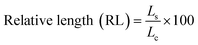Hg bioavailability and impact on bacterial communities in a long-term polluted soil
P.
Ruggiero
*a,
R.
Terzano
a,
M.
Spagnuolo
a,
L.
Cavalca
b,
M.
Colombo
b,
V.
Andreoni
b,
M. A.
Rao
c,
P.
Perucci
d and
E.
Monaci
d
aDip. di Biologia e Chimica Agro-forestale ed Ambientale, Università degli Studi di Bari Aldo Moro, Via Amendola 165/a—70126, Bari, Italy. E-mail: pruggier@agr.uniba.it; Fax: +39 080 5442850; Tel: +39 080 5442852
bDip. di Scienze e Tecnologie Alimentari e Microbiologiche, Università degli Studi di Milano, Milan, Italy
cDip. di Sci. del Suolo, della Pianta e dell'Ambiente e delle Produzioni Animali, Università degli Studi di Napoli Federico II, Portici, Italy
dDip. di Scienze Ambientali e Produzioni Vegetali, Università delle Marche, Ancona, Italy
First published on 8th November 2010
Abstract
Different soil samples characterised by a long-term Hg-pollution were studied for Hg total content, fractionation, phytotoxicity and influence on the bacterial community. Hg pollution ranged from 1 to 50 mg kg−1 and most of it was speciated in scarcely soluble forms. In agreement with this, the biochemical quality indexes were investigated (biomass, enzyme activities) and the bacterial community (viable heterotrophic (VH) bacteria, functional diversity) apparently was not influenced by the degree of Hg pollution. In particular, the investigated soils exhibited a low percentage of Hg-resistant (HgR) bacteria ranging from less than 0.001% to 0.25% of the VH and the addition of available Hg in the form of HgCl2 induced an enrichment of resistant HgR populations. The general biodiversity of the bacterial community was evaluated by denaturing gradient gel electrophoresis of DNA of Hg spiked soil microcosms and of control soils. HgR bacteria capable to grow in a minimal medium containing HgCl2 were also isolated and identified. MerA and merB gene PCR fragments were obtained from different HgR strains and the range of similarities at the DNA level and at the deduced amino acid level showed that they carried mercuric reductase and lyase. Differently from bacteria, some influence of soil Hg content on seeds' germination and root elongation was observed for Lepidium sativum L. and Solanum lycopersicum L. In conclusion, most of the Hg in these long-term polluted soils was scarcely mobile and available and did not significantly influence the soil bacterial community. The risk of potential Hg remobilisation over time, that could be naturally favoured by the activity of plant roots or other inorganic processes occurring in soil, can be extenuated since bacterial community was resistant and resilient to subsequent Hg stress.
Environmental impactCombined chemical, biological and ecotoxicological studies on contaminated soils are necessary to predict mercury fate and its detrimental effects in the environment. In this context, soil biological properties are sensitive early indicators of soil metabolic disturbances. The stability of the microbial community to external disturbances is of outmost importance for the risk assessment in relation to environmental management of polluted areas. In addition, potential Hg remobilisation, that could be naturally favoured by the activity of plant roots or other processes occurring in soil, should be considered. A combined multidisciplinary approach using chemical methods, seed germination tests, biochemical investigations and advanced molecular biology techniques was adopted to study polluted soils collected nearby an industrial site where a long-term pollution was present. |
Introduction
Even though mercury (Hg) occurs naturally in the environment (mineral deposits, volcanoes, forest fires, and oceanic emissions), human activities and disposal of chemical wastes have caused extensive anthropogenic Hg-pollution of soils.1 It is generally recognised that Hg toxicity and bioavailability depend largely on the chemical state of the metal rather than on the mere Hg total concentration. However, beside chemical investigations, the actual Hg toxicity and bioavailability in soil can only be assessed by studying the impact of the pollutant on soil biochemical properties and/or on selected organisms living in/on soil (e.g., bacteria, plants, earthworms, small biota, etc.).In the last years, combined chemical, biological and ecotoxicological studies on contaminated soils have increased importance to predict metal fate in the environment. This type of approach is based on toxicity tests with various species from different trophic levels, with different exposure routes. Most of these papers have regarded polluted soils in the vicinity of chlor–alkali plants,2 one of the main source of Hg-pollution throughout the world. All these studies demonstrate that the results obtained with these different approaches can be then used and compared to better assess potential risk.
In this context, soil biological and biochemical properties are sensitive and early indicators of soil metabolic disturbances.3 They can be useful tools to evaluate soil quality and functionality as well as to monitor soil reclamation.4
It is generally accepted that accumulation of heavy metals reduces soil microbial biomass5 by directly killing or bio-chemically disabling microorganisms.6 A decrease in soil functional diversity and changes in microbial community structure can concurrently occur.7 The study of soil microbial biomass behaviour and its metabolic activity is up today a crucial key able to relate the influence of various pollutants with the soil ecosystem and to monitor soil productivity and sustainability. Thus, soil microbial biomass carbon content, soil basal respiration, and enzymatic activities can be considered an indirect measure of the bio-availability of heavy metals in soil and a suitable indicator tool to study the recovery of polluted soils.
With respect to Hg, bacteria mediate three primary transformations of mercury: reduction of Hg(II), Hg-methylation and demethylation.8 These enzymatic reactions are codified by a cluster of genes organized in the mer operon, present on the plasmid or flanked by transposons. In general, most mercury resistant isolates have merP, merR, and merA genes, coding for the transport, extracellular binding and mercuric reductase functions, respectively. In some organisms, additional genes like merB code for organomercurial lyase for the cleavage of certain organomercuric compounds.
Among living organisms, other effective bio-indicators of soil pollution are plants which show a peculiar sensitivity to heavy metals. Though some metals are essential or beneficial nutrients, at high concentrations all metals can strongly affect plant growth already in the germination phase. In this first phase of the biological cycle seeds are sensitive, strictly depending on the capacity of metals to overcome the natural barriers of seed coats and thereby on the tolerance of each plant species according to structural features.
The aim of this study was to assess the impact of Hg stress on the bacterial community of polluted soils collected nearby an industrial site in the south of Italy where a long-term pollution, dating back to the activities of a chlor–alkali plant in the 1960s–1980s, was present. In order to reach this goal, a combined approach using chemical methods, seed germination tests, biochemical investigations and studies on the bacterial community, was adopted.
Specifically, the following aspects have been investigated: (i) preliminary Hg speciation by means of sequential extractions; (ii) impact of long-term Hg pollution on soil microbial biomass and on several soil biochemical parameters related to metabolic activity; (iii) phytotoxicity effects of Hg-contaminated soils on the germination of seeds belonging to different plant species; (iv) effect of the addition of available forms of Hg on the structure and diversity of bacterial communities and the population dynamics of the soil microorganisms; and (v) characterization of HgR bacterial strains possibly involved in the biogeochemical cycle of Hg in the soils.
Materials and methods
Soil sampling and physico-chemical characterisation
Soil samples were collected in 2006 from five different points (1–5) around the industrial site of Ferrandina (MT), located within the larger Val Basento industrial area, in the south of Italy. In this area, a chlor–alkali plant was active in the 1960s–1980s and no more existing since several years.All samples were collected at two depths (A: 0–10 cm and B: 40–50 cm), air dried, sieved at 2 mm, homogenized, and stored in HDPE vessels at 4 °C. For sample 5, only one composite sample was collected at 0–10 cm (5A), owing to the shallowness of the soil profile.
Soil texture, pH, organic carbon content (Corg), total nitrogen (Ntot) and available phosphorus (Pav) were determined for each soil sample according to the standard methods of soil analysis.
