Metabolic fate of orally administered enzymatically synthesized glycogen in rats
Takashi
Furuyashiki
*a,
Hiroki
Takata
a,
Iwao
Kojima
a,
Takashi
Kuriki
a,
Itsuko
Fukuda
b and
Hitoshi
Ashida
bc
aInstitute of Health Sciences, Ezaki Glico Co., Ltd., 4-6-5, Utajima, Nishiyodogawa-ku, Osaka, Japan 555-8502. E-mail: furuyashiki-takashi@glico.co.jp; takata-hiroki@glico.co.jp; kojima-iwao@glico.co.jp; kuriki-takashi@glico.co.jp
bResearch Center for Food Safety and Security, Graduate School of Agricultural Science, Kobe University, 1-1 Rokkodai-cho, Nada-ku, Kobe, Hyogo, Japan 657-8501. E-mail: itsuko@silver.kobe-u.ac.jp; ashida@kobe-u.ac.jp
cDepartment of Agrobioscience, Graduate School of Agricultural Science, Kobe University, 1-1 Rokkodai-cho, Nada-ku, Kobe, Hyogo, Japan 657-8501
First published on 26th January 2011
Abstract
We developed a new process for enzymatically synthesized glycogen (ESG), which is equivalent in physicochemical properties to natural-source glycogen (NSG) except its resistant property to degradation by α-amylase in vitro. In this study the metabolic fates of orally administered ESG in rats were investigated by a single oral administration test and a 2 week ingestion test. The glycemic index of ESG was 79. After the 2 week ingestion of ESG, the cecal content and production of short chain fatty acids were significantly increased, the pH value of cecal content was lowered, and the counts of Bifidobacterium and Lactobacillus in feces were significantly increased. Additionally, plasma levels of triacylglycerol and total cholesterol were significantly reduced by ESG. In contrast, NSG did not affect these parameters at all. The results collectively suggest that around 20% of orally administered ESG was transferred to the cecum in the form of polymer and assimilated into short chain fatty acids by microbiota and the polymer affected lipid metabolism.
Introduction
Glycogen, which is a highly branched α-D-glucan containing α-1,4 and α-1,6 linkages, is a major storage form of glucose found in many organisms ranging from bacteria to primates. Many researchers have studied the metabolism and intracellular functions of glycogen in the liver and muscles. However, except for some reports on the immunomodulatory effects of glycogen,1–3 few studies have investigated the effects of orally administered glycogen. Among common foods, shellfish and animal livers are known to be rich in glycogen. In addition, phytoglycogen, a glycogen-like polysaccharide, has also been isolated from several higher plants.4Our group recently developed an in vitro method to synthesize glycogen from starch using isoamylase (EC 3.2.1.68), branching enzyme (EC 2.4.1.18), and amylomaltase (EC 2.4.1.25).5 The enzymatically synthesized glycogen (ESG) is equivalent to natural-source glycogen (NSG) in structural parameters, physicochemical properties, and molecular shape.5,6 In an in vitro test, treatment of ESGs with excess α-amylase resulted in the formation of high molecular weight resistant fractions (>1000 kDa) in a roughly 40% yield even after prolonged reaction. However, the content and size of the resistant fractions of NSGs were found to vary: 1 of 6 NSGs possessed high molecular weight resistant fractions, as do ESGs, but the others showed only small resistant fractions (<10 kDa) such as branched oligosaccharides.7 Evaluation of ESG by the AOAC method 2002-02 revealed that it contains about 20% of dietary fiber content.7
Dietary fiber is defined as carbohydrate polymers that are neither digested nor absorbed in the small intestine, and which have beneficial physiological effects in humans. The benefits of dietary fiber ingestion are well studied and many effects have been clarified: some soluble dietary fibers reduce plasma cholesterol levels, especially LDL cholesterol, while insoluble dietary fibers are known to increase the bulk of feces.8,9 Dietary fibers such as oligofructose, inulin, and resistant starch have been reported to function as prebiotics, which increase intestinal bifidobacteria in animals and humans.10–12 Moreover, it has been suggested that appropriate consumption of dietary fiber reduces the risk of some diseases, including cardiovascular diseases, colorectal cancer, and diabetes mellitus.13–15
In this study, the metabolic fate of orally administered ESG and the effects of ESG ingestion on intestinal fermentation and lipid metabolism were investigated in rats. In order to elucidate the role of the molecular size of digestion-resistant fractions, the effect of ingestion of NSG from mussel, which had the smallest resistant fractions among the 6 types of NSG tested previously in vitro, were also studied.
Materials and methods
Chemicals
ESG was synthesized in our laboratory using previously described methods.5 NSG from mussel was obtained from Laboratories Serobiologique (France). The molecular weight and in vitro digestibility of ESG and NSG reported previously are summarized in Table 1. Standards of acetic, propionic, and butyric acids for gas/liquid chromatography (GLC) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). α-Amylase, isoamylase, and glucoamylase were obtained from Sigma Chemical Co. (St. Louis, MO, USA), Hayashibara Biochemical Laboratories (Okayama, Japan), and TOYOBO Co. Ltd. (Osaka, Japan), respectively. All other chemicals were analytical grade and were obtained from Wako Pure Chemical Industries.Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of Ezaki Glico Co., Ltd., and performed in accordance with Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan). Six-week-old male Sprague Dawley (SD) rats were purchased from Japan SLC, Inc. (Shizuoka, Japan) and provided with commercially available standard chow and tap water in the same housing for 1 week to allow acclimatization to the environment before starting the experiments. All animal experiments were performed in a temperature-controlled room (23 ± 1 °C) with a 12 h light and 12 h dark cycle. Experiment 1: for the single oral administration test, 14 rats were divided into 2 groups of 7 rats each with approximately equal body weights. After the rats were fasted for 18 h, glucose or ESG were administered to the rats at a dosage of 2 g per kg body weight by gavage. Blood samples were collected from the tail vein before and 10, 20, 30, and 60 min after the administration of glucose or ESG; these samples were centrifuged (900 × g, 5 min), and plasma samples were obtained for the measurement of blood glucose and insulin levels. Plasma glucose and insulin levels were measured using commercial kits purchased from Wako and Shibayagi Co. Ltd. (Gunma, Japan), respectively.Experiment 2: for the 2 week ingestion test, 21 rats were divided into 3 groups of 7 rats each with approximately equal average body weights. The rats were housed in plastic cages and fed the control, high, or low ESG diets as shown in Table 2 for 2 weeks. During the feeding period, body weight and food intake were measured every 2–3 days. A few fresh fecal pellets excreted on day 10 were used for analyzing fecal microbiota and all feces excreted on days 11 and 12 were collected to determine α-glucan content. At the end of the 2 week feeding, the rats were euthanized under anesthesia without fasting; subsequently, their livers were removed and weighed, and the epididymal fat and cecal contents were collected.
| Diet group | |||
|---|---|---|---|
| Control | High ESG | Low ESG | |
| a Mineral mix and vitamin mix were the AIN93 composition obtained from Oriental Yeast Co., Ltd. (Tokyo, Japan). | |||
| α-Corn starch | 63 | — | 47.2 |
| ESG | — | 63 | 15.8 |
| Casein | 20 | 20 | 20 |
| Cellulose | 5 | 5 | 5 |
| Corn oil | 5 | 5 | 5 |
| Mineral mixa | 5 | 5 | 5 |
| Vitamin mixa | 2 | 2 | 2 |
| Calorie (kcal per 100 g) | 377 | 377 | 377 |
Experiment 3: to compared ESG and NSG, we substituted NSG from mussel for ESG in the high ESG group diet (Table 2). Twenty one rats were divided into 3 groups of 7 rats each with approximately equal average body weights, then rats were fed control, high ESG or high NSG diets for 2 weeks under the same conditions as Experiment 2.
Experiment 4: to evaluate the effect of ESG on plasma lipids, 14 rats were divided into 2 groups of 7 rats. The rats were housed in plastic cages and fed with the control or high ESG diets as shown in Table 2 for 2 weeks. After 6 h of fasting, rats were euthanized under anesthesia, blood samples were collected from the heart, and plasma concentrations of triacylglycerol, total cholesterol, HDL cholesterol, and non-esterified fatty acids (NEFA) were measured using the relevant commercial kits purchased from Wako.
Glucose and α-glucan analyses
The fecal samples and cecal contents were lyophilized and crushed, and 100–200 mg of these samples were dissolved in phosphate-buffered saline (PBS) and homogenized. The homogenate was centrifuged (900 × g, 10 min), and the glucose concentration in the supernatant was determined. To determine the α-glucan concentration in terms of glucose residues, a 200 μl aliquot of the supernatant was incubated with α-amylase (200 mU) and isoamylase (30 U) for 2 h at 37 °C in 0.2 M acetic buffer (pH 5.5), and subsequently with glucoamylase (20 U) for 16 h at 37 °C. It has been shown that successive treatment by 3 types of enzymes converts glycogen to glucose quantitatively.7 The glucose concentration in the reaction mixture was determined and the α-glucan concentration was calculated by subtracting the glucose concentration of the untreated supernatant from that of the reaction mixture.The excretion rate of α-glucan in the feces was estimated by the following calculation: (total amount of daily feces) × (fecal concentration of α-glucan)/(total daily intake of α-glucan).
Short-chain fatty acid (SCFA) analysis
For measurement of the cecal concentration of SCFAs, samples were prepared for GLC analysis according to the method of Chen and Lifschitz16 with slight modification. Briefly, 1 g of cecal content was homogenized in 5 ml of distilled deionized water, and the pH of this solution was measured. The homogenates were acidified to pH 2.0 with 2 N H2SO4 and centrifuged (6000 × g, 15 min, 4 °C); the supernatant was filtered through a microconcentrator (Vivascience AG, Hannover, Germany) with a 5000 Da molecular-mass cut-off membrane by centrifugation (8000 × g, 1 h, 4 °C). To measure the SCFA concentration, 2 μl of the filtrate was injected into a GLC (Shimadzu GC-2014, Shimadzu Co., Kyoto, Japan) equipped with a flame ionization detector and a Thermon-3000 glass column packed with Chromosorb W (Shimadzu Co., Kyoto, Japan). Nitrogen was used as the carrier gas at a flow rate of 50 ml min−1. The analysis conditions were as follows: the injection port and the detector were maintained at 250 °C, temperature of the column oven was maintained at 130 °C for 13 min, and then at 240 °C for 6 min to remove other organic compounds.Measurement of fecal microbiota
To assess the effect of ESG on fecal microbiota, 1 g of fresh feces collected on day 10 was diluted 10-fold with PBS containing 0.1% (w/v) purified agar and 0.05% (w/v) cysteine-HCl. Then, Bifidobacterium, Bacteroidaceae, Enterobacteriaceae, Streptococcus, Staphylococcus, and Lactobacillus were cultured in their respective selective media and the colonies were counted, as described by Ikeda et al.17Statistical analysis
All data are presented as the mean ± SD. The relationships between pairs of data sets; i.e., plasma glucose after the single oral administration test and the effects of ESG ingestion on plasma lipids, were analyzed by Student's t-test. The other effects of ESG ingestion were evaluated by one-way ANOVA and subsequently by a post-hoc Fisher's protected least significant difference test, using Stat View software (Abacus Concepts, Berkeley, CA). p values of <0.05 were considered to be statistically significant.Results
Single oral administration test
ESG or glucose was administered to rats at a dosage of 2 g per kg body weight, and plasma glucose and insulin levels were determined at the indicated intervals (Fig. 1). Although plasma glucose levels increased immediately after administration in both groups, glucose levels in the ESG-treated group were significantly lower than those in the control group at 20 and 30 min after administration (p = 0.043 and 0.031, respectively) as shown in Fig. 1A. The glycemic index was calculated from the area under the curve (AUC) of plasma glucose level. The glycemic index of ESG was 79.0 and significantly lower than that of glucose (p = 0.046) (Fig. 1B). The changes in the plasma insulin level were similar to those in the plasma glucose level, but there was no significant difference between the control and ESG-treated groups (Fig. 1C). The AUC of insulin level was also not changed.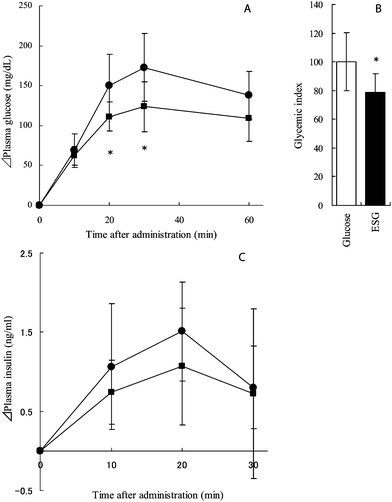 | ||
| Fig. 1 Plasma glucose and insulin concentration in glucose-treated and ESG-treated rats. Rats were administered glucose (control) or ESG at a dosage of 2 g per kg body weight. Before administration and then at 10, 20, 30, and 60 min after, blood samples were collected and centrifuged to obtain plasma. Concentrations of glucose (A) and insulin (C) were determined. Closed circles and squares indicate control and ESG-treated rats, respectively. The glycemic index was calculated and is shown in B. Values are mean ± S.D.; n = 7. Asterisks indicate significant difference from the control group: *p < 0.05. | ||
Effects of 2 week ingestion of ESG
As shown in Table 3, after the 2 week ingestion test, the body weight gain in the high ESG group was significantly lower than that in the control group (p = 0.043). Food intake in the high ESG group was slightly lower than that in the control group, but the difference was not statistically significant. The epididymal fat weight of the high ESG group was significantly reduced to 81.6% of that of the control group (p = 0.031). However, there was no significant change in the liver weight in any group (Table 3).| Diet group | |||
|---|---|---|---|
| Control | High ESG | Low ESG | |
| a Values are mean ± S D; n = 7. b The means in each row with different superscript letters are significantly different (p < 0.05). | |||
| Body weight gain (g) | 95.4 ± 8.4b | 85.1 ± 9.6a | 96.7 ± 8.5b |
| Food intake (g day−1/7 rats) | 109 ± 10 | 103 ± 12 | 111 ± 9 |
| Organ weight (% of body weight) | |||
| Liver | 4.06 ± 0.38 | 4.12 ± 0.25 | 4.20 ± 0.38 |
| Epididymal fat | 1.41 ± 0.25b | 1.15 ± 0.17a | 1.42 ± 0.20b |
The quantity of cecal content increased significantly in the high ESG group compared with the control (p < 0.0001) (Fig. 2A); the cecal content of the low ESG group was 127% of that of the control group, but this change was not statistically significant. The pH of cecal content in both high ESG (7.27) and low ESG groups (7.61) was significantly lower (p < 0.0001 and p = 0.0008, respectively) than that in the control group (8.14) (Fig. 2B).
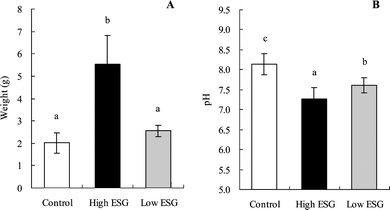 | ||
| Fig. 2 Weight and pH value of cecal content were altered by ESG ingestion. Rats were fed high or low ESG diets for 2 weeks. The cecal content was weighed (A) and pH (B) measured, as described in the materials and methods section. Values are mean ± S.D.; n = 7. Bars with different letters are significantly different from each other (p < 0.05). | ||
Since these changes suggested modification of the fermentation in the cecum by ESG ingestion, the major fecal microbes were analyzed (Table 4). Although there were no changes in the counts of Bacteroidaceae, Enterobacteriaceae, Streptococcus or Staphylococcus, the viable counts of Bifidobacterium and Lactbacillus in the high ESG group were significantly increased compared to the control group (p < 0.0001 and p = 0.0065).
| Diet group | |||
|---|---|---|---|
| Control | High ESG | Low ESG | |
| a Values are mean ± S D; n = 7. b The means in each row with different superscript letters are significantly different (p < 0.05). | |||
| log10 per g wet feces | |||
| Bifidobacterium | 7.1 ± 0.66a | 10.7 ± 0.25b | 7.8 ± 1.30a |
| Bacteroidaceae | 9.5 ± 0.48 | 9.7 ± 0.52 | 9.5 ± 0.21 |
| Enterobacteriaceae | 6.8 ± 0.66 | 6.4 ± 0.43 | 6.4 ± 0.43 |
| Streptococcus | 7.4 ± 0.71 | 6.7 ± 0.57 | 7.1 ± 0.45 |
| Staphylococcus | 6.0 ± 0.44 | 4.4 ± 0.92 | 5.9 ± 0.60 |
| Lactobacillus | 7.6 ± 0.43a | 9.1 ± 0.45b | 8.4 ± 0.43a |
It is recognized that changes in fermentation often result in increases in short-chain fatty acids (SCFAs). To clarify this point, the concentrations of acetate, propionate, and butyrate in the cecal contents were determined by GLC analysis (Fig. 3). In the high ESG group, the amounts of acetate, propionate, and butyrate were significantly increased to 269% (p = 0.0002), 516% (p < 0.0001), and 766% (p = 0.0006), respectively, of those in the control group, and the total amount of SCFAs was 350% (p < 0.0001) of that in the control group. Although the increases in acetate, propionate, and butyrate levels in the low ESG group were not significantly increased compared to those in the control group, the total amount of SCFAs was significantly (p = 0.031) increased to 186% of the control group.
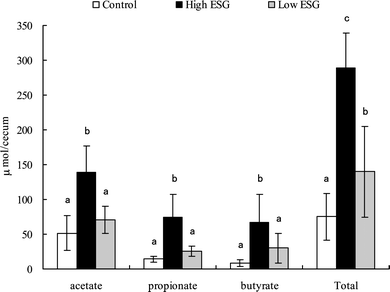 | ||
| Fig. 3 The amounts of cecal SCFAs were increased by ESG ingestion. Rats were fed high or low ESG diets for 2 weeks. The amounts of the SCFAs, acetate, propionate, and butyrate in the cecal content were measured with GLC as described in the materials and methods section. The total SCFA amount is the sum of the amounts of these 3 acids. Values are mean ± S.D.; n = 7. Bars with different letters are significantly different from each other (p < 0.05). | ||
All the above results strongly suggested that indigestible fractions of ESG were transferred to the cecum and assimilated by enteric bacteria. To examine this possibility, the concentrations of α-glucan and glucose in the cecal content and fecal samples were measured. As shown in Table 5, the concentration of α-glucan in the cecal content was significantly increased (more than 10-fold) in the high ESG group as compared to that in the control group (p = 0.0093), while the increase in the low ESG group (more than 2.6-fold) was not significant (p = 0.62) when compared to that in the control group. However, glucose concentrations in the cecal content of the high ESG (9.1 mg per g content) and low ESG groups (9.6 mg per g content) were significantly higher (p = 0.030 and p = 0.021, respectively) than that of the control group (3.6 mg per g content). The concentrations of α-glucan and glucose in the feces of the high ESG group were significantly increased to 878% and 244%, respectively, as compared to those in the control group. The amounts of excreted α-glucan in the feces of the control, high ESG and low ESG groups were estimated to be 0.06%, 0.48% and 0.13% of the carbohydrate intake, respectively.
| Diet group | |||
|---|---|---|---|
| Control | High ESG | Low ESG | |
| a Values are mean ± S D; n = 7. b The means in each row with different superscript letters are significantly different (p < 0.05). | |||
| mg per g cecal content | |||
| α-Glucan | 0.77 ± 0.50a | 8.0 ± 7.8b | 2.0 ± 1.9a |
| Glucose | 3.6 ± 2.4a | 9.1 ± 5.0b | 9.6 ± 5.7b |
| mg per g feces | |||
| α-Glucan | 2.7 ± 1.7a | 23.7 ± 12.6b | 7.2 ± 2.5a |
| Glucose | 3.6 ± 3.0a | 8.8 ± 1.0b | 2.8 ± 2.1a |
Comparison of ESG and NSG from mussel
As summarized in Table 1, an in vitro digestion test revealed that the molecular size of the resistant fractions produced by α-amylase degradation of NSG from mussel was much smaller than that from ESG. Additionally, NSG from mussel was degraded most easily by α-amylase in 6 types of NSG tested in the previous in vitro study.7 To investigate the participation of the molecular size of the resistant fractions in the fiber-like effects, we substituted NSG for ESG in the high ESG group (Table 2), and rats were fed control, high ESG or high NSG diets for 2 weeks under the same conditions as previously. After the 2 week feeding, increased amount and reduced pH value of the cecal content of the ESG group were again observed, but the values for the NSG group were similar to those of the control group (Fig. 4A, B). Additionally, SCFA production, α-glucan concentration in the cecum, and epididymal fat weight were not altered in the NSG group as compared to the corresponding values in the control group (Table 6).| Diet group | |||
|---|---|---|---|
| Control | ESG | NSG | |
| a Values are mean ± S D; n = 7. b The means in each row with different superscript letters are significantly different (p < 0.05). | |||
| Cecal SCFA production (μmol per cecum) | |||
| Acetate | 48.2 ± 24.1a | 230.1 ± 139.2b | 38.1 ± 11.4a |
| Propionate | 16.0 ± 6.2a | 80.4 ± 43.6b | 14.5 ± 3.5a |
| Butyrate | 10.1 ± 4.6a | 62.0 ± 29.0b | 14.5 ± 3.5a |
| Cecal α-glucan (mg per g cecal content) | 0.54 ± 0.16a | 5.42 ± 7.12b | 0.28 ± 0.20a |
| Epididymal fat (g) | 1.6 ± 0.2a | 1.1 ± 0.1b | 1.6 ± 0.3a |
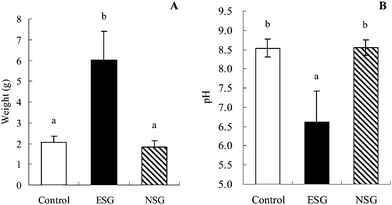 | ||
| Fig. 4 Weight and pH value of cecal content were altered by ESG but not NSG ingestion. Rats were fed ESG or NSG diets for 2 weeks. The cecal content was weighed (A) and pH was measured (B), as described in the materials and methods section. Values are mean ± S.D.; n = 7. Bars with different letters are significantly different from each other (p < 0.05). | ||
Plasma lipid profiles
Finally, the effects of ESG on plasma lipids profile were evaluated. As shown in Table 7, plasma triacylglycerol and total cholesterol concentrations in the high ESG group were significantly reduced (p = 0.008 and 0.0007, respectively) to 51.8 and 47.2 mg dL−1 as compared to the control group (86.2 and 62.2 mg dL−1), after a 2 week ingestion of experimental diets. Although the concentration of plasma HDL-cholesterol was also significantly (p = 0.002) reduced in the high ESG group, the ratio of HDL/total cholesterol in the high ESG group was significantly higher than that in the control group (p = 0.022). The concentration of NEFA was not significantly reduced by ESG.| Diet group | ||
|---|---|---|
| Control | High ESG | |
| a Values are mean ± S D; n = 7. b Mean values were significantly different from those of control group by Student's t-test (* p < 0.05, ** p < 0.01). | ||
| Triacylglycerol (mg dL−1) | 86.2 ± 15.7 | 51.8 ± 11.7** |
| Cholesterol | ||
| Total (mg dL−1) | 62.2 ± 5.5 | 47.2 ± 4.2** |
| HDL (mg dL−1) | 42.1 ± 3.1 | 34.2 ± 3.3** |
| HDL/total ratio (%) | 67.8 ± 3.0 | 72.5 ± 2.8* |
| NEFA (mEq L−1) | 0.68 ± 0.19 | 0.53 ± 0.12 |
Discussion
In the present study, the metabolic fate of orally administered ESG in rats was investigated. The glycemic index of ESG was found to be 79, which was consistent with the dietary fiber content (Table 1) determined by the AOAC method.7In vivo, in addition to pancreatic α-amylase, maltase, isomaltase and glucoamylase acting in the intestinal surface18 can work to degrade glycogen, and we concluded that the combination of these digestive enzymes degraded approximately 80% of the glucosyl moieties of ESG to glucose. The molecular character of the remaining 20% of ESG remains to be studied. In vitro study using pancreatic α-amylase revealed that around 40% of ESG remained as a resistant fraction (α-macrodextrin) with a high molecular weight (>1000 kDa) (Table 1).7 We hypothesized that the in vivo remnant from ESG has a similar structure to the in vitro α-macrodextrin, a highly branched polymer. Various changes, such as the quantity and pH of cecum contents and the microbiota in feces, strongly indicated that the polymers were transferred to the cecum and assimilated to SCFAs by microbiota, especially Bifidobacterium and Lactobacillus. Only a negligible amount (<0.5% of ingestion) was excreted in feces.We also carried out oral administration tests with NSG from mussel, which displayed the smallest molecular of α-amylase resistant fractions in an in vitro study.7 The quantity and pH of cecal contents were not altered after the rats ingested a diet containing 63% NSG from mussel for 2 weeks; and the amounts of α-glucan in the cecum was not increased (Table 6). Our previous study showed that the physicochemical properties of ESGs were equivalent to NSGs, but the fine structures of ESGs and NSGs were slightly different. In short, in the ESG molecule, the α-1,6-branch linkages were more regularly distributed than those in the NSG molecules.7 Consequently, most of spans between the branches in ESG molecules should be 1 or 2 glucose units, which can not be attacked by α-amylase.7 On the other hand, the spans in NSG molecules should be varied (0–4 units), and some parts with relatively long spans could be degraded. Thus, the difference should be related to the different resistant properties of ESGs and NSGs. However, the in vitro study showed that at least 1 out of 6 types of NSG possessed high molecular weight resistant fractions.7 It is possible that such NSGs having high resistance to α-amylase would be similarly metabolized in gut with ESG and affect lipid metabolism. We surmise that the molecular size of the resistant fractions of NSGs could be affected by the physiological conditions of organisms, such as their nutritional status; therefore, studies of the effects of ingestion of NSG with high molecular weight resistant fractions should be of great interest.
In this study, it was demonstrated that ESG ingestion affected lipid metabolism by decreasing plasma triacylglycerol, total cholesterol, and body fat accumulation, and increasing the HDL/total cholesterol ratio. These effects of ESG appeared to be similar to those of dietary fiber. However, the effects of fiber are known to be varied. Soluble fibers, pectin, guar gum, oat gum, and psyllium, reduced cholesterol concentrations in serum and liver, but did not change triacylglycerol concentration in cholesterol-fed SD rats.19 Epidemiological studies and dietary intervention studies have revealed that appropriate soluble dietary fiber lowers total and LDL cholesterol concentration without affecting HDL and triacylglycerol.8,13,20,21 However, a study in rodents revealed that resistant starch decreases both total serum cholesterol and triacylglycerol, with reduction of epididymal fat pad weight.22 Potato pulp, which is rich in resistant starch, also lowers serum cholesterol and triacylglycerol levels.23 Inulin and inulin-type fructans reduce plasma cholesterol as well as triacylglycerol levels through the downregulation of hepatic lipogenesis.24 In addition, Keenan et al.25 reported that consumption of a large amount of resistant starch reduced abdominal fat accumulation in rats. The mechanism underlying these effects of dietary fibers on lipid metabolism may be at least partly related to their viscosity and/or colonic fermentation. Thus, differences in colonic fermentation should contribute to different effects on lipid metabolism. Among the natural water-soluble fibers, resistant starch, inulin and inulin-type fructans have been recognized as major prebiotics,10–12 which are defined as nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or the activity of one or a few bacteria such as Bifidobacterium in the colon.26 In the present study, it was suggested that ESG ingestion increased intestinal Bifidobacterium and Lactobacillus. The effects of ESG on lipid metabolism and on the microbiota appeared to be similar to those of resistant starch, inulin and inulin-type fructans. Most studies evaluating the effects of dietary fiber on lipid metabolism have used diets containing 5%–10% dietary fiber. In this study, we used 63% and 17.8% ESG in the diet. Assuming that 20% of ESG could have a dietary-fiber like effect, these amounts could correspond to 12% and 3.5%, respectively, and were thus comparable with the amounts of dietary fiber in the previous studies. Interestingly, NSG from mussel did not affect lipid metabolism at all after a 2 week ingestion (data not shown). Thus, it was considered that the high molecular weight resistant fractions of ESG critically contribute to the observed effects on lipid metabolism.
This study demonstrated that ESG ingestion greatly increased SCFA production in the cecum. Acetate, a principal SCFA in the colon, has been reported to have favorable effects on energy metabolism.27–29 Acetate serves as a lipogenic substrate when incorporated into hepatocytes, whereas propionate is a competitive inhibitor of the transport of acetate into hepatocytes.30 With respect to cholesterol metabolism, propionate inhibits cholesterol synthesis, while acetate is the primary substrate for cholesterol synthesis.31 Thus, the increased ratio of propionate/acetate in the gut is likely to reduce the plasma triacylglycerol and cholesterol concentrations. It was reported that the molar proportion of cecal SCFA concentrations in rats fed a 65% cornstarch diet for 15 days is in the order of acetate > propionate > butyrate with an approximate molar ratio of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.32 In this study, although the relative cecal concentrations of acetate, propionate, and butyrate in the control group were about 70
10.32 In this study, although the relative cecal concentrations of acetate, propionate, and butyrate in the control group were about 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, those in the high ESG group and low ESG group were about 48
10, those in the high ESG group and low ESG group were about 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 and 60
26 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22, respectively. Hence, we suggest that not only the increased SCFA quantities, but also the change in the ratios of individual SCFAs contribute to the effects on lipid metabolism.
22, respectively. Hence, we suggest that not only the increased SCFA quantities, but also the change in the ratios of individual SCFAs contribute to the effects on lipid metabolism.
A recent report revealed that SCFAs stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41, and propionate administration increases plasma leptin level in mice.33 Leptin is a well-known secretory hormone in adipose tissue, with anti-obesity properties; it is known to be related to the negative control of energy intake and the positive control of energy consumption.34 Although total SCFA production in the cecum was increased by more than 3-fold after ESG ingestion, the food intake was not affected in this study. In contrast, in our following study, plasma leptin concentration decreased slightly after ESG ingestion (unpublished data). This discrepancy can be explained on the basis of the reduction in the weight of subcutaneous adipose tissue that secretes leptin.34 Otherwise, acetate and propionate are reported to stimulate adipogenesis in adipocyte-like cell, 3T3-L1 cells.35 Further studies are warranted to clarify the mechanism of the anti-obesity effect of ESG.
Abbreviations
| ESG | Enzymatically synthesized glycogen |
| NSG | Natural-source glycogen |
| AUC | Area under the curve |
| GLC | Gas/liquid chromatography |
| SCFA | Short-chain fatty acid |
| NEFA | Non-esterified fatty acids |
Acknowledgements
This work was supported in part by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.References
- K. Ryoyama, Y. Kidachi, H. Yamaguchi, H. Kajiura and H. Takata, Anti-tumor activity of an enzymatically synthesized alpha-1,6 branched alpha-1,4-glucan, glycogen, Biosci., Biotechnol., Biochem., 2004, 68, 2332–2340 CrossRef CAS.
- R. Kakutani, Y. Adachi, H. Kajiura, H. Takata, T. Kuriki and N. Ohno, Relationship between structure and immunostimulating activity of enzymatically synthesized glycogen, Carbohydr. Res., 2007, 342, 2371–2379 CrossRef CAS.
- Y. Takaya, H. Uchisawa, H. Ichinohe, J. Sasaki, K. Ishida and H. Matsue, Antitumor glycogen from scallops and the interrelationship of structure and antitumor activity, J. Mar. Biotechnol., 1998, 6, 208–213 Search PubMed.
- D. J. Manners, Recent developments in our understanding of glycogen structure, Carbohydr. Polym., 1991, 16, 37–82 CrossRef CAS.
- H. Kajiura, R. Kakutani, T. Akiyama, H. Takata and T. Kuriki, A novel enzymatic process for glycogen production, Biocatal. Biotransform., 2008, 26, 133–140 CrossRef CAS.
- H. Kajiura, H. Takata, T. Kuriki and S. Kitamura, Structure and solution properties of enzymatically synthesized glycogen, Carbohydr. Res., 2010, 345, 817–824 CrossRef CAS.
- H. Takata, H. Kajiura, T. Furuyashiki, R. Kakutani and T. Kuriki, Fine structural properties of natural and synthetic glycogens, Carbohydr. Res., 2009, 344, 654–659 CrossRef CAS.
- E. Theuwissen and R. P. Mensink, Water-soluble dietary fibers and cardiovascular disease, Physiol. Behav., 2008, 94, 285–292 CrossRef CAS.
- D. P. Burkitt, A. R. Walker and N. S. Painter, Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease, Lancet, 1972, 2, 1408–1412 CrossRef CAS.
- J. M. Campbell, G. C. Fahey Jr and B. W. Wolf, Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats, J. Nutr., 1997, 127, 130–136 CAS.
- G. R. Gibson, E. R. Beatty, X. Wang and J. H. Cummings, Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin, Gastroenterology, 1995, 108, 975–982 CrossRef CAS.
- O. Murphy, Non-polyol low-digestible carbohydrates: food applications and functional benefits, Br. J. Nutr., 2001, 85, 47–53.
- J. Mann, Dietary carbohydrate: relationship to cardiovascular disease and disorders of carbohydrate metabolism, Eur. J. Clin. Nutr., 2007, 61, 100–111.
- T. J. Key and E. A. Spencer, Carbohydrates and cancer: an overview of the epidemiological evidence, Eur. J. Clin. Nutr., 2007, 61, 112–121.
- M. O. Weickert and A. F. Pfeiffer, Metabolic effects of dietary fiber consumption and prevention of diabetes, J. Nutr., 2008, 138, 439–442 CAS.
- H. M. Chen and C. H. Lifschitz, Preparation of fecal samples for assay of volatile fatty acids by gas-liquid chromatography and high-performance liquid chromatography, Clin. Chem., 1989, 35, 74–76 CAS.
- N. Ikeda, Y. Saito, J. Shimizu, A. Ochi, J. Mizutani and J. Watabe, Variations in concentrations of bacterial metabolites, enzyme activities, moisture, pH and bacterial composition between and within individuals in faeces of seven healthy adults, J. Appl. Bacteriol., 1994, 77, 185–194 CAS.
- G. M. Gray, Starch digestion and absorption in nonruminants, J. Nutr., 1992, 122, 172–177 CAS.
- J. W. Anderson, A. E. Jones and S. Riddell-Mason, Ten different dietary fibers have significantly different effects on serum and liver lipids of cholesterol-fed rats, J. Nutr., 1994, 124, 78–83 CAS.
- J. W. Anderson and T. J. Hanna, Impact of nondigestible carbohydrates on serum lipoproteins and risk for cardiovascular disease, J. Nutr., 1999, 129, 1457S–1466 CAS.
- A. T. Erkkila and A. H. Lichtenstein, Fiber and cardiovascular disease risk: how strong is the evidence?, J. Cardiovasc. Nurs., 2006, 21, 3–8.
- E. A. de Deckere, W. J. Kloots and J. M. van Amelsvoort, Resistant starch decreases serum total cholesterol and triacylglycerol concentrations in rats, J. Nutr., 1993, 123, 2142–2151 CAS.
- N. Hashimoto, Y. Ito, K. H. Han, K. Shimada, M. Sekikawa, D. L. Topping, A. R. Bird, T. Noda, H. Chiji and M. Fukushima, Potato pulps lowered the serum cholesterol and triglyceride levels in rats, J. Nutr. Sci. Vitaminol., 2006, 52, 445–450 Search PubMed.
- M. Beylot, Effects of inulin-type fructans on lipid metabolism in man and in animal models, Br. J. Nutr., 2005, 93, 163–168.
- M. J. Keenan, J. Zhou, K. L. McCutcheon, A. M. Raggio, H. G. Bateman, E. Todd, C. K. Jones, R. T. Tulley, S. Melton, R. J. Martin and M. Hegsted, Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat, Obesity, 2006, 14, 1523–1534 CrossRef CAS.
- G. R. Gibson and M. B. Roberfroid, Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics, J. Nutr., 1995, 125, 1401–1412 CAS.
- H. Yamashita, K. Fujisawa, E. Ito, S. Idei, N. Kawaguchi, M. Kimoto, M. Hiemori and H. Tsuji, Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats, Biosci., Biotechnol., Biochem., 2007, 71, 1236–1243 CrossRef CAS.
- T. Fushimi, K. Tayama, M. Fukaya, K. Kitakoshi, N. Nakai, Y. Tsukamoto and Y. Sato, Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats, J. Nutr., 2001, 131, 1973–1977 CAS.
- S. Sakakibara, T. Yamauchi, Y. Oshima, Y. Tsukamoto and T. Kadowaki, Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice, Biochem. Biophys. Res. Commun., 2006, 344, 597–604 CrossRef CAS.
- N. M. Delzenne and C. M. Williams, Prebiotics and lipid metabolism, Curr. Opin. Lipidol., 2002, 13, 61–67 CrossRef CAS.
- J. M. Wong, R. de Souza, C. W. Kendall, A. Emam and D. J. Jenkins, Colonic health: fermentation and short chain fatty acids, J. Clin. Gastroenterol., 2006, 40, 235–243 CrossRef CAS.
- T. Morita, S. Kasaoka, A. Ohhashi, M. Ikai, Y. Numasaki and S. Kiriyama, Resistant proteins alter cecal short-chain fatty acid profiles in rats fed high amylose cornstarch, J. Nutr., 1998, 128, 1156–1164 CAS.
- Y. Xiong, N. Miyamoto, K. Shibata, M. A. Valasek, T. Motoike, R. M. Kedzierski and M. Yanagisawa, Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1045–1050 CrossRef CAS.
- J. M. Friedman and J. L. Halaas, Leptin and the regulation of body weight in mammals, Nature, 1998, 395, 763–770 CrossRef CAS.
- Y. H. Hong, Y. Nishimura, D. Hishikawa, H. Tsuzuki, H. Miyahara, C. Gotoh, K. C. Choi, D. D. Feng, C. Chen, H. G. Lee, K. Katoh, S. G. Roh and S. Sasaki, Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43, Endocrinology, 2005, 146, 5092–5099 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
