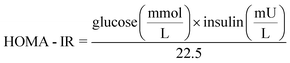(−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice
Sudathip
Sae-tan
a,
Kimberly A.
Grove
a,
Mary J.
Kennett
b and
Joshua D.
Lambert
*a
aDepartment of Food Science, The Pennsylvania State University, 332 Food Science Building, University Park, PA 16802, USA. E-mail: jdl134@psu.edu; Fax: (+814)863-6132
bDepartment of Veterinary and Biomedical Science, The Pennsylvania State University, University Park, PA 16802, USA
First published on 4th January 2011
Abstract
(−)-Epigallocatechin-3-gallate (EGCG), the major polyphenol in green tea, has been shown to prevent the development of obesity in rodent models. Here, we examined the effect of EGCG on markers of fat oxidation in high fat-fed C57bl/6J mice. High fat-fed mice treated with 0.32% dietary EGCG for 16 weeks had reduced body weight gain and final body weight (19.2% and 9.4%, respectively) compared to high fat-fed controls. EGCG-treatment decreased fasting blood glucose, plasma insulin, and insulin resistance by 18.5%, 25.3%, and 33.9%, respectively. EGCG treatment also reduced markers of obesity-related fatty liver disease in high fat-fed mice. Gene expression analysis of skeletal muscle showed that EGCG increased mRNA levels of nuclear respiratory factor (nrf)1, medium chain acyl coA decarboxylase (mcad), uncoupling protein (ucp)3, and peroxisome proliferator responsive element (ppar)α by 1.4–1.9-fold compared to high fat-fed controls. These genes are all related to mitochondrial fatty acid oxidation. In addition, EGCG increased fecal excretion of lipids in high fat-fed mice. In summary, it appears that EGCG modulates body weight gain in high fat-fed mice both by increasing the expression of genes related fat oxidation in the skeletal muscle and by modulating fat absorption from the diet.
1.0 Introduction
(−)-Epigallocatechin-3-gallate (EGCG, Fig. 1) is the most abundant and widely-studied catechin in green tea (Camellia sinensis, Theaceae).1 Previous studies have shown that green tea and EGCG inhibit the development of obesity in laboratory animal models, and may modulate body weight in human subjects [reviewed in 2].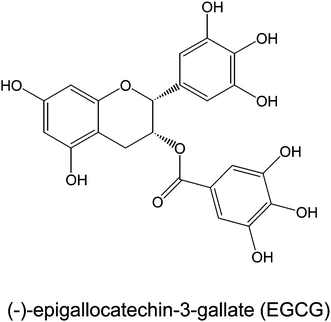 | ||
| Fig. 1 Chemical structure of EGCG. | ||
Treatment of C57bl/6J mice with 0.32% dietary EGCG for 16 wk has been shown to reduce high fat diet induced body weight gain, markers of Type II diabetes, and severity of obesity-related fatty liver disease (ORLFD).3 Analysis of fecal lipid content showed that EGCG treatment increased fecal lipid excretion, and that these increases strongly correlated with decreased body weight gain. Pancreatic lipase is the major digestive enzyme responsible for the cleavage of triglycerides in the small intestine.4EGCG has previously been shown to inhibit pancreatic lipasein vitro.5,6 We have recently found that EGCG-mediated inhibition of pancreatic lipase is non-competitive with respect to substrate concentration (Grove et al., unpublished results).