Total mercury (Hgtot) concentration in soil samples was determined using an Automatic Mercury Analyzer (AMA 254, Altec, FKV). This instrument allowed the direct Hg determination without any sample chemical pre-treatment.
Mercury sequential extractions
A seven step sequential extraction procedure (SEP)9 was performed on 0.5 g of each soil sample sieved at 2 mm to determine the partitioning of Hg associated with different soil constituents and to elucidate the potential availability and mobility of Hg according to the solubilisation promoted by various extracting solutions at increasing strength. Briefly, the adopted SEP enables to discriminate among the following operative pools: water-soluble (F1: deionised H2O); exchangeable (F2: NH4Cl 1 M); carbonates (F3: ammonium acetate 1 M, pH 4.5); easily reducible (F4: Tamm's solution); 6 M HCl soluble (F5); oxidizable (F6: H2O2, ammonium acetate, HNO3, pH 2); and residue (F7: dry analysed by AMA 254).Seed germination tests
The phytotoxicity of the Hg-contaminated soils was evaluated by static-type germination assays using cress (Lepidium sativum L.), tomato (Solanum lycopersicum L.), and cucumber (Cucumis sativus L.) seeds. A randomized complete block experimental design with three replicates was used. Ten grams of each soil sample were placed in 10 × 90 mm Petri dishes, and wet with distilled water until reaching the water holding capacity of each soil. Fifteen seeds were placed on pre-moistened (with 5 ml of distilled water) Whatman No. 1 filter paper disc laying on wet soil. Control tests were performed with distilled water as germination medium. The samples were incubated at 25 ± 0.5 °C in darkness for 3 days (cress and cucumber seeds) or 5 days (tomato seeds).After incubation, the number of germinated seeds and the length of the roots were measured to calculate the following indexes:10
where Gs and Gc are the number of germinated seeds in the sample and in the control, respectively; Ls and Lc are the length of the roots in the sample and in the control, respectively.
Soil biochemical analyses
Daily basal respiration (DBR) of soils was monitored at 22 °C for 48 days.11 Microbial biomass-C content (Cmic) was determined using the fumigation extraction method.12 Metabolic (qCO2) and microbial (MQ) quotient were calculated as reported by Anderson and Domsch13 and Smith and Paul,14 respectively.The hydrolysis rate of fluorescein diacetate (FDAH) was estimated using the method described by Perucci et al.15 The activities of β-glucosidase (Glu—EC 3.2.1.21) and arylsulfatase (ArS—EC 3.2.6.1) were measured as described by Rao et al.16 Alkaline and acid phosphatase (AlkP and AcP—EC 3.1.3.1 and EC 3.1.3.2, respectively) activities were determined according to Tabatabai.17 The activity of urease (Ure—EC 3.5.1.5) was determined according to Nannipieri et al.18 Dehydrogenase (DH—EC 1.1) activity was measured with tetrazolium salts as substrate according to Trevors19 and o-diphenol oxidase (o-DPO) activity was measured following the method of Perucci et al.20
Enumeration of heterotrophic bacteria
Plate count method was used to monitor the number of viable heterotrophic (VH) and Hg-resistant (HgR) bacteria according to Dell'Amico et al.21 Plates were incubated at 28 °C and the number of CFU was counted and expressed on a dry weight basis (105 °C, 24 h). The mercury resistance of the heterotrophs was expressed as percentage of growth on 0.1 strength TSA medium (Difco) with HgCl2 addition (50 µM). For the investigated soils, fast- (number of CFU after 2 days) and slow- (number of CFU after ten days minus CFU after 2 days) growing VH and HgR bacteria were numbered to calculate the colony development index as CD = ∑(pi/i) × 100, where pi = colony number at time i/total colony number.Preparation of soil microcosms and characterization of the bacterial community
For these experiments, soil samples were spiked with HgCl2 in order to assess responses of VH and HgR bacterial communities to the addition of soluble Hg. Furthermore, the genetic diversity of the bacterial community was analysed in microcosms of soil samples 2A and 2B, chosen because of the higher variability in the bacterial community and a starting Hg content intermediate between the less contaminated and the most contaminated soil samples.Fifty grams of each soil sample were placed in 500 ml vials sealed with a rubber stopper. Hg-spiked soils were obtained by adding 1 mM HgCl2 solution into the sample in order to add 10 mg kg−1 of Hg. Unspiked control microcosms were also prepared. Soil microcosms were remoistened at 60% of their water holding capacity and kept at 28 °C up to 50 days. On days 0, 14, and 50 each soil sample was mixed thoroughly and aliquots were collected and analyzed for the enumeration of viable (VH) and of Hg-resistant (HgR) heterotrophic bacteria as well as for DNA extraction. On day 28, the samples were aerated by mixing to ensure aerobic conditions. After 50 days, soil aliquots were sampled for DNA extraction. At this time, the enumeration of VH and HgR bacteria was performed only on spiked soil samples 1A, 1B, 2A, 2B, 4A, and 4B. Each microcosm experiment was carried out in triplicate.
Genetic diversity of the bacterial community was analysed at successive incubation times (0, 14 and 50 days) in duplicate experiments by DGGE of the hyper variable region V3 of bacterial 16S rRNA gene. Total DNA was extracted from soil samples (2 g) by a bead-beating method (MOBIO, USA). Amplification of V3 regions was performed by using primer pair P341f-GC clamped and P534r.22 PCR mixtures, amplification conditions and DGGE runs were performed as previously reported23 in a DCODE Universal Mutation Detection System (Bio-Rad, USA). The V3 region of the 16S rDNA fragments of single isolates was run on DGGE gels and checked to co-migrate with prominent bands.
Shannon–Weaver (H′)24 and Sørensen (S)25 indices were used to evaluate the general biodiversity and the similarity of controls vs. Hg-spiked soils, respectively. The H′ index of bacterial community was calculated on the basis of the number and of the intensity of bands present on DGGE profiles, run on the same gel, as follows: H′ = −∑pi logepi, where pi is the importance probability of bands in a gel lane and it was calculated as follows: pi = ni/N, where ni is the band intensity for each individual band and N is the sum of intensities of bands in a lane. S index (S = 2c/[a + b], with c = common DGGE bands between two patterns and [a + b] = total band number of the two patterns) was calculated on the basis of the presence/absence of bands in DGGE profiles of Hg-spiked vs. not spiked microcosms after 50 days of incubation. S = 0 indicates that two samples are completely different, whereas S = 1 indicates identical samples. Bands were analyzed by the Diversity Database software of the Gel Doc image 234 analyzer system (Bio-Rad, USA). Statistical comparison of different DGGE profiles was done on duplicate samples by the QuantityOne GelDoc software package after assigning bands to the gel tracks.
Characterization of Hg-resistant strains and detection of mer genes
Bacterial colonies were randomly selected from HgR plates of soil microcosms on the basis of colony morphology and further characterized by determining their Hg-resistance when growing in Tris-mineral medium containing 0.6% gluconate (TMMG)26 and different amounts of HgCl2 (1, 3 and 15 µM). DNA was extracted from TMMG liquid cultures grown overnight with proteinase K digestion (1 mg ml−1). Polymerase chain reaction (PCR) amplification of the 16S rRNA gene was performed on the extracted DNA using the eubacterial universal primers P27f and P1495r referred to Escherichia coli nucleotide gene sequence according to Weisburg et al.27 Strains were identified by sequence analysis (1200 bp) of 16S rRNA gene.Amplification of genes for mercuric reductase (merA) in HgR strains was performed in 25 µl final volume containing: 10× buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.3 µM forward primer A2-n.F (5′-CCATCGGCGGCWSYTGCGTSAA-3′) and reverse primer A5-n.R (5′-ACCATCGTCAGRTARGGRAAVA-3′) each, 1 U Taq polymerase and 2 µl of template DNA. The thermal profile was: 94 °C for 3 min followed by 35 cycles (94 °C for 10 s, 57 °C for 60 s, and 72 °C for 30 s) and 72 °C for 5 min. The expected size of the PCR fragment was 1200 bp. Amplification of genes for organomercurial lyase (merB) was carried out using two different sets of primer. The first set was appositely designed taking into account the gene sequence variations among the Streptomyces genus: P15 forward (5′-CCAGCAGCTCGCCACCC-3′) and P640 reverse (5′-ACGCGG(G/T)CGGGGTGTCG-3′). The reaction was performed in 25 µl final volume containing: 10× buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.4 µM forward and reverse primers each, 1.5 U Taq polymerase and 2 µl of template DNA. The thermal profile was: 94 °C for 5 min followed by 45 cycles (94 °C for 45 s, 56 °C for 45 s, and 72 °C for 90 s) and 72 °C for 7 min. The size of the expected fragment was 625 bp. The second set of primer for merB amplification was designed to encompass the known diversity of merB within the Pseudomonas genus (Bestetti, personal communication): merBK62 forward (5′-TCCAAAAAGATTGCCGAAAG-3′) and merBK62 reverse (5′-TTTCCTCGCAGTCCTCTAGC-3′). The reaction was performed in 25 µl final volume containing: 10× buffer, 1.75 mM MgCl2, 0.2 mM dNTPs, 0.2 µM forward and reverse primers each, 1.5 U Taq polymerase and 2 µl of template DNA. The thermal profile was: 94 °C for 5 min followed by 40 cycles (94 °C for 10 s, 54 °C for 60 s, and 72 °C for 60 s) and 72 °C for 7 min. The size of the expected fragment was 600 bp. Taq polymerase and related buffer were purchased from Invitrogen (Glasgow, UK) and nucleotides from GE Healthcare (Uppsala, Sweden); primers were synthesised by Primm (Milano, Italy). PCR reactions were performed on T-Gradient Biometra apparatus (Gottingen, Germany).
Nucleotide sequence analysis
Prominent bands of the DGGE patterns of 2A and 2B microcosms were excised from DGGE gels with a sterile scalpel under UV light; the DNA was extracted in 100 µl deionised water by shaking for 1 h at 37 °C. DNA solution (10 µl) was used as a template to amplify the V3 fragments with not GC-clamped primers. PCR products were ligated into pCR®II-TOPO TA cloning vector (Invitrogen). After chemical transformation into library efficiency DH5αTM competent cells (Invitrogen), positive clones were directly sequenced by using M13 forward and reverse primers.PCR products were purified from agarose gel by QIAquick columns (Quiagen, Germany) before sequencing. Taq Dye-Deoxy Terminator Cycle Sequencing kit (Applied Biosystems, USA) was used according to the ABI Prism protocol (Applied Biosystems). The forward and reverse samples were run on a 310A sequence analyser (Applied Biosystems). A similarity analysis of the nucleotide sequences was obtained using the Advanced Blast Search program (GenBank, NCBI). Results were checked for errors on both strands and the translated amino acidic sequences were searched for homology by using the NCBI-Blast2—Protein Database Query program (http://www.ebi.ac.uk/blastall/%20index.html).
Statistical analysis
Low significance differences were calculated by one-way ANOVA followed by Tukey's test (p < 0.05, JMP 7.0-SAS). Spearman correlation coefficients were calculated by multivariate analysis-non-parametric correlations (JMP 7.0-SAS).Results
Soil chemical characterisation
The soils under investigation were characterised by a loam or sandy-loam texture (USDA), an alkaline pH and, in general, a low content of organic matter, except for samples 1A and 5A where the Corg content was 2.80% and 2.98%, respectively (Table 1). Mercury concentration ranged from 1.2 mg kg−1 in sample 1B to 50 mg kg−1 in sample 5A. These amounts exceeded the levels imposed by the Italian legislation for residential/public sites (1 mg kg−1) or for industrial sites (5 mg kg−1). There was no relationship between the content of organic matter and Hg concentration. The amount of Hg was higher at 40–50 cm than at 0–10 cm for samples 2 and 4, while no significant differences were observed for samples 1 and 3 at different depths. Sample 5 could be collected only at 0–10 cm, because of the shallowness of the soil profile in that point.| Soil sample | pH | C org (dw%) | N tot (dw%) | P ass (mg kg−1) | Hgtot (mg kg−1) | HgF1–F4 (Hgtot%) | HgF5 (Hgtot%) | HgF6 (Hgtot%) | HgRES (Hgtot%) |
|---|---|---|---|---|---|---|---|---|---|
| 1A | 7.9 | 2.80 | 0.23 | 10 | 1.5 ± 0.3 | 0 | 97 | 0 | 3 |
| 1B | 8.5 | 0.59 | 0.03 | 1 | 1.2 ± 0.2 | 0 | 70 | 0 | 30 |
| 2A | 8.2 | 0.62 | 0.04 | 3 | 7.3 ± 0.6 | 0 | 90 | 0 | 10 |
| 2B | 8.5 | 0.68 | 0.04 | 2 | 12.1 ± 1.7 | 0 | 90 | 0 | 10 |
| 3A | 8.2 | 0.85 | 0.04 | 3 | 1.35 ± 0.08 | 0 | 84 | 0 | 16 |
| 3B | 8.2 | 1.06 | 0.04 | 2 | 1.4 ± 0.2 | 0 | 86 | 0 | 14 |
| 4A | 8.5 | 1.00 | 0.07 | 2 | 1.8 ± 0.2 | 0 | 90 | 0 | 10 |
| 4B | 8.9 | 0.38 | 0.03 | 1 | 4.9 ± 0.6 | 0 | 97 | 0 | 3 |
| 5A | 8.0 | 2.98 | 0.16 | 13 | 50 ± 5 | 0 | 87 | 3 | 10 |
Most of the mercury (70–97%) could be extracted only by using concentrated acid (6 M HCl: HgF5) or remained in the residual fraction (HgRES). Only for sample 5A a small percentage of Hg (3%) could be extracted in the oxidisable fraction (HgF6). Essentially no mercury was recovered in the more labile fractions (HgF1–F4: water soluble, exchangeable, weak acidic conditions and complexing agents) (Table 1).
Phytotoxicity
The Hg content of the studied soils had no significant effect (p < 0.05) on the relative germination of C. sativus seeds and an enhancement of relative root length, especially in soil sample 5A (RL = 227%), was observed (Fig. 1). | ||
| Fig. 1 Relative germination (RG), relative root length (RL), and germination index (GI) of cucumber (Cucumis sativus L.), cress (Lepidium sativum L.) and tomato (Solanum lycopersicum L.) seeds in Hg polluted soils. | ||
Conversely, L. sativum showed higher sensitivity already at low Hg concentrations (Fig. 1). In the presence of low Hg-contaminated soil samples, RG and RL mean values were lower than the control (ca. 80 and 70%, respectively). Differently, in the most contaminated soil sample (5A) 54% RG and 283% RL were observed. Therefore, sample 5A evidenced a mean GI value even higher than the control (147%).
An intermediate behaviour was observed with S. lycopersicum seeds (Fig. 1). RG values declined by less than 20% in all soils except in 5A that did not affect the number of germinated seeds but strongly enhanced the root length (RL = 553%). As a result GI of S. lycopersicum increased over 500% in soil sample 5A and it remained lower than 100% in the other soils.
Soil biochemical characterisation
Table 2 reports Cmic, DBR, MQ and qCO2 values determined in the investigated soils at the two sampling depths. Cmic values ranged from 161 to 334 mg kg−1 in the soils sampled at 0–10 cm and from 48 to 135 mg kg−1 at the depth of 40–50 cm. By comparing the values of Cmic with those of Hgtot (Table 1), no direct relationship could be observed. Noteworthy, the soil sample with the highest Hg concentration (5A) showed the highest amount of Cmic. Indeed, in all soils Cmic appeared to be more influenced by Corg rather than by Hg content. This assumption is confirmed by the values of the microbial quotient obtained from samples 1 and 4, since MQ did not differ within the same soil at 0–10 cm and at 40–50 cm by varying Hg concentration, although Corg and Cmic decreased with the depth. Conversely, in samples 2 and 3 the MQ decreased at 40–50 cm. Nonetheless, MQ decreased by increasing Hg concentration only in sample 2.| Soil samples | C mic (mg kg−1) | DBR (mg CO2 kg−1 day−1) | MQ (%) | qCO2 (g CO2/g Corg) | FDAH (µg FDA g−1 h−1) | AcP (µg p-NP g−1 h−1) | AlkP (µg p-NP g−1 h−1) | Glu (µg p-NP g−1 h−1) | ArS (µg p-NP g−1 h−1) | Ure (µmol NH4+ g−1 h−1) | DPO (µmol catechol g−1 10 min−1) | DH (µg TPF g−1 h−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a C mic = microbial biomass C content; DBR = daily basal respiration; MQ = microbial quotient; qCO2 = metabolic quotient; FDAH = FDA hydrolysis activity; AcP and AlkP= acid and alkaline phosphatase; Glu = glucosidase, ArS = arylsulfatase; Ure = urease; DPO = o-diphenol oxidase; and DH = dehydrogenase. | ||||||||||||
| 1A | 217 ± 50 | 128 ± 22 | 0.8 | 0.6 | 89 ± 5 | 237 ± 8 | 339 ± 14 | 424 ± 7 | 78 ± 2 | 75 ± 1 | 4.4 ± 0.2 | 1.06 ± 0.64 |
| 1B | 48 ± 11 | 21 ± 5 | 0.8 | 0.5 | 50 ± 4 | 87 ± 4 | 220 ± 26 | 54 ± 5 | 14 ± 1 | 14 ± 1 | 3.1 ± 0.5 | 0.58 ± 0.05 |
| 2A | 161 ± 31 | 41 ± 3 | 2.6 | 0.3 | 62 ± 7 | 61 ± 3 | 184 ± 19 | 95 ± 13 | 13 ± 3 | 45 ± 1 | 4.5 ± 0.3 | 0.03 ± 0.01 |
| 2B | 69 ± 23 | 23 ± 3 | 1.0 | 0.3 | 50 ± 7 | 56 ± 12 | 207 ± 21 | 37 ± 2 | 8 ± 0 | 11 ± 0 | 5.1 ± 0.2 | 0.05 ± 0.02 |
| 3A | 173 ± 12 | 59 ± 6 | 2.3 | 0.4 | 73 ± 4 | 93 ± 2 | 226 ± 22 | 144 ± 12 | 31 ± 6 | 24 ± 1 | 5.7 ± 0.02 | 0.02 ± 0.01 |
| 3B | 135 ± 12 | 30 ± 2 | 1.3 | 0.2 | 62 ± 3 | 89 ± 5 | 205 ± 44 | 46 ± 4 | 12 ± 1 | 19 ± 0 | 5.3 ± 0.12 | 0.01 ± 0.00 |
| 4A | 173 ± 33 | 48 ± 7 | 1.7 | 0.3 | 69 ± 4 | 95 ± 4 | 273 ± 45 | 112 ± 11 | 16 ± 3 | 25 ± 2 | 4.4 ± 0.3 | 0.01 ± 0.00 |
| 4B | 57 ± 17 | 14 ± 4 | 1.5 | 0.2 | 8 ± 5 | 40 ± 5 | 100 ± 6 | 8 ± 3 | 5 ± 0 | 15 ± 0 | 4.2 ± 0.2 | 0.073 ± 0.01 |
| 5A | 334 ± 48 | 130 ± 16 | 1.1 | 0.4 | 73 ± 4 | 105 ± 4 | 443 ± 15 | 241 ± 15 | 36 ± 1 | 3 ± 0 | 7.4 ± 0.2 | 0.307 ± 0.05 |
Daily basal respiration (DBR) rate, which represents a measure of the metabolic activity of soil microbial community, decreased at 40–50 cm in all soils and according to the decreasing amount of Cmic. Despite the highest amount of Hg (50 mg kg−1), sample 5 was characterized by a DBR quite similar to that of sample 1A which contained only 1.5 mg kg−1 of Hg, thus evidencing no toxic effect of such an elevated amount of Hg. The qCO2 values also slightly differed among the soil samples but they did not increase by increasing the Hg concentration, as sample 5A evidenced a lower value of qCO2 than sample 1.
The activities of the main enzymes involved in the biogeochemical cycles of C, N, P, and S were measured and, as expected, they were generally higher at 0–10 cm than at 40–50 cm for all the sampled soils (Table 2). In agreement with the Corg and Cmic values, all the tested enzymatic activities were generally higher in samples 1A and 5A than in the other soil samples.
VH and HgR bacteria in soils and impact of Hg spiking
Numbers of VH in the soils were in the range from 0.16 × 107 to 3.81 × 107 CFU g−1 soil dw (Table 3). HgR bacteria were present at levels ranging from 0.1 × 103 to 95.4 × 103 CFU g−1 soil dw in the lowest and in the highest polluted soils, respectively. All the nine soils exhibited percentages of HgR bacteria ranging from less than 0.001% to 0.25% of the VH bacteria, reflecting a basic range of tolerance to mercury of these populations.| Soil sample | Hgtot (mg kg−1) | t = 0 | t = 14 | HgR enrichment factor between t = 0 and t = 14 | VH enrichment factor between t = 0 and t = 14 | HgR/VH (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VH (107 CFU g−1) | HgR (103 CFU g−1) | Control | Spiked soil | Control | Spiked soil | Control | Spiked soil | t = 0 | t = 14 | ||||
| VH (107 CFU g−1) | HgR (103 CFU g−1) | VH (107 CFU g−1) | HgR (103 CFU g−1) | ||||||||||
| a Each value is the mean of three determinations ± standard deviation; *, significantly different data from HgR of t = 0 (Student-t, p ≤ 0.05); and n.s., not significantly different. | |||||||||||||
| 1A | 1.5 | 2.75 ± 0.89 | 0.20 ± 0.001 | 1.03 ± 0.26* | 0.36 ± 0.001* | 2.43 ± 0.17 | 7.46 ± 3.32* | 1.8 | 37 | 0.37 | n.s. | <0.001 | 0.031 |
| 1B | 1.2 | 0.53 ± 0.07 | 0.10 ± 0.04 | 0.54 ± 0.06 | 0.08 ± 0.05 | 0.25 ± 0.16 | 15.50 ± 1.41* | n.s. | 155 | n.s. | n.s. | 0.002 | 0.62 |
| 2A | 7.3 | 1.61 ± 0.57 | 18.20 ± 1.01 | 0.69 ± 0.07 | 3.92 ± 0.25* | 0.45 ± 0.10* | 33.40 ± 4.30* | 0.21 | 1.80 | n.s. | 0.3 | 0.11 | 0.74 |
| 2B | 12.1 | 0.60 ± 0.14 | 4.71 ± 1.89 | 0.41 ± 0.02* | 1.21 ± 0.21* | 0.48 ± 0.21 | 9.27 ± 8.86 | 0.26 | n.s. | 0.68 | n.s. | 0.08 | 0.19 |
| 3A | 1.35 | 0.51 ± 0.08 | 12.30 ± 7.80 | 1.35 ± 0.86 | 0.39 ± 0.22* | 0.43 ± 0.04 | 0.78 ± 0.09* | 0.03 | 0.06 | n.s. | n.s. | 0.24 | 0.018 |
| 3B | 1.4 | 0.16 ± 0.008 | 3.10 ± 0.15 | 0.23 ± 0.11 | 1.14 ± 0.55* | 0.28 ± 0.06* | 0.50 ± 0.28* | 0.37 | 0.16 | n.s. | 1.8 | 0.20 | 0.018 |
| 4A | 1.8 | 1.37 ± 0.45 | 3.48 ± 0.95 | 0.83 ± 0.06 | 3.94 ± 2.81 | 0.87 ± 0.41 | 67.5 ± 19.80* | n.s. | 19.40 | n.s. | n.s. | 0.03 | 0.77 |
| 4B | 4.9 | 1.18 ± 0.34 | 14.90 ± 1.55 | 0.36 ± 0.08* | 15.10 ± 9.56 | 0.45 ± 0.18* | 18.9 ± 5.42 | n.s. | n.s. | 0.30 | 0.4 | 0.13 | 0.42 |
| 5A | 50 | 3.81 ± 0.05 | 95.40 ± 0.75 | 3.19 ± 0.06* | 7.24 ± 2.83* | 2.63 ± 0.21 | 11.40 ± 0.40* | 0.08 | 0.12 | 0.8 | 0.7 | 0.25 | 0.043 |
Slow-growing bacteria were predominant in all the investigated soils and CD index values of VH and HgR bacteria were not related to the Hg content of the soils (Fig. 2).
 | ||
| Fig. 2 Colony development (CD) index for HgR and VH bacteria related to the total Hg content of the investigated soils. | ||
After 14 days from spiking with HgCl2, the number of VH bacteria decreased by factors from 0.3 to 0.7; only in the soil sample 3B it increased 1.8 fold, while did not change significantly in most soils (Table 3). The number of HgR bacteria significantly increased in most of the soil samples regardless their Hgtot content. This could indicate that Hg spiking would have favoured the spread of effective Hg resistant bacteria, while negatively affecting the populations tolerating the Hg content of soils. Between t = 14 and t = 50 only in the soil sample 1B the number of HgR bacteria decreased (11.30 × 103 ± 0.8), while it did not change significantly in the other samples analyzed. The number of VH bacteria instead changed in the soil samples 1A and 4A (0.52 × 107 ± 0.02 and 1.33 × 107 ± 0.52, respectively), while it did not change significantly in samples 1B, 2A, 2B, and 4B.
Between t = 0 and t = 14, the percentage of HgR bacteria increased in the soils with low starting HgR/VH ratios and not in those with high HgR/VH ratios (≥0.20%), thus indicating that some HgR populations, already under a stress condition, died when exposed to a pulse of readily available Hg.
Effect of Hg spiking on the genetic diversity of 2A and 2B soils
Preliminary DGGE analysis of soil microcosms (data not shown) indicated that the addition of available Hg promoted the strongest modifications in the DNA profile of samples 2A and 2B. These samples presented a content of total Hg (7.3 and 12.1 mg kg−1, respectively) intermediate among all the investigated samples, and were then chosen as models for evaluating the impact of Hg spiking on the genetic diversity of bacterial communities by DGGE analysis (Fig. 3). | ||
| Fig. 3 DGGE profiles of 16S rRNA gene fragments of single isolates from soil sample 2A (2A/1, Arcicella sp.; 2A/10, Rhizobium giardinii; 2A/14 and 2A/18 Agrobacterium tumefaciens; 2A/29, Methylobacterium radiotolerans) and of samples 2A and 2B soil microcosms in mercury-spiked (+Hg) and control (C) replicates (a and b) after 14 and 50 days of incubation. DNA of sample 14b in 2B failed to be extracted. S: soil sample before spiking. Side numbers indicate the bands discussed in the Result section. | ||
Bands corresponding to different bacterial species were evidenced and used to calculate the Shannon–Weaver diversity index (H′) and the Sørensen similarity index (S). H′ was higher in the 0–10 cm profile (H′2A = 2.9) than in the 40–50 cm profile (H′2B = 2.5) due to the presence of a total of 37 and 24 bands, respectively. Some bands were visible at the two soil depths (i.e. numbers 2, 3, 6, 7, 10 and 11), indicating the presence of common bacterial species. Mercury addition decreased the diversity after 14 days of incubation (H′2A = 2.1 and H′2B = 2.0) measured as a reduction of the total band number detected on the DGGE gel of the Hg-spiked soils. Some bands decreased in their intensity or disappeared in the Hg-spiked samples and not in the controls (i.e. numbers 2, 3, 8, 9, and 10), while others increased (i.e. numbers 6, 7, 11 and 13), indicating that Hg addition led to an adaptation of the bacterial communities to the stressor. From the 14th to the 50th day of incubation, H′ values in the Hg-spiked microcosms passed to H′2A = 2.3 and to H′2B = 2.2, indicative of a resilience capacity of the bacterial communities. Recovery in the genetic diversity was due to the appearance of new bands in the Hg-spiked soils (i.e. numbers 4 and 5) or to the presence of intense bands not dominating in the controls (i.e. numbers 1, 6 and 7). Similarity analysis, performed by means of the S index, was calculated between control and Hg-spiked microcosms after 50 days of incubation. A relatively high S value (0.67) was calculated for sample 2A indicating that small changes occurred in the bacterial community due to the Hg addition. Low S values (0.46) were instead calculated for sample 2B.
Predominant bands of the profile in the Hg-spiked microcosm 2A (i.e. numbers 1, 4, 5, 6 and 7) were cloned and sequenced and the similarity analysis towards 16S rRNA gene sequence databases revealed that they belonged, respectively, to: Arcicella sp. (99% to Acc. Num. AJ46140), uncultured Bacteroidetes clone AKYG501 (98% to Acc. Num. AY921927), to unidentified bacterium clone TBS17 (98% to Acc Num. AJ005988), to Rhizomonas sp. K6 Alphaproteobacteria (99% to Acc. Num. AJ000918) and to Methylobacterium radiotolerans (99% to Acc. Num. DQ447780). The first and the last sequences corresponded to HgR genera isolated from soil 2A.
Identification of HgR bacteria and characterization of mer genotypes of HgR bacteria
Forty morphologically different bacteria were isolated from soil HgR bacteria plates. Among these, twelve strains, belonging to Alphaproteobacteria, Gammaproteobacteria, Bacteroidetes, Actinobacteria and Firmicutes, were able to grow in liquid TMMG medium containing different concentrations of Hg (Table 4).| Isolate | Identification (% homology) | Hg resistance (µM) | BLASTP results of merA | BLASTP results of merB | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 15 | Similarity% (Acc. num.) | Closest relative | Similarity% (Acc. num.) | Closest relative | ||
| a +, growth and −, absence of growth. | ||||||||
| 2A/1 | Arcicella sp. (100%) | + | + | − | ||||
| 2A/10 | Rhizobium giardinii (99%) | + | + | 99 (Q8KLZ4) | P. putida Spi4 | 98 (Q7CVL1) | A tumefaciens C58 | |
| 2A/14 | Agrobacterium tumefaciens (98%) | + | + | − | 96 (Q8KLZ4) | P. putida Spi4 | 93 (Q7CVL1) | A. tumefaciens C58 |
| 2A/29 | Methylobacterium radiotolerans (100%) | + | − | − | ||||
| 1A | Bradyrhizobium japonicum (100%) | + | − | − | ||||
| 4A/1 | Streptomyces humidus (98%) | + | + | – | ||||
| 4A/8 | S. humidus (100%) | + | + | − | ||||
| 4A/9 | Bacillus samanii (99%) | + | − | − | 65 (Q8KLZ4) | P. putida Spi4 | 99 (Q7CVL1) | Agrobacterium tumefaciens C58 |
| 3A | Agromyces cerinus (100%) | + | − | − | ||||
| 5A/1 | Pseudomonas sp. (100%) | + | + | + | 88 (Q9F3W8) | Pseudomonas sp. ED23-33 | 95 (Q8G9P0) | P putida MU10-2 |
| 5A/2 | Pseudomonas putida (100%) | + | + | + | 98 (Q8KLZ4) | P. putida Spi4 | 91 (Q8G9P0) | P putida MU10-2 |
| 5A/3 | Pseudomonas plecoglossicida (99%) | + | + | + | 100 (Q8KLZ4) | P. putida Spi4 | 98 (Q8G9P0) | P putida MU10-2 |
MerA and merB PCR gene fragments were obtained from three moderately HgR strains Rhizobium giardinii 2A/10, Agrobacterium tumefaciens 2A/14, and Bacillus samanii 4A/9 and from three highly HgR strains Pseudomonas spp. 5A/1, 5A/2 and 5A/3 (Table 4). The merA fragments were homologous to mercuric reductase genes of Pseudomonas putida Spi4 and of Pseudomonas sp. ED23-33 present in databases. The similarities ranged between 94 and 99% at the DNA level and from 65 to 100% at the deduced amino acid level. MerB fragments in strains 5A/1, 5A/2 and 5A/3 had homologies to merB-related fragments ranging between 96 and 98% at nucleotide level and from 91 to 98% at deduced amino acid level to merB gene for alkylmercury lyase of P. putida MU10-2. The sequences of the merB fragments obtained in R. giardinii 2A/10, A. tumefaciens 2A/14 and in B. samanii 4A/9 were related to an ABC transporter/substrate binding protein of A. tumefaciens C58, whose involvement in Hg transformation is unknown.
Repeated attempts to amplify mer genes from the remaining HgR strains failed, suggesting that sequence divergence between our isolates and the known mer sequences was too large for annealing of the primers.
Discussion
According to Sanchez et al.,9 data from sequential extractions (Table 1) suggested that Hg is most likely speciated in highly insoluble forms such as HgS (either as cinnabar and/or metacinnabar), associated to crystalline Fe/Mn oxyhydroxides and/or present as other hardly soluble Hg-compounds (e.g., Hg (0), Hg2Cl2, mixed Hg–S–Cl minerals). In addition, it seemed that organic matter did not play a significant role in Hg-complexation.The results indicated that the organic carbon content rather than the Hg concentration influenced soil enzyme activities in the investigated samples (Table 5).
| C org | C mic | DBR | FDAH | AcP | AlkP | ArS | Glu | Ure | DH | DPO | Hgtot | VH | HgR | CD VH | CD HgR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a *p < 0.05, **p < 0.01, and ***p < 0.001. FDAH = FDA hydrolysis activity; AcP and AlkP= acid and alkaline phosphatase; ArS = arylsulfatase; Glu = glucosidase; Ure = urease; DH = dehydrogenase; and DPO = o-diphenol oxidase. | ||||||||||||||||
| C mic | 0.91*** | |||||||||||||||
| DBR | 0.86** | 0.96*** | ||||||||||||||
| FDAH | 0.97*** | 0.92*** | 0.91*** | |||||||||||||
| AcP | 0.91*** | 0.87*** | 0.84** | 0.89*** | ||||||||||||
| AlkP | 0.65* | 0.76* | 0.77** | 0.82** | 0.84** | |||||||||||
| ArS | 0.77** | 0.83** | 0.89*** | 0.83** | 0.91*** | 0.89*** | ||||||||||
| Glu | 0.84** | 0.89*** | 0.93*** | 0.91*** | 0.88*** | 0.78** | 0.96*** | |||||||||
| Ure | 0.48 | 0.37 | 0.39 | 0.54 | 0.32 | −0.08 | 0.24 | 0.42 | ||||||||
| DH | 0.09 | 0.19 | 0.21 | 0.03 | 0.26 | 0.53 | 0.39 | 0.30 | −0.34 | |||||||
| DPO | 0.27 | 0.44 | 0.44 | 0.25 | 0.22 | 0.36 | 0.26 | 0.21 | −0.43 | 0.00 | ||||||
| Hgtot | −0.17 | 0.12 | 0.07 | −0.19 | −0.26 | 0.10 | −0.16 | −0.14 | −0.51 | 0.30 | 0.55 | |||||
| VH | 0.35 | 0.60 | 0.53 | 0.41 | 0.40 | 0.45 | 0.47 | 0.53 | 0.13 | 0.48 | 0 | 0.65 | ||||
| HgR | 0 | 0.32 | 0.23 | 0.02 | −0.18 | −0.12 | −0.10 | 0.02 | −0.23 | −0.14 | 0.52 | 0.73* | 0.42 | |||
| CD VH | −0.37 | −0.38 | −0.37 | −0.28 | −0.07 | −0.07 | −0.07 | −0.18 | −0.12 | −0.02 | −0.50 | −0.60 | −0.35 | −0.30 | ||
| CD HgR | 0.39 | 0.143 | 0.23 | 0.27 | 0.58 | 0.67* | 0.50 | 0.38 | −0.13 | 0.25 | −0.22 | −0.32 | 0.09 | −0.62 | 0.33 |
As a confirmation of the presence of scarcely soluble, and thus hardly available, Hg-forms in the investigated soil samples, it was observed that Hg concentration had no influence on the soil biochemical parameters related to the metabolic functionality of the soil microbial community (Table 5). Thus, Hg does not seem to represent a stress factor for the microbial biomass of these soils.
Although soil microorganisms are highly sensitive to elevated concentrations of heavy metals which usually cause a decrease of soil microbial biomass,5 the prolonged exposure to the contaminant can change the microbial community structure towards metal tolerant microbial pools.7 As suggested by several authors, in field situation many factors could influence biological properties and enzyme activities and minimize the heavy metal effects.28
The lack of Hg toxic effects could be due to an adaptation of the soil microflora to the pollutant, since all the tested soils were long-term contaminated by the heavy metal, but due also to the possible non-bioavailability of Hg, caused by the quite insoluble forms in which Hg is speciated. It is known that changes in the form and quantity of heavy metals may reduce the toxicity to soil enzymes.29 Anyway, it cannot be excluded that already in its origin Hg-pollution was determined by insoluble and unavailable Hg-chemical forms.
In a previous study carried out in the same site, Cattani et al.30 found that the soluble and root available fractions of mercury were generally very low with respect to the total mercury concentration. By using the diffusive gradient in thin films (DGT) technique that allows the maximum potential root availability to be estimated, they established that only the 0.01% of the total Hg content is available for plant uptake.
The response of the phytotoxicity tests showed a different sensitivity among the used plants. The stronger phytotoxic effect was observed in L. sativum. This plant has seeds characterized by an external mucilaginous layer that could favour a major soaking immediately at the water contact and consequently a more direct effect of the contaminant potentially present. Nevertheless, the negative effects on germination indices reported in this study were not so marked as reported in literature.31,32 Street et al.32 indicated 0.5 mg L−1 as the critical concentration of Hg for germination of some medicinal plants. According to Munzuroglu and Geckil31 even C. sativus underwent a strong reduction of the germination percentage until falling down to zero in the presence of Hg concentrations higher than 1.6 mM. However, it is important to highlight that in the majority of these papers mercury was present in solution and a direct interaction with plant tissue was favoured. Conversely, in our experimental design seeds were sown in contaminated soils in which the soluble Hg fraction is only a very small fraction of the total content30 and, as observed in the enzymatic activity behaviour, also seed germination seemed to be scarcely affected by the Hg presence. In the case of L. sativum seeds the correlation with Hg content was significantly negative (−0.622, p < 0.05) for RG but positive for RL as well as for GI (0.769 and 0.801, p < 0.05, respectively). Although no significant correlation was found for RG of S. lycopersicum seeds with Hg content, there was a positive correlation for both RL and GI values (0.805 and 0.842, p < 0. 05, respectively).
Our results agree with Zagury et al.,2 who similarly studied the effect of contaminated soils on barley (Hordeum vulgare). Highly Hg polluted soils (568 mg kg−1 and 295 mg kg−1) induced a low inhibition of seed germination (5.6 and 11%, respectively) and a reduction of 39% was observed only with a soil containing 11500 mg kg−1. The authors found positive correlation between toxic effect and water-soluble and exchangeable concentration of Hg and not with total Hg content.2
Similarly even soil enzyme activities in the investigated samples were not affected by Hg concentration as demonstrated by no correlation between these parameters. A mitigation effect of Corg on soil biochemical properties could be hypothesized as confirmed by the high positive correlation of this parameter with the majority of the measured properties (Table 5). In fact, enzyme activities were highly positively correlated with Corg and Cmic except for o-diphenol oxidase, dehydrogenase, and urease activities (Table 5).
No significant correlation was found between the number of VH, HgR bacteria, CD index and most chemical and biochemical soil parameters. Instead, HgR bacteria and CD HgR index were positively correlated with Hg content and alkaline phosphatase, respectively (Table 5).
Some HgR bacteria (especially of the Pseudomonas genus) harbor FP plasmids that, beside conferring resistance to Hg ions, have also chromosome-mobilizing ability. These plasmids can significantly increase the production of alkaline phosphatase.33 The higher alkaline phosphatase activity detected in soils would have been promoted by the presence of HgR bacteria possibly harboring FP-like plasmids. This could represent advantages for HgR bacteria in relation to phosphate uptake, to the presence of more active specific substrate-transporting systems or to effective hydrolysis-uptake coupling systems.
HgR bacteria were in the range of 0.001% to 0.25% of the VH bacteria (Table 3), and were related to the Hg content of the soil. Amounts of HgR bacteria in the range of 0.01–0.07% have been found in uncontaminated environments.34
This study shows that the addition of available Hg rapidly affected indigenous soil bacterial communities. The bacterial response to the addition of Hg is unevenly distributed among soils with comparable levels of total Hg. In soils with a lower initial HgR/VH ratio, the addition of HgCl2 promoted the enrichment of an effective Hg resistant community (Table 3), which grew mainly on the soil organic carbon. However we cannot exclude that some microorganisms could also have grown on the available carbon increase due to the cell lyses of the sensitive bacteria. The long term exposition to Hg concentrations could have selected different bacterial communities with a different dominance level of HgR phenotypes, as shown by the increasing factor of HgR bacteria in soil samples 1. Selection and multiplication of pre-existing HgR bacteria and gene transfer and/or stress induced gene rearrangements are among the mechanisms responsible for the HgR enrichment.
The genetic diversity of soil samples 2A and 2B was affected by the addition of available Hg as reported by Ranjard et al.35 in mercury-polluted soils. Although the microbial community diversity expressed by H′ decreased in the first 14 days of incubation, a partial recovery was observed after 50 days, as found in a mercury-spiked soil microcosms study by Rasmussen and Sørensen.36 The observed changes were caused by the disappearance of some bands, probably belonging to sensitive strains, and by the appearance of new dominating bands not present in the control soils and belonging to possible mercury resistant strains. As resulted from similarity analysis of nucleotide sequences, those bands belonged to uncultured environmental clones retrieved from agricultural soils37 and to chlorophenol-degrading strain isolated from contaminated groundwater.38 Although a tendency to the resilience was observed, genetic diversity did not completely recover within the time span of the experiment, probably for the selective pressure still exerted by the spiked available mercury on the bacterial species. The mercury addition caused a shift in the structure of the bacterial community, as indicated by low values of the Sørensen similarity index between mercury-spiked and non-spiked microcosms at 50 days, and the effect was more evident in the bacterial community of soil 2B than 2A, thus indicating that the former was affected more severely by the pulse of available Hg. This was probably due to the Hg content and to most of the biological characteristics of the soil before the spiking.
The Hg-resistant Alphaproteobacteria and Gammaproteobacteria, Bacteroidetes, Actinobacteria and Firmicutes microorganisms isolated in the investigated soils represent soil bacteria recently characterised for their involvement in the Hg biogeochemistry.36 The three highly resistant Pseudomonas strains were isolated from the most contaminated soil.
Genes for Hg resistance were detected in some HgR strains. Sequence homologies of the mer gene fragments were not consistent with the phylogenetic affiliation of the closest relative bacteria, indicating that inter-species gene transfer exists in the bacterial community of these Hg-polluted soils. Mercury resistance genes are normally located on plasmid or flanked by transposons which are responsible for gene transfer events in natural environments.39 Although Hg has been shown to negatively affect the conjugal transfer of plasmids,40 long-term pollution and low concentration of bioavailable Hg in soil have been recently proposed to cause horizontal transfer of mobile genetic elements carrying mer genes.41 The merA sequences obtained in the isolates were homologous to two different sequences for mercuric reductase genes: one to that present on Tn5058 transposon of Pseudomonas sp. ED23-33 isolated from Russian permafrost42 and the remaining five to that of P. putida Spi4 isolated from an industrial wastewater-treating biocatalyzer.43 Also B. samanii 4A/9 carried a merA gene with deduced amino acid homology of 65% to merA of Spi4. Repeated attempts to amplify the mer genes in 4A/9 strain with primers specific for this phylum44 did not get any successful result, a difficulty evidenced also by other recent works.41,45Pseudomonas strains 5A/1, 5A/2 and 5A/3 were characterised by the presence of putative merB related to a Tn5041D-carried alkylmercury lyase of P. putida MU10-2.42 However, in this work we did not demonstrate the ability of these strains to volatilize Hg(II) and to grow on organomercurials.
The artificial contamination with available Hg changed the microbial community structure and highlighted the presence of resistant phenotypes. The heterotrophic HgR population probably plays an important role in the development of the culturable diversity during environmental stress, supporting the proposed view that HgR culturable bacteria provide a rapid useful assessment of biological responses to heavy metal pollution.7
Conclusions
In this study, the response of already adapted soil microorganisms to Hg stress in long-term polluted industrial site has been assessed. In these soils, highly insoluble, and thus hardly available forms of Hg were present. Considering that a relevant control soil (without Hg) was not found, as usual in field studies of long-term contaminated sites, the results obtained showed that the different amounts of Hg in these soils did not influence the soil microbial parameters and enzyme activities.Nonetheless, the bioavailability and toxic effects, as widely known, are largely influenced by metal speciation and even in the presence of scarcely soluble and immobilized metal forms there is a need of further relevant issues under field conditions aimed to monitor and predict possible remobilisation processes in the soil–plant system. So, we tested also whether the bacterial community was resistant or resilient to a subsequent Hg stress by spiking experiments where soluble Hg forms to the same soils were added. Our results highlighted the potentiality of indigenous bacterial communities to withstand Hg stress by enhancing their capacity for resistance and to recover after this stress by expressing a capacity of resilience. At the same time, a selection of resistant well adapted strains carrying mer genes was observed. The worry of long-term changes (over time) in Hg solubility can be considered extenuated.
Acknowledgements
This research was financed by the Ministry of University and Research of Italy (COFIN, 2005) project “Innovative chemical, physical, and biological methods to characterize and remediate soils polluted by heavy metals (MICROS)”. The authors thank Nicola Carella for technical support in sampling and sample preparation, Cristina Romagnoli for PCR analysis and Anna Santoro for analytical studies.References
- A. Renzoni, F. Zino and E. Franchi, Environ. Res., 1998, 77, 68–72 CrossRef CAS.
- G. J. Zagury, C.-M. Neculita, C. Bastien and L. Deschenes, Environ. Toxicol. Chem., 2006, 25, 1138–1147 CrossRef CAS.
- P. C. Brookes, Biol. Fertil. Soils, 1995, 19, 269–279 CrossRef CAS.
- M. B. Hinojosa, J. A. Carreira, R. García-Ruíz and R. P. Dick, Soil Biol. Biochem., 2004, 36, 1559–1568 CrossRef CAS.
- K. Chander and P. C. Brookes, Soil Biol. Biochem., 1993, 25, 587–597 CrossRef.
- Z. L. He, X. E. Yang and P. J. Stoffella, J. Trace Elem. Med. Biol., 2005, 19, 125–140 CrossRef CAS.
- R. J. Ellis, P. Morgan, A. J. Weightman and J. C. Fry, Appl. Environ. Microbiol., 2003, 59, 3605–3617.
- T. Barkay, S. M. Miller and A. O. Summers, FEMS Microbiol. Rev., 2003, 27, 355–384 CrossRef CAS.
- D. M. Sanchez, A. J. Quejido, M. Fernandez, C. Hernandez, T. Schmid, R. Millan, M. Gonzalez, M. Aldea, R. Martin and R. Morante, Anal. Bioanal. Chem., 2005, 381, 1507–1513 CrossRef CAS.
- G. Iamarino, M. A. Rao and L. Gianfreda, Chemosphere, 2009, 74, 216–223 CrossRef CAS.
- S. Dumontet and S. P. Mathur, Soil Biol. Biochem., 1989, 21, 431–436 CrossRef CAS.
- E. D. Vance, P. C. Brooks and D. S. Jenkinson, Soil Biol. Biochem., 1987, 19, 703–707 CrossRef CAS.
- J. P. E. Anderson and K. H. Domsch, Soil Biol. Biochem., 1990, 22, 251–255 CrossRef.
- J. L. Smith and E. A. Paul, in Soil Biochemistry, ed. J. M. Bollag and G. Stotzky, Marcel Dekker Inc., New York, 1990, pp. 359–396 Search PubMed.
- P. Perucci, S. Dumontet, S. A. Bufo, A. Mazzatura and C. Casucci, Biol. Fertil. Soils, 2000, 32, 17–23 CrossRef CAS.
- M. A. Rao, F. Sannino, G. Nocerino, E. Puglisi and L. Gianfreda, Biol. Fertil. Soils, 2003, 38, 327–332 CrossRef.
- M. A. Tabatabai, in Methods of Soil Analysis, Part 2 Agronomy Monograph 9, ed. A. L. Page, R. H. Miller and D. R. Keeney, ASA/SSSA, Madison, WI, 1998 Search PubMed.
- P. Nannipieri, B. Ceccanti, S. Cervelli and E. Matarrese, Soil Sci. Soc. Am. J., 1980, 44, 1011–1016 CAS.
- J. T. Trevors, Plant Soil, 1984, 77, 285–293 CrossRef CAS.
- P. Perucci, C. Casucci and S. Dumontet, Soil Biol. Biochem., 2000, 32, 1927–1933 CrossRef CAS.
- E. Dell'Amico, M. Mazzocchi, L. Cavalca, L. Allievi and V. Andreoni, Microbiol. Res., 2008, 163, 671–683 CrossRef CAS.
- G. Muyzer, E. C. de Waal and G. G. Uitterlindem, Appl. Environ. Microbiol., 1993, 59, 695–700 CAS.
- L. Cavalca, M. A. Rao, S. Bernasconi, M. Colombo, V. Andreoni and L. Gianfreda, Biodegradation, 2008, 19, 1–13 CrossRef CAS.
- C. E. Shannon and W. Weaver, The Mathematical Theory of Communication, University of Illinois Press, Urbana, IL, 1949 Search PubMed.
- T. A. Sørensen, Kongelige Danske Videnskabernes Selskabs Biologiske Skrifter, 1984, 5, 1–34 Search PubMed.
- A. Sadouk and M. Mergeay, Mol. Gen. Genet., 1990, 240, 181–187.
- W. G. Weisburg, S. M. Barns, D. A. Pelletier and D. J. Lane, J. Bacteriol., 1991, 173, 697–703 CAS.
- K. E. Giller, E. Witter and S. P. McGrath, Soil Biol. Biochem., 1998, 30, 1389–1414 CrossRef CAS.
- M. R. Banerjee, D. L. Burton and S. Depoe, Agric., Ecosyst. Environ., 1997, 66, 241–249 CrossRef.
- I. Cattani, S. Spalla, G. M. Beone, A. A. M. Del Re, R. Boccelli and M. Trevisan, Talanta, 2008, 74, 1520–1526 CrossRef CAS.
- O. Munzuroglu and H. Geckil, Arch. Environ. Contam. Toxicol., 2002, 43, 203–213 CrossRef CAS.
- R. A. Street, M. G. Kulkarni, W. A. Stirk, C. Southway and J. Van Staden, Bull. Environ. Contam. Toxicol., 2007, 79, 371–376 CrossRef CAS.
- J. Johnson, R. L. Warren and A. A. Branstrom, J. Clin. Microbiol., 1991, 29, 940–944 CAS.
- W. J. Kelly and D. C. Reanney, Soil Biol. Biochem., 1984, 16, 1–8 CrossRef CAS.
- L. Ranjard, S. Nazaret, F. Gourbiere, J. Thioulouse, P. Linet and A. Richaume, FEMS Microbiol. Ecol., 2000, 31, 107–115 CrossRef CAS.
- R. D. Rasmussen and S. J. Sørensen, FEMS Microbiol. Ecol., 2001, 36, 1–9 CrossRef.
- S. G. Tringe, C. von Mering, A. Kobayashi, A. A. Salamov, K. Chen, H. W. Chang, M. Podar, J. M. Short, E. J. Mathur, J. C. Detter, P. Bork, P. Hugenholtz and E. M. Rubin, Science, 2005, 308, 554–557 CrossRef CAS.
- M. K. Mannisto, M. A. Tiirola, M. S. Salkinoja-Salonen, M. S. Kulomaa and J. A. Puhakka, Arch. Microbiol., 1999, 171, 189–197 CrossRef CAS.
- S. J. Sørensen, M. Bailey, L. H. Hansen, N. Kroer and S. Wuertz, Nat. Rev. Microbiol., 2005, 3, 700–710 Search PubMed.
- A. R. Johnsen and N. Kroer, FEMS Microbiol. Ecol., 2007, 59, 718–728 CrossRef CAS.
- G. Oregaard and S. J. Sørensen, ISME J., 2007, 1, 453–467 CrossRef CAS.
- G. Kholodii, Z. Gorlenko, S. Mindlin, J. Hobman and V. Nikiforov, Microbiology, 2002, 148, 3569–3582 CAS.
- A. D. M. Felske, W. Fehr, B. V. Pauling, H. von Canstein and I. Wagner-Döbler, BMC Microbiol., 2003, 3, 22–33 CrossRef.
- C. C. Huang, M. Narita and T. Yamagata, Gene, 1999, 239, 361–366 CrossRef CAS.
- L. D. Rasmussen, C. Zawadsky, S. J. Binnerup, G. Oregaard, S. J. Sorensen and N. Kroer, Appl. Environ. Microbiol., 2008, 74, 3795–3803 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |