physiol., Nutr., Metab., 2007, 32, 619–634 CrossRef CAS.
- F. Calon, G. P. Lim, T. Morihara, F. Yang, O. Ubeda, N. Salem, Jr, S. A. Frautschy and G. M. Cole, Eur. J. Neurosci., 2005, 22, 617–626 CrossRef.
- C. C. Chiu, K. P. Su, T. C. Cheng, H. C. Liu, C. J. Chang, M. E. Dewey, R. Stewart and S. Y. Huang, Progress Neuro-Psychopharmacol. Biol. Psychiatry, 2008, 32, 1538–1544 Search PubMed.
- S. C. Dyall, Int. J. Alzheimers Dis., 2010, 274128 Search PubMed.
- S. C. Cunnane, M. Plourde, F. Pifferi, M. Bégin, C. Féart and P. Barberger-Gateau, Prog. Lipid Res., 2009, 48, 239–256 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