Other recent studies have suggested that EGCG and green tea may modulate expression of genes related to lipid metabolism. For example, Klaus et al. have reported that treatment of New Zealand black mice with 1% dietary EGCG for 4 wks reduced high fat diet-induced increases in body weight and body fat mass.7 Analysis of fecal energy content showed that EGCG-treated mice had higher energy levels in the feces than high fat-fed controls indicating that EGCG caused malabsorption of dietary energy intake. The authors also reported that EGCG increased the mRNA expression of uncoupling protein (ucp)2 and ucp3 in the liver and skeletal muscle, respectively. These genes are related to fatty acid oxidation and increased expression may explain some of the effects of EGCG on body weight gain. EGCG treatment also down-regulated several genes related to fatty acid synthesis and storage in the liver and white adipose tissue including: stearoyl coA dehydrogenase 1, malic enzyme, and glucokinase. Similar effects on gene expression in adipose tissue were also observed in EGCG-treated, high fat-fed C57bl/6J mice.8
Comparatively little has been reported on the effect of EGCG on the expression of genes related to obesity in skeletal muscle. Treatment of obese beagle dogs with 80 mg kg−1, p.o. green tea extract prior to feeding for 12 wks had no significant effect on body weight or body fat mass, but did reduce plasma triglyceride levels and improve insulin sensitivity. Gene expression analysis showed that green tea treatment increased the mRNA expression of peroxisome proliferator-activated receptor (ppar)α and lipoprotein lipase in the muscle. No significant effect on the mRNA levels of the glucose transporter 4 was observed.9 By contrast, Chen et al., found no significant effect of EGCG of green tea on the expression of pparα or ucp3 in the skeletal muscle of high fat-fed rats treated for 27 wks.10
The differences between effects observed in the dog and those observed in the rat may be the result of differences in the bioavailability of tea polyphenols in these species.11,12 The absolute oral bioavailability of EGCG in the rat is only 1.6%, whereas in the dog oral bioavailability is much higher. Our previous studies showing that the mouse is more similar to humans than the rat in terms of biotransformation and bioavailability of EGCG.13,14 For this reason and due to the widespread use of mouse models for the study of obesity prevention, we examined the expression of several genes related to lipid oxidation in the skeletal muscle of high fat-fed mice. We compared these changes to observed effects on physiological markers of obesity, type II diabetes and ORLFD. Herein, we report the results of our study.
2.0 Experimental
2.1 Chemicals and diet
EGCG (93% pure) was purchased from Taiyo Green Power Company (Jiangsu, China). Diets were prepared by Research Diets, Inc. (New Brunswick, NJ) and the formulations have been previously reported.3 Primers for real-time PCR were synthesized by the Genomics Core Facility at The Pennsylvania State University (University Park, PA). All other chemicals were of the highest grade commercially-available.2.2 Animals and treatment
Male C57BL/6J mice (5 wk old) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained on 12 h light/dark with access to food and waterad libitum. Mice were housed in shoebox cages on corn cob bedding. All experiments were approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University (IACUC #28962). After a two wk acclimatization period, mice were divided into 3 treatment groups: low fat (LF, 10% kcal fat, n = 16), high fat (HF, 60% kcal fat, n = 22) and high fat plus 0.32% EGCG (HFE, n = 22). Mice were maintained on experimental diets for 15 wk, and body weight and diet consumption were recorded weekly. Rate of body weight gain was calculated by subtracting initial body weight from final body weight and dividing the difference by 15 weeks. Fecal samples (24 h total cage sample) were collected during weeks 10, 12 and 14 of the study. At the end of the study, mice were fasted for 7 h and blood was taken by cardiac puncture from anesthetized mice. Plasma samples were isolated by centrifugation at 700 × g for 15 min and stored at −80 °C for later analysis. Livers were harvested, rinsed and weighed. Sections of livers were fixed in 10% formalin. The remaining liver sample was frozen at −80 °C for biochemical analysis. Muscle samples were collected from the rear leg, washed with saline, and frozen at −80 °C for biochemical analysis.2.3 Fasting blood glucose, plasma insulin, and insulin resistance
Fasting blood glucose measurements were recorded at weeks 0, 4, 8, 10, 12 and 14 for each treatment group using a hand-held Contour glucose monitor (Bayer Healthcare, Tarrytown, NY). Mice were fasted for 7 h after the cage bedding was changed (to prevent copraphagy) and blood was sampled from the tail vein. Fasting plasma insulin was determined at the completion of the experiment using an ELISA for Rat/Mouse Insulin (Millipore, Billerica, MA) according to the manufacturer's protocol. Insulin resistance was estimated from the final blood glucose and insulin values by the Homeostasis model assessment of insulin resistance (HOMA-IR):152.4 Analysis of obesity-related fatty liver disease
ORFLD was assessed using both biochemical and histopathological methods. Hepatic triglycerides were determined by homogenizing liver tissue (50–100 mg) in 2 mL isopropanol. The homogenate was centrifuged at 2000 × g for 10 min and the supernatant was analyzed with L-Type Triglyceride M kit (Wako Diagnostics, Richmond, VA). Lipid concentrations were normalized to tissue wet weight. Plasma alanine aminotransferase (ALT) levels were determined using a spectrophotometric method (Catachem, Inc., Bridgeport, CT).For histopathological diagnosis, formalin-fixed liver sections were dehydrated and embedded in paraffin blocks. Sections (6 μm) were cut and stained with hematoxylin and eosin. Samples were blinded and read by a board-certified laboratory animal veterinarian with expertise in rodent pathology (MJK). Hepatic lipidosis, vacuolization and focal necrosis were determined as criteria for liver disease. Severity of lipidosis was determined semi-quantitatively based on the degree of lipid accumulation and the area of involvement. Lipidosis was scored on a scale of 0 = no significant lesions, 1 = minimal (1–20%), 2 = slight (21–40%), 3 = moderate (41–60%), 4 = marked (61–80%), 5 = severe (81–100%).
2.5 Real-time PCR analysis of gene expression
Total RNA was isolated from leg muscle samples by using Tri reagent (Sigma) according to the manufacturer's instruction. Isolated RNA was quantified using the NanoDrop ND-1000 spectrophotometer and cDNA was synthesized using reverse transcriptase. After cDNA synthesis, Real Time PCR was performed by using the SYBR Green PCR Master Mix according to the manufacturer's protocol and amplified on the ABI Prism 7000 sequence detection system. mRNA levels were normalized to β-actin. Standard curves were made by using serial dilutions from pooled cDNA samples. The sequences for the primers used are listed in Table 1.| Gene | Forward primer | Reverse primer |
|---|---|---|
| mcad | GAGCCTGGGAACTCGGCTTGA | GCCAAGGCCACCGCAACTTT |
| nrf1 | TGCAGCAGGGAGCCACTGTC | ATGGGCGGCAGCTTCACTGT |
| ucp3 | GAGCGGACCACTCCAGCGTC | TCACCACATCCGTGGGCTGG |
| pparα | ATCGGCCTGGCCTTCTAAAC | TCCCCTCCTGCAACTTCTCA |
2.6 Fecal lipid analysis
Fecal samples were combined with deionized water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w
2, w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) and incubated overnight at 4 °C. The samples were vortexed and extracted twice with equal volume of methanol:cholorform (2
v) and incubated overnight at 4 °C. The samples were vortexed and extracted twice with equal volume of methanol:cholorform (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). The organic phase was filtered through 0.45 μm PTFE membrane and dried under vacuum. The residue was weighed and normalized to fecal weight.
v). The organic phase was filtered through 0.45 μm PTFE membrane and dried under vacuum. The residue was weighed and normalized to fecal weight.
2.7 Statistical analysis
All plots show the mean ± standard error of the mean (SEM). One-way ANOVA with Tukey's post-test was used to compare BW gain, insulin, HOMA-IR, liver triglycerides, ALT, fecal lipids and hepatomegaly. Two-way ANOVA with Bonferroni's post-test was used for BW and blood glucose over the course of the study. Statistical significance was achieved at p < 0.05. All analyses were performed using GraphPad Prism (San Diego, CA).3.0 Results and discussion
In the present study, we examined the effect of dietary EGCG on markers of obesity, insulin resistance and fat oxidation in high fat-fed mice. The dose of EGCG used in the study (0.32%) corresponds to human consumption of approximately 10 cups of green tea per day (assuming a 200 mL cup and 2 g of green tea leaves) based on allometric scaling.16 Overall, there was no significant difference in the food intake between the treatment groups (data not shown). HF treatment significantly increased final body weight and rate of body weight gain compared to LF-fed mice (Table 2). By contrast high fat-fed mice treated with EGCG showed a 21.7% reduction in the rate of body weight gain and a 9.4% decrease in final body weight (p < 0.05, Table 1). Interestingly, there was no significant effect of EGCG on the weight of retroperitoneal and epididymal fat pads (data not shown). These effects on final body weight and body weight gain are similar to previously reported results, although not as dramatic in magnitude.3 The reasons for the differences are unclear, but it may be due to the relatively large standard deviation observed in experiments with this model. Neither the intestinal nor subcutaneous fat depots were examined, so it is possible that the observed changes in body weight correlate with changes in those fat depots.| LF (n = 16) | HF (n = 22) | HFE (n = 22) | |
|---|---|---|---|
| a Values represent the mean ± SEM. Values with different superscripts are statistically significantly different by one-way ANOVA with Tukey's post-test, P < 0.05. Biomarkers of Type II diabetes were collected in the fasted state. | |||
| Initial Body Weight (g) | 19.1 ± 0.3a | 18.8 ± 0.2a | 18.8 ± 0.3a |
| Final Body Weight (g) | 31.8 ± 0.7a | 49.6 ± 0.5b | 44.9 ± 1.1c |
| Rate of Weight Gain (g/wk) | 0.9 ± 0.1a | 2.3 ± 0.1b | 1.8 ± 0.1c |
| Blood Glucose (mg/dl) | 119.1 ± 5.1a | 206.7 ± 6.9b | 168.6 ± 6.1c |
| Plasma Insulin (ng mL−1) | 1.2 ± 0.1a | 6.6 ± 0.3b | 4.9 ± 0.5c |
| HOMA-IR | 6.5 ± 0.8a | 57.2 ± 3.2b | 37.8 ± 4.4c |
High fat diet significantly increased fasting blood glucose values at week 4 and continued for the rest of the treatment (Table 2, p < 0.05). HF mice had a 42.4% increase in final fasting blood glucose compared to LF mice. EGCG treatment blunted the high fat diet-mediated hyperglycemia. At the end of the experiment, the fasting blood glucose of HFE mice was 18.5% lower than HF mice. Treatment with EGCG also significantly decreased fasting plasma insulin (25.3% decrease) compared to HF (Table 2). To estimate insulin resistance, HOMA-IR was calculated with final fasting insulin and blood glucose values. HF mice had an 88.6% increase in insulin resistance compared to LF fed mice. This increase in insulin resistance was blunted in HFE mice (33.9% decrease compared to HF mice) (Table 2, p < 0.05).
ORFLD was assessed both biochemically and histopathologically (Fig. 2). Plasma ALT values were measured to assess liver damage. Average plasma ALT levels in LF and HF mice were 3.3 and 100.1 U/L, respectively (Fig. 2A). Plasma ALT levels were decreased by 50% in HFE compared to HF control mice. In HF mice, liver weight was increased by 26.1% compared to LF mice (Fig. 2B). EGCG treatment blunted the effects of the high fat diet and the mean liver weight of the EGCG treated mice was 22% less than the high fat-fed mice. Liver triglycerides were reduced by 27% in HFE compared to HF (Fig. 2C).
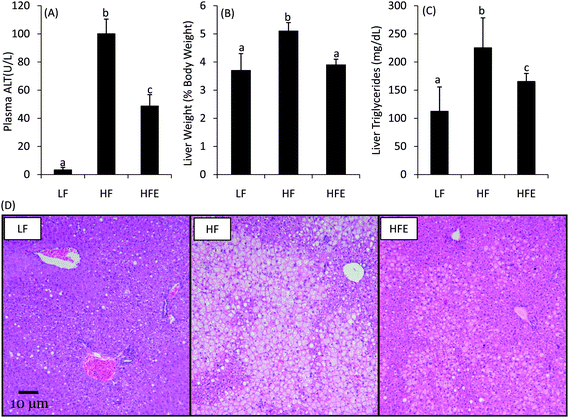 | ||
| Fig. 2 Effect of EGCG on ORFLD in high fat-fed C57bl/6J mice. EGCG supplementation reduced plasma ALT (A), hepatomegaly (B), and liver triglycerides (C) after 15 wk treatment compared to HF control mice. Bars represent the mean of n = 16–22. Error bars represent the SEM. Different superscripted letters indicate statistically significant differences by one-way ANOVA with Tukey's post-test. Histopathological analysis (D) showed that EGCG treatment reduced the severity and area of hepatic lipidosis. Photo micrographs of representative liver samples are shown at 100× magnification. | ||
Histopathological analysis confirmed the biochemical diagnosis of ORFLD (Fig. 2D). The HF mice had severe centrilobular hepatic lipidosis with focal necrosis. HFE mice had visibly less fat accumulation and smaller areas of involvement. Semi-quantitative analysis of hepatic lipidosis showed a reduction in severity score from 4.8 ± 0.1 to 3.5 ± 0.5 in HFE mice (p < 0.05).
These changes in biochemical and histological parameters of obesity, diabetes and ORFLD are similar to those previously reported in the high fat diet-fed mouse model (reviewed in ref. 2). Although previous studies have examined the role of EGCG-mediated gene expression changes in the liver and adipose tissue in the prevention of obesity, the skeletal muscle had largely been ignored. Green tea consumption has been shown in both humans and animal models to increase energy expenditure and decrease respiratory quotient.7,17,18
In the present study, we assessed the impact of EGCG treatment on the expression in the skeletal muscle of several genes related to fat oxidation. We found that, compared to high fat-fed controls, EGCG-treated mice had higher expression of mcad (1.4-fold increase), nrf1 (1.5-fold increase), ucp3 (1.9-fold increase) and pparα (1.9-fold increase) (Fig. 3). These four genes are all related to fatty acid oxidation or mitochondrial gene expression. Mutations in ucp3 have been associated with decreased fat oxidation and increased risk of morbid obesity and diabetes.19 Heilbronn et al. have also shown that expression of ucp3 and nrf1 are decreased in the skeletal muscle of overweight and obese insulin-resistant individuals.20 Deficiency in mcad is a serious genetic metabolic disorder that prevents utilization of fatty acids. Based on the key biological function that these genes play in the metabolism of fatty acids, it seems clear that enhanced expression might be an effective means of increasing fat oxidation and ameliorating the effects of a high fat-diet. The present results suggest that EGCG enhances basal metabolism and increases lipid oxidation. Such gene changes may help explain the effect of EGCG on body weight gain in high fat-fed mice. Similar results were for the effect the expression of pparα in the skeletal muscle of green tea extract-supplemented obese Beagle dogs.9 The authors do not report whether the green tea used in the study contained caffeine, which represents a potential confounder. By contrast, a previous study in rats found no effect of EGCG on the expression of ucp3 or pparα.10 To our knowledge, the present data is the first report of pure EGCG enhancing the expression of genes related to fatty oxidation in the skeletal muscle.
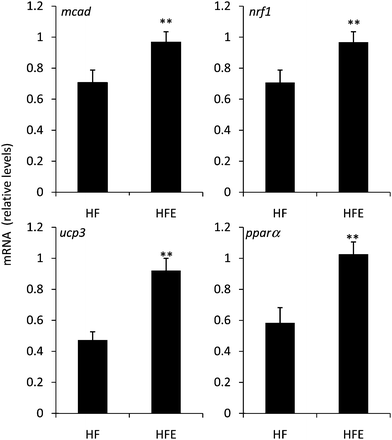 | ||
| Fig. 3 Effect of EGCG on the expression of genes related to lipid oxidation in the skeletal muscle of high fat-fed C57bl/6J mice. EGCG treatment enhanced the mRNA expression of mcad, nrf1, pparα, and ucp3 in the skeletal muscle compared to HF control mice. Values represent the mean ± SEM (n = 22). ** indicates statistical difference (p < 0.01) from HF group by Student's T-test. | ||
The underlying mechanisms for these changes in gene expression are unclear, but may be related to direct stimulation or indirect effects that modulate energy homeostasis. For example, EGCG has been reported to potently inhibit catechol-O-methyltransferase activity, and may therefore enhance sympathetic nervous system output.21,22 Increased sympathetic signaling results in increased adenosine monophosphate-activated protein (AMP) kinase signaling and enhanced fatty acid oxidation in the mitochondria.23EGCG and green tea have been shown in other models to stimulate activity of AMPK, but the underlying mechanisms have not been reported.24AMPK activation has been shown to increase expression of mcad via increased PPARα.25 It is possible that EGCG works via this mechanism in the present study, but additional work is needed to test this hypothesis. Alternatively, the observed changes in gene expression may be a compensatory response to changes in dietary nutrient absorption. Starvation state decreases adipogenesis and increases β-oxidation.26 Although treatment with EGCG clearly does not induce starvation in the present study, it does modulate lipid absorption as discussed below, which may in turn increase β-oxidation. Again, such a hypothesis needs to be tested in the present system.
Previous studies have shown that long-term treatment with EGCG can increase fecal excretion of lipids. We determined fecal lipid content gravimetrically and found an average fecal lipid concentration of 8.6 and 10.8 mg g−1 in HF and HFE, respectively. The average fecal lipid content significantly increased by 20.4% with EGCG treatment compared to HF control group (p < 0.05). This shows that in addition to the gene changes reported above, EGCG treatment affects lipid absorption. Overall the changes observed in body weight gain and markers of hyperglycemia by EGCG-treatment are likely a combination of modulation of energy absorption and fat oxidation.
In summary, in the present study we demonstrate for the first time that EGCG-mediated changes in body weight gain and markers of Type II diabetes in high fat-fed mice are associated with increased expression of fatty acid oxidation-related genes in the skeletal muscle. These changes may explain the effect of green tea on respiratory quotient and energy expenditure observed in human subjects. Further studies are needed to assess the relative impact on body weight of EGCG-mediated changes in gene expressionversusEGCG-mediated changes in nutrient absorption in human subjects.
4.0 Conclusions
Here we observed that the green tea polyphenol, EGCG, increases the skeletal muscle expression of several genes related to fatty acid oxidation in the high fat-fed C57bl/6J mouse model. This increased expression, in conjunction with the ability of EGCG to reduce dietary fat absorption from the intestine, may underlie the observed modulation of body weight gain, severity of hyperglycemia/hyperinsulinemia, and ORFLD in this model.Abbreviations
| ALT | alanine aminotransferase |
| EGCG | (−)-epigallocatechin-3-gallate |
| HF | high fat diet |
| HFE | high fat diet supplemented with 0.32% EGCG |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| LF | low fat diet |
| mcad | medium chain acyl coA dehydrogenase |
| nrf1 | nuclear respiratory factor 1 |
| ORFLD | obesity-related fatty liver disease |
| pparα | peroxisome proliferator-activated receptor α |
| ucp | uncoupling protein |
5.0 Acknowledgements
This work was supported by NIH grant AT004678 (to JDL).6.0 References
- D. A. Balentine, S. A. Wiseman and L. C. Bouwens, The chemistry of tea flavonoids, Crit. Rev. Food Sci. Nutr., 1997, 37, 693–704 CrossRef CAS.
- K. A. Grove and J. D. Lambert, Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity, J. Nutr., 2010, 140, 446–453 CrossRef CAS.
- M. Bose, J. D. Lambert, J. Ju, K. R. Reuhl, S. A. Shapses and C. S. Yang, The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice, J Nutr, 2008, 138, 1677–1683 CAS.
- R. B. Birari and K. K. Bhutani, Pancreatic lipase inhibitors from natural sources: unexplored potential, Drug Discov Today, 2007, 12, 879–889 CrossRef CAS.
- I. Ikeda, K. Tsuda, Y. Suzuki, M. Kobayashi, T. Unno, H. Tomoyori, H. Goto, Y. Kawata, K. Imaizumi, A. Nozawa and T. Kakuda, Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats, J Nutr, 2005, 135, 155–159 CAS.
- M. Nakai, Y. Fukui, S. Asami, Y. Toyoda-Ono, T. Iwashita, H. Shibata, T. Mitsunaga, F. Hashimoto and Y. Kiso, Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro, J. Agric. Food Chem., 2005, 53, 4593–4598 CrossRef CAS.
- S. Klaus, S. Pultz, C. Thone-Reineke and S. Wolfram, Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation, International Journal of Obesity, 2005, 29, 615–623 Search PubMed.
- S. Wolfram, D. Raederstorff, Y. Wang, S. R. Teixeira, V. Elste and P. Weber, TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass, Ann. Nutr. Metab., 2005, 49, 54–63 CrossRef CAS.
- S. Serisier, V. Leray, W. Poudroux, T. Magot, K. Ouguerram and P. Nguyen, Effects of green tea on insulin sensitivity, lipid profile and expression of PPARalpha and PPARgamma and their target genes in obese dogs, Br. J. Nutr., 2008, 99, 1208–1216 CAS.
- N. Chen, R. Bezzina, E. Hinch, P. A. Lewandowski, D. Cameron-Smith, M. L. Mathai, M. Jois, A. J. Sinclair, D. P. Begg, J. D. Wark, H. S. Weisinger and R. S. Weisinger, Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet, Nutr. Res., 2009, 29, 784–793 CrossRef CAS.
- L. Chen, M. J. Lee, H. Li and C. S. Yang, Absorption, distribution, elimination of tea polyphenols in rats, Drug Metab Dispos, 1997, 25, 1045–1050 CAS.
- I. M. Kapetanovic, J. A. Crowell, R. Krishnaraj, A. Zakharov, M. Lindeblad and A. Lyubimov, Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs, Toxicology, 2009, 260, 28–36 CrossRef CAS.
- H. Lu, X. Meng, C. Li, S. Sang, C. Patten, S. Sheng, J. Hong, N. Bai, B. Winnik, C. T. Ho and C. S. Yang, Glucuronides of tea catechins: enzymology of biosynthesis and biological activities, Drug Metab. Dispos., 2003, 31, 452–461 CrossRef CAS.
- J. D. Lambert, M. J. Lee, H. Lu, X. Meng, J. J. Hong, D. N. Seril, M. G. Sturgill and C. S. Yang, Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice, J Nutr, 2003, 133, 4172–4177 CAS.
- B. Mlinar, J. Marc, A. Janez and M. Pfeifer, Molecular mechanisms of insulin resistance and associated diseases, Clin. Chim. Acta, 2007, 375, 20–35 CrossRef CAS.
- K. Schneider, J. Oltmanns and M. Hassauer, Allometric principles for interspecies extrapolation in toxicological risk assessment–empirical investigations, Regul. Toxicol. Pharmacol., 2004, 39, 334–347 CrossRef CAS.
- A. G. Dulloo, C. Duret, D. Rohrer, L. Girardier, N. Mensi, M. Fathi, P. Chantre and J. Vandermander, Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans, Am J Clin Nutr, 1999, 70, 1040–1045 CAS.
- M. Boschmann and F. Thielecke, The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study, J Am Coll Nutr, 2007, 26, 389S–395S CAS.
- G. Argyropoulos, A. M. Brown, S. M. Willi, J. Zhu, Y. He, M. Reitman, S. M. Gevao, I. Spruill and W. T. Garvey, Effects of mutations in the human uncoupling protein 3 gene on the respiratory quotient and fat oxidation in severe obesity and type 2 diabetes, J. Clin. Invest., 1998, 102, 1345–1351 CrossRef CAS.
- L. K. Heilbronn, S. K. Gan, N. Turner, L. V. Campbell and D. J. Chisholm, Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects, J. Clin. Endocrinol. Metab., 2007, 92, 1467–1473 CrossRef CAS.
- H. Lu, X. Meng and C. S. Yang, Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate, Drug Metab. Dispos., 2003, 31, 572–579 CrossRef CAS.
- A. G. Dulloo, J. Seydoux, L. Girardier, P. Chantre and J. Vandermander, Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity, International Journal of Obesity, 2000, 24, 252–258 Search PubMed.
- D. Carling, M. J. Sanders and A. Woods, The regulation of AMP-activated protein kinase by upstream kinases, International Journal of Obesity, 2008, 32(Suppl. 4), S55–59 Search PubMed.
- D. K. Singh, S. Banerjee and T. D. Porter, Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells, J. Nutr. Biochem., 2009, 20, 816–822 CrossRef CAS.
- R. S. Meng, Z. H. Pei, R. Yin, C. X. Zhang, B. L. Chen, Y. Zhang, D. Liu, A. L. Xu and Y. G. Dong, Adenosine monophosphate-activated protein kinase inhibits cardiac hypertrophy through reactivating peroxisome proliferator-activated receptor-alpha signaling pathway, Eur. J. Pharmacol., 2009, 620, 63–70 CrossRef CAS.
- J. Kerner, W. K. Parland, P. E. Minkler and C. L. Hoppel, Rat liver mitochondrial carnitine palmitoyltransferase-I, hepatic carnitine, and malonyl-CoA: effect of starvation, Arch. Physiol. Biochem., 2008, 114, 161–170 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |