Polyethylene glycol in water: a simple and environment friendly medium for C–P bond formation
Kunda Uma Maheswara
Rao
a,
Soora Harinath
Jayaprakash
a,
Sandip Kumar
Nayak
b and
Cirandur Suresh
Reddy
*a
aDepartment of Chemistry, Sri Venkateswara University, Tirupati-517 502, A.P., India. E-mail: csrsvu@gmail.com; Fax: +91-877 2289555; Tel: +91-9849694958
bBio-Organic Division, BARC, Mumbai, India
First published on 10th October 2011
Abstract
A simple and highly efficient protocol for C–P bond formation under catalyst free conditions at an ambient temperature is reported by using polyethylene glycol in water as an efficient recyclable medium without using any organic co-solvent or additive.
Introduction
Phosphorus–carbon bond formation reactions have attracted serious attention because of their application in the synthesis of α-functionalized phosphonic acids and their derivatives since they are valuable intermediates for the preparation of compounds of synthetic importance.1,2 The main interest in phosphonyl and related derivatives resides in their potential biological activity as antibiotics,3a herbicides,3b insecticides,3c fungicides,3d and antiviral agents.3e Since α-aminophosphonates are structural mimics of α-amino acids, they exhibit very high enzyme inhibiting potency including HIV protease.3f Some of these compounds have already been found to act as antibacterial, neuroactive, anticancer agents, and pesticides while some of them being commercialized.4 The altered activity of such enzymes has been associated with the inhibition of HIV infections and several pathological disorders, including cancers and cataracts.5A number of synthetic methods for α-amino phosphonates have been developed.6 The Kabachnik–Fields and Pudovik reactions are the most general, straightforward and widely applied methods for construction of C–P bonds.7 Of these, the nucleophilic addition of phosphites to imines, catalyzed by a base or an acid, is the most convenient route. Lewis acids such as SnCl4,8a BF3·OEt2,8b and ZnCl28c have been used for this transformation. However, these reactions could not be carried out in a one-pot single step operation with a carbonyl compound, an amine and a dialkyl phosphite because the amine and water formed during imine formation decompose or deactivate the Lewis acid.8d This drawback has been partly overcome in some recent methods using lanthanide triflates/MgSO4,9a InCl3,9b TaCl5–SiO2,9c bismuth nitrate pentahydrate,9d MgClO4,9e TiO2,9f Amberlite-IR 120,9g sulfamic acid,9h H3PW12O40,9i trimethylanilinium chloride,9j lithium perchlorate,9k Amberlyst-15,9l and ZrOCl2·8H2O.9m However, the continual search for a new and greener synthetic protocol that is simple, efficient and cost effective remains an ever-challenging objective. In continuation of our program on the development of novel organic synthetic methodologies,10 we have investigated the synthesis of α-aminophosphonate and α-hydroxyphosphonate derivatives by using polyethylene glycol-600 (PEG-600) in water as a recyclable medium without adding any organic solvent and catalyst.
In the recent years, PEG, a biologically acceptable polymer, emerged as a powerful phase transfer catalyst and performs many useful organic transformations under mild reaction conditions. Moreover, PEG and its monomethyl ethers are inexpensive, thermally stable, recoverable, and are proved to be non-toxic phase-transfer catalysts. The PEG has hitherto not been widely used as a solvent medium but has been used as a support for various transformations.11 The use of water as a reaction medium has received considerable attention in the context of green chemistry, because it is a cheap, safe and environmentally benign medium.12 This background prompted us to use PEG in water as reaction medium and surprisingly it worked successfully even without any catalyst.
An efficient C–P bond formation by using PEG in water in the absence of a catalyst is not yet reported. This report on the C–P bond formation using PEG in water at an ambient temperature is a successful endeavor that realised this goal.
Results and discussion
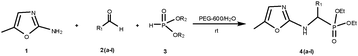
We investigated the reaction between 2-amino-5-methyl oxazole, 3-nitro benzaldehyde and diethyl phosphite by stirring equimolar quantities in different solvents at ambient temperature (Table 1) and low yield of desired α-amino phosphonate is obtained in all these experiments. The best result was obtained when the reaction was carried out using PEG in water. We also studied the influence of temperature on the reaction and observed that there is a significant change in the yields of products at elevated temperatures (Table 2). PEG catalyses by increasing the electrophilicity of the![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N carbon through hydrogen bonding of hydroxyl oxygen with the imine nitrogen and water plays the role of a proton source. After this success, several aldehydes and diethyl/dimethyl phosphite were reacted using PEG in water. The results are summarized in Table 3. In all the cases, the three component coupling proceeded smoothly and the corresponding α-amino phosphonate was isolated. In these experiments, the catalyst was isolated by cooling the reaction mixture in a dry ice–acetone bath to precipitate the PEG and extracted with ether. The ether extract was dried over MgSO4 and could be reloaded with fresh reagents for further runs, thus, recyclization of the PEG is possible without significant loss of activity (Table 3, entry 4a).13 Surprisingly, from the reaction of aldehydes (2a–l) and dialkyl phosphite (3) in the presence of an amine (1) (Scheme 1), no α-hydroxyphosphonate formation is observed.14 The examples studied included electron-rich aromatic aldehydes (entries 4e, 4f, 4g, 4h), electron deficient aromatic aldehydes (entries 4a, 4b), heterocyclic aldehydes (entries 4c, 4d, 4i, 4j) and aliphatic aldehydes (entries 4k, 4l). All of them gave α-amino phosphonates in good yields at room temperature only. Ketones do not react under these conditions.
N carbon through hydrogen bonding of hydroxyl oxygen with the imine nitrogen and water plays the role of a proton source. After this success, several aldehydes and diethyl/dimethyl phosphite were reacted using PEG in water. The results are summarized in Table 3. In all the cases, the three component coupling proceeded smoothly and the corresponding α-amino phosphonate was isolated. In these experiments, the catalyst was isolated by cooling the reaction mixture in a dry ice–acetone bath to precipitate the PEG and extracted with ether. The ether extract was dried over MgSO4 and could be reloaded with fresh reagents for further runs, thus, recyclization of the PEG is possible without significant loss of activity (Table 3, entry 4a).13 Surprisingly, from the reaction of aldehydes (2a–l) and dialkyl phosphite (3) in the presence of an amine (1) (Scheme 1), no α-hydroxyphosphonate formation is observed.14 The examples studied included electron-rich aromatic aldehydes (entries 4e, 4f, 4g, 4h), electron deficient aromatic aldehydes (entries 4a, 4b), heterocyclic aldehydes (entries 4c, 4d, 4i, 4j) and aliphatic aldehydes (entries 4k, 4l). All of them gave α-amino phosphonates in good yields at room temperature only. Ketones do not react under these conditions.
| Entry | R1 | R2 | Time/min | Yield (%) |
|---|---|---|---|---|
| a Isolated yields after recycling of catalysts. | ||||
| 4a | 3-NO2–C6H4 | Et | 65 | 93 (90, 87, 88, 89)a |
| 4b | 3-NO2–C6H4 | Me | 72 | 89 |
| 4c | 2-Imidazolyl | Et | 80 | 89 |
| 4d | 2-Imidazolyl | Me | 85 | 82 |
| 4e | 4-N(CH3)2–C6H4 | Et | 75 | 91 |
| 4f | 4-N(CH3)2–C6H4 | Me | 83 | 86 |
| 4g | 4-OH–C6H4 | Et | 83 | 85 |
| 4h | 4-OH–C6H4 | Me | 90 | 79 |
| 4i | 3-Indolyl | Et | 74 | 92 |
| 4j | 3-Indolyl | Me | 78 | 86 |
| 4k | C6H5CH2CH2– | Et | 87 | 89 |
| 4l | CH3–CH–CH3 | Et | 90 | 84 |
 | ||
| Scheme 1 | ||
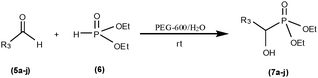
In assessing the catalytic activity of PEG in water, we extended the reaction and studied the addition of diethyl phosphite to aldehydes. This reaction proceeds smoothly and gave the corresponding α-hydroxy phosphonates in excellent yields. In recent times, α-hydroxyphosphonates are drawing increased attention due to their enzyme inhibitory bioactivity towards rennin,15a enolpyruvylshikimate-3-phosphate synthase,15b farnesyl protein transferase,15c human immunodeficiency virus protease and polymerase.15d They also exhibit anti-virus15e and anti-cancer activity.15f Furthermore, α-hydroxy phosphonates are useful precursors for the preparation of a variety of α-functionalized phosphonates, such as α-amino,16a α-keto,16b α-halo16c and α-acetoxyphosphonates16d and their extended derivatives. The available methods for the synthesis of α-hydroxyphosphonates involve the nucleophilic addition of di or trialkylphosphite to different carbonyl compounds in the presence of various catalysts, such as, enzymes,17a alkaloids,17b phosphoric acids,17c Lewis acids,17d alumina,17e SALEN,17f BINOL,17g alumina/potassium fluoride,17h NH4VO317i and polymer/solid supported base.17j When we tried to improve the yields, none of them was satisfactory. Hence, there arose still a need to search for an efficient method for their preparation. Herein, we report our results for the preparation of α-hydroxy phosphonates from aldehydes and diethyl phosphite using PEG in water at room temperature (Scheme 2).
 | ||
| Scheme 2 | ||
Reaction of 3-nitro benzaldehyde with diethyl phosphite in the absence of PEG afforded low (<20%) yield of diethyl 1-hydroxy-3-nitrophenylmethylphosphonate. The same reaction when performed in the presence of PEG in water at room temperature resulted in the formation of the desired products in high yields (Table 4). Further, no byproduct formation occurred in these reactions.
| Entry | R3 | Time/min | Yield (%) |
|---|---|---|---|
| 7a | 3-NO2–C6H4 | 90 | 92 |
| 7b | 4-F–C6H4 | 122 | 83 |
| 7c | 2-Imidazolyl | 95 | 88 |
| 7d | 3-Indolyl | 115 | 86 |
| 7e | 2,4-Cl–C6H3 | 130 | 90 |
| 7f | 4-Cl–C6H4 | 135 | 83 |
| 7g | 4-OH–C6H4 | 110 | 86 |
| 7h | 4-Isopropyl–C6H4 | 120 | 89 |
| 7i | 4-(NCH3)2–C6H4 | 95 | 84 |
| 7j | 3,5-OMe–C6H3 | 130 | 85 |
When this reaction was performed in organic solvents such as ethanol, MeCN, CH2Cl2, CHCl3, THF, dioxane, low (<20%) yields of the α-hydroxy phosphonates were obtained. Ketones and triethyl phosphite did not react under these conditions.
Experimental
General
NMR spectra were recorded on a Bruker instrument at 500 MHz for 1H NMR, 75.4 MHz for 13C NMR, and 121.4 MHz for 31P NMR in CDCl3 solution, using tetramethylsilane as internal and 85% H3PO4 as external standard respectively. Positive chemical shifts occurred downfield from that of external 85% H3PO4 for 31P NMR spectra. Chemical shifts (δ) are indicated in parts per million (ppm) and coupling constants (J) in Hz. Mass spectra were recorded on an ESI-MS mass spectrometer and IR spectra were measured on a Perkin-Elmer FT-IR 240-c spectrophotometer using KBr optics. Elemental analyses were performed on a Thermo Finnigan Instrument. Melting points were determined in open capillaries and are uncorrected. All reagents were purchased from Sigma Aldrich and were used without further purification.Typical procedure for the synthesis of 4a
A mixture of 2-amino-5-methylisoxazole (1 mmol), 3-nitro benzaldehyde (1 mmol), diethylphosphite (1.2 mmol), PEG-600 (3 g) and water (2 ml) was placed in a 25 ml round-bottomed flask. The mixture was kept for stirring at room temperature. After completion of the reaction, the reaction mixture was cooled in a dry ice–acetone bath to precipitate the PEG and extracted with ether. The ether extract was dried over MgSO4 and evaporated. The resulting product, though seen as a single compound by TLC, was further purified by passing it over a silica gel column with EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) as an eluent. The recovered PEG has been reused.13
4) as an eluent. The recovered PEG has been reused.13
Typical procedure for the synthesis of 7a
A mixture of 3-nitro benzaldehyde (1 mmol), diethylphosphite (1.2 mmol), PEG-600 (3 g) and water (2 ml) was placed in a 25 ml round-bottomed flask. The mixture was kept for stirring at room temperature. After completion of the reaction, the reaction mixture was cooled in a dry ice–acetone bath to precipitate the PEG and extracted with ether. The ether extract was dried over MgSO4 and evaporated. The resulting product, though seen as a single compound by TLC, was further purified by passing it over a silica gel column with EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) as an eluent. The recovered PEG has been reused.12
6) as an eluent. The recovered PEG has been reused.12
Physical and spectral data of the products
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 752 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 7.65–7.82 (m, 4Ar–H), 6.23 (s, isoxazole H), 5.54 (brs, NH), 4.89 (d, JP-C = 7.2 Hz, P-CH), 3.81–4.19 (m, 4H, P(O)CH2CH3), 1.94 (s, -CH3), 1.21 (t, 6H, 3J = 7.3 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 161.21 (C-5′), 154.16 (C-2′), 141.13 (C-1), 133.86 (C-3), 129.37 (C-6), 122.36 (C-5), 121.72 (C-2), 115.23 (C-4), 113.5 (C-4′), 64.5 (d, 2JP-C = 7.6 Hz, P(O)CH2CH3), 45.9 (P-C), 16.9 (d, 3JP-C = 5.9 Hz, P(O)CH2CH3), 15.2 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 20.56; ESI-MS: 369.25; anal. calcd for C15H20N3O6P (369): C, 48.78; H, 5.46; N, 11.38%. Found: C, 48.72; H, 5.43; N, 11.35%.
O), 752 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 7.65–7.82 (m, 4Ar–H), 6.23 (s, isoxazole H), 5.54 (brs, NH), 4.89 (d, JP-C = 7.2 Hz, P-CH), 3.81–4.19 (m, 4H, P(O)CH2CH3), 1.94 (s, -CH3), 1.21 (t, 6H, 3J = 7.3 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 161.21 (C-5′), 154.16 (C-2′), 141.13 (C-1), 133.86 (C-3), 129.37 (C-6), 122.36 (C-5), 121.72 (C-2), 115.23 (C-4), 113.5 (C-4′), 64.5 (d, 2JP-C = 7.6 Hz, P(O)CH2CH3), 45.9 (P-C), 16.9 (d, 3JP-C = 5.9 Hz, P(O)CH2CH3), 15.2 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 20.56; ESI-MS: 369.25; anal. calcd for C15H20N3O6P (369): C, 48.78; H, 5.46; N, 11.38%. Found: C, 48.72; H, 5.43; N, 11.35%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 758 (P–C); 1H NMR (DMSO-d6, 500 MHz): δ 7.62–7.81 (m, 4Ar-H), 6.26 (s, isoxazole H), 5.49 (brs, NH), 4.78 (d, JP-C = 7.2 Hz, P-CH), 3.55 (d, 3JP-H= 10.5 Hz, P-OCH3), 3.73 (d, 3JP-H= 10.9 Hz, P-OCH3), 1.92 (s, -CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 24.80; ESI-MS: 341.02; anal. calcd for C13H16N3O6P (341): C, 45.75; H, 4.73; N, 12.31%. Found: C, 45.69; H, 4.69; N, 12.25%.
O), 758 (P–C); 1H NMR (DMSO-d6, 500 MHz): δ 7.62–7.81 (m, 4Ar-H), 6.26 (s, isoxazole H), 5.49 (brs, NH), 4.78 (d, JP-C = 7.2 Hz, P-CH), 3.55 (d, 3JP-H= 10.5 Hz, P-OCH3), 3.73 (d, 3JP-H= 10.9 Hz, P-OCH3), 1.92 (s, -CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 24.80; ESI-MS: 341.02; anal. calcd for C13H16N3O6P (341): C, 45.75; H, 4.73; N, 12.31%. Found: C, 45.69; H, 4.69; N, 12.25%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 761 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 5.90 (s, 1H, NH), 7.45–7.65 (m, 3Ar-H), 5.60 (d, JP-C = 10.8 Hz, P-CH), 3.81–4.09 (m, 4H, P(O)CH2CH3), 1.82 (s, -CH3), 1.17 (t, 6H, 3J = 7.3 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 159.64 (C-5′), 148.01 (C-2′), 145.72 (C-2), 129.2 (C-4), 120.8 (C-5), 112.79 (C-4′), 63.5 (d, 2JP-C = 7.3 Hz, P(O)CH2CH3), 44.7 (P-C), 14.6 (d, 3J = 6.1 Hz, P(O)CH2CH3), 14.1 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 21.61; ESI-MS: 314.16; anal. calcd for C12H19N4O4P (314): C, 45.86; H, 6.09; N, 17.83%. Found: C, 45.84; H, 6.04; N, 17.82%.
O), 761 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 5.90 (s, 1H, NH), 7.45–7.65 (m, 3Ar-H), 5.60 (d, JP-C = 10.8 Hz, P-CH), 3.81–4.09 (m, 4H, P(O)CH2CH3), 1.82 (s, -CH3), 1.17 (t, 6H, 3J = 7.3 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 159.64 (C-5′), 148.01 (C-2′), 145.72 (C-2), 129.2 (C-4), 120.8 (C-5), 112.79 (C-4′), 63.5 (d, 2JP-C = 7.3 Hz, P(O)CH2CH3), 44.7 (P-C), 14.6 (d, 3J = 6.1 Hz, P(O)CH2CH3), 14.1 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 21.61; ESI-MS: 314.16; anal. calcd for C12H19N4O4P (314): C, 45.86; H, 6.09; N, 17.83%. Found: C, 45.84; H, 6.04; N, 17.82%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 759 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 5.85 (s, 1H, NH), 7.28–7.39 (m, 3Ar-H), 5.48 (d, JP-C = 10.8 Hz, P-CH), 3.44 (d, 3JP-H= 10.5 Hz, P-OCH3), 3.69 (d, 3JP-H= 11.1 Hz, P-OCH3), 1.84 (s, -CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.82; ESI-MS: 286.07; anal. calcd for C10H15N4O4P (286): C, 41.96; H, 5.28; N, 19.57%. Found: C, 41.91; H, 5.26; N, 19.52%.
O), 759 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 5.85 (s, 1H, NH), 7.28–7.39 (m, 3Ar-H), 5.48 (d, JP-C = 10.8 Hz, P-CH), 3.44 (d, 3JP-H= 10.5 Hz, P-OCH3), 3.69 (d, 3JP-H= 11.1 Hz, P-OCH3), 1.84 (s, -CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.82; ESI-MS: 286.07; anal. calcd for C10H15N4O4P (286): C, 41.96; H, 5.28; N, 19.57%. Found: C, 41.91; H, 5.26; N, 19.52%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 751 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.81–7.01 (m, 4Ar-H), 2.82 (s, 6H), 5.54 (s, NH), 5.12 (d, JP-C = 11.2 Hz, P-CH), 3.81–4.10 (m, 4H, P(O)CH2CH3), 1.18 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 160.16 (C-5′), 153.42 (C-3′), 139.72 (C-4), 131.14 (C-2 and C-6), 128.83 (C-1), 123.58 (C-3 and C-5), 116.75 (C-4′), 63.6 (d, 2JP-C = 7.6 Hz, P(O)CH2CH3), 46.1 (P-C), 16.9 (d, 3JP-C = 5.9 Hz, P(O)CH2CH3), 14.8 (-N(CH3)2), 15.7 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 20.42; ESI-MS: 367.04; anal. calcd for C17H26N3O4P (367): C, 55.58; H, 7.13; N, 11.44%. Found: C, 55.54; H, 7.08; N, 11.39%.
O), 751 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.81–7.01 (m, 4Ar-H), 2.82 (s, 6H), 5.54 (s, NH), 5.12 (d, JP-C = 11.2 Hz, P-CH), 3.81–4.10 (m, 4H, P(O)CH2CH3), 1.18 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 160.16 (C-5′), 153.42 (C-3′), 139.72 (C-4), 131.14 (C-2 and C-6), 128.83 (C-1), 123.58 (C-3 and C-5), 116.75 (C-4′), 63.6 (d, 2JP-C = 7.6 Hz, P(O)CH2CH3), 46.1 (P-C), 16.9 (d, 3JP-C = 5.9 Hz, P(O)CH2CH3), 14.8 (-N(CH3)2), 15.7 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 20.42; ESI-MS: 367.04; anal. calcd for C17H26N3O4P (367): C, 55.58; H, 7.13; N, 11.44%. Found: C, 55.54; H, 7.08; N, 11.39%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 766 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.75–7.93 (m, 4Ar-H), 2.85 (s, 6H), 5.49 (s, NH), 5.19 (d, JP-C = 11.2 Hz, P-CH), 3.56 (d, 3JP-H= 10.4 Hz, P-OCH3), 3.74 (d, 3JP-H= 11.1 Hz, P-OCH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.35; ESI-MS: 339.18; anal. calcd for C15H22N3O4P (339): C, 53.09; H, 6.53; N, 12.38%. Found: C, 52.09; H, 6.48; N, 12.33%.
O), 766 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.75–7.93 (m, 4Ar-H), 2.85 (s, 6H), 5.49 (s, NH), 5.19 (d, JP-C = 11.2 Hz, P-CH), 3.56 (d, 3JP-H= 10.4 Hz, P-OCH3), 3.74 (d, 3JP-H= 11.1 Hz, P-OCH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.35; ESI-MS: 339.18; anal. calcd for C15H22N3O4P (339): C, 53.09; H, 6.53; N, 12.38%. Found: C, 52.09; H, 6.48; N, 12.33%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 763 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.58–6.72 (m, 4Ar-H), 5.61 (s, 1H, NH), 5.04 (d, 1H, 2JP-C = 11.3 Hz, P-CH), 3.72–3.98 (m, 4H, P(O)CH2CH3), 1.89 (s, 3H), 1.08 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.83; ESI-MS: 340.16; anal. calcd for C15H21N2O5P (340): C, 52.94; H, 6.22; N, 8.23%. Found: C, 52.90; H, 6.17; N, 8.19%.
O), 763 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.58–6.72 (m, 4Ar-H), 5.61 (s, 1H, NH), 5.04 (d, 1H, 2JP-C = 11.3 Hz, P-CH), 3.72–3.98 (m, 4H, P(O)CH2CH3), 1.89 (s, 3H), 1.08 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 22.83; ESI-MS: 340.16; anal. calcd for C15H21N2O5P (340): C, 52.94; H, 6.22; N, 8.23%. Found: C, 52.90; H, 6.17; N, 8.19%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 779 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.52–6.68 (m, 4Ar-H), 5.65 (s, 1H, NH), 4.98 (d, 1H, 2JP-C = 11.3 Hz, P-CH), 3.46 (d, 3JP-H= 10.2 Hz, P-OCH3), 3.76 (d, 3JP-H= 10.9 Hz, P-OCH3), 1.86 (s, 3H); 13C NMR (DMSO-d6, 75.4 MHz): δ 161.36 (C-5′), 151.61 (C-2′), 146.03 (C-4), 131.75 (C-1), 129.53 (C-2 and C-6), 124.91 (C-3 and C-5), 110.36 (C-4′), 54.8 (P-OCH3, d, J = 6.5 Hz), 46.5 (P-C), 14.6 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 21.76; ESI-MS: 312.27; anal. calcd for C13H17N2O5P (312): C, 50.00; H, 5.49; N, 8.97%. Found: C, 49.62; H, 5.42; N, 8.91%.
O), 779 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.52–6.68 (m, 4Ar-H), 5.65 (s, 1H, NH), 4.98 (d, 1H, 2JP-C = 11.3 Hz, P-CH), 3.46 (d, 3JP-H= 10.2 Hz, P-OCH3), 3.76 (d, 3JP-H= 10.9 Hz, P-OCH3), 1.86 (s, 3H); 13C NMR (DMSO-d6, 75.4 MHz): δ 161.36 (C-5′), 151.61 (C-2′), 146.03 (C-4), 131.75 (C-1), 129.53 (C-2 and C-6), 124.91 (C-3 and C-5), 110.36 (C-4′), 54.8 (P-OCH3, d, J = 6.5 Hz), 46.5 (P-C), 14.6 (-CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 21.76; ESI-MS: 312.27; anal. calcd for C13H17N2O5P (312): C, 50.00; H, 5.49; N, 8.97%. Found: C, 49.62; H, 5.42; N, 8.91%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 751 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.75–7.58 (m, 6H, Ar-H), 9.98 (s, 1H, Ar-NH), 5.59 (s, 1H, Aliph-NH), 4.88 (d, 1H, 2JP-C = 11.2 Hz, PCH); 3.68–3.81 (m, 4H, P(O)CH2CH3), 2.10 (s, 3H), 1.23 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 24.75; ESI-MS: 363.28; anal. calcd for C17H22N3O4P (363): C, 56.19; H, 6.10; N, 11.56%. Found: C, 56.13; H, 6.06; N, 11.51%.
O), 751 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.75–7.58 (m, 6H, Ar-H), 9.98 (s, 1H, Ar-NH), 5.59 (s, 1H, Aliph-NH), 4.88 (d, 1H, 2JP-C = 11.2 Hz, PCH); 3.68–3.81 (m, 4H, P(O)CH2CH3), 2.10 (s, 3H), 1.23 (t, 6H, 3J = 7.8 Hz, P(O)CH2CH3); 31P NMR (DMSO-d6, 121.4 MHz): δ 24.75; ESI-MS: 363.28; anal. calcd for C17H22N3O4P (363): C, 56.19; H, 6.10; N, 11.56%. Found: C, 56.13; H, 6.06; N, 11.51%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 746 (P-CH); 1H NMR (DMSO-d6, 500 MHz): δ 6.84–7.70 (m, 6H, Ar-H), 10.12 (s, 1H, Ar-NH), 5.70 (s, 1H, Aliph-NH), 4.76–4.88 (m, 1H, PCH), 3.28 (d, 3JP-H= 10.0 Hz, P-OCH3), 3.57 (d, 3JP-H= 11.1 Hz, P-OCH3), 2.10 (s, 3H, Ar-CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 122.7 (C-2), 112.5 (C-3), 121.8 (C-4), 120.6 (C-5), 119.6 (C-6), 111.5 (C-7), 131.2 (C-8), 179 (C-9), 151.5 (C-3′), 113.5 (C-4′), 160.9 (C-5′), 54.76 (P-CH), 53.9 (P-OCH3, d, J = 6.5 Hz), 15.8 (Ar-CH3); 31P NMR (DMSO-d6, 121.4 MHz); δ 31.29; ESI-MS: 315.11; anal. calcd for C15H18N3O4P (315): C, 53.73; H, 5.41; N, 12.53%. Found: C, 53.53; H, 5.31; N, 12.50%.
O), 746 (P-CH); 1H NMR (DMSO-d6, 500 MHz): δ 6.84–7.70 (m, 6H, Ar-H), 10.12 (s, 1H, Ar-NH), 5.70 (s, 1H, Aliph-NH), 4.76–4.88 (m, 1H, PCH), 3.28 (d, 3JP-H= 10.0 Hz, P-OCH3), 3.57 (d, 3JP-H= 11.1 Hz, P-OCH3), 2.10 (s, 3H, Ar-CH3); 13C NMR (DMSO-d6, 75.4 MHz): δ 122.7 (C-2), 112.5 (C-3), 121.8 (C-4), 120.6 (C-5), 119.6 (C-6), 111.5 (C-7), 131.2 (C-8), 179 (C-9), 151.5 (C-3′), 113.5 (C-4′), 160.9 (C-5′), 54.76 (P-CH), 53.9 (P-OCH3, d, J = 6.5 Hz), 15.8 (Ar-CH3); 31P NMR (DMSO-d6, 121.4 MHz); δ 31.29; ESI-MS: 315.11; anal. calcd for C15H18N3O4P (315): C, 53.73; H, 5.41; N, 12.53%. Found: C, 53.53; H, 5.31; N, 12.50%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 749 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.48–6.92 (m, 5Ar-H), 6.12 (s, oxazole H), 5.62 (brs, NH), 4.56 (d, JP-C = 7.2 Hz, P–CH), 3.67–4.07 (m, 4H, P(O)CH2CH3), 2.16–2.54 (m, 2H), 1.87 (t, 3JC-H = 7.0 Hz, 2H), 1.28 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3);31P NMR (DMSO-d6, 121.4 MHz): δ 21.35; ESI-MS: 352; anal. calcd for C17H25N2O4P (352): C, 57.95; H, 7.15; N, 7.95%. Found: C, 57.91; H, 7.10; N, 7.91%.
O), 749 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.48–6.92 (m, 5Ar-H), 6.12 (s, oxazole H), 5.62 (brs, NH), 4.56 (d, JP-C = 7.2 Hz, P–CH), 3.67–4.07 (m, 4H, P(O)CH2CH3), 2.16–2.54 (m, 2H), 1.87 (t, 3JC-H = 7.0 Hz, 2H), 1.28 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3);31P NMR (DMSO-d6, 121.4 MHz): δ 21.35; ESI-MS: 352; anal. calcd for C17H25N2O4P (352): C, 57.95; H, 7.15; N, 7.95%. Found: C, 57.91; H, 7.10; N, 7.91%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 732 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.44 (s, oxazole H), 5.17 (brs, NH), 4.34 (d, JP-C = 7.2 Hz, P-CH), 3.55–3.85 (m, 4H, P(O)CH2CH3), 2.31–2.48 (m, 1H), 1.28 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3), 1.08 (m, 6H); 31P NMR (DMSO-d6, 121.4 MHz): δ 17.68; ESI-MS: 290; anal. calcd for C12H23N2O4P (290): C, 49.65; H, 7.99; N, 9.65%. Found: C, 49.59; H, 7.95; N, 9.60%.
O), 732 (P-C); 1H NMR (DMSO-d6, 500 MHz): δ 6.44 (s, oxazole H), 5.17 (brs, NH), 4.34 (d, JP-C = 7.2 Hz, P-CH), 3.55–3.85 (m, 4H, P(O)CH2CH3), 2.31–2.48 (m, 1H), 1.28 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3), 1.08 (m, 6H); 31P NMR (DMSO-d6, 121.4 MHz): δ 17.68; ESI-MS: 290; anal. calcd for C12H23N2O4P (290): C, 49.65; H, 7.99; N, 9.65%. Found: C, 49.59; H, 7.95; N, 9.60%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1009 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.63–7.79 (m, 4Ar-H), 5.23 (d, 2JP-H = 10.2 Hz, P-C-H), 3.93–4.08 (m, 4H, P(O)CH2CH3), 1.185 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 147.47 (C-3), 141.13 (C-1), 133.86 (C-6), 129.37 (C-5), 122.36 (C-2), 121.72 (C-4), 69.05 (P–C), 52.59 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 18.53 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.61 (P
O, phosphonate), 1009 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.63–7.79 (m, 4Ar-H), 5.23 (d, 2JP-H = 10.2 Hz, P-C-H), 3.93–4.08 (m, 4H, P(O)CH2CH3), 1.185 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 147.47 (C-3), 141.13 (C-1), 133.86 (C-6), 129.37 (C-5), 122.36 (C-2), 121.72 (C-4), 69.05 (P–C), 52.59 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 18.53 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.61 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 289.09; anal. calcd for C11H16NO6P (289): C, 45.68; H, 5.58%. Found: C, 45.65; H, 5.54%.
O); ESI-MS: 289.09; anal. calcd for C11H16NO6P (289): C, 45.68; H, 5.58%. Found: C, 45.65; H, 5.54%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1065 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.09–7.17 (m, 4Ar-H), 4.69 (d, 2JP-H = 10.2 Hz, P-C-H), 3.86–4.12 (m, 4H, P(O)CH2CH3), 1.18 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 148.5 (C-4), 139.23 (C-1), 129.36 (C-2), 128.42 (C-6), 121.73 (C-3), 121.54 (C-5), 73.4 (P-C), 56.9 (d, 2JP-C = 7.5 Hz, P-OCH2CH3), 17.8 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.52 (P
O), 1065 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.09–7.17 (m, 4Ar-H), 4.69 (d, 2JP-H = 10.2 Hz, P-C-H), 3.86–4.12 (m, 4H, P(O)CH2CH3), 1.18 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 148.5 (C-4), 139.23 (C-1), 129.36 (C-2), 128.42 (C-6), 121.73 (C-3), 121.54 (C-5), 73.4 (P-C), 56.9 (d, 2JP-C = 7.5 Hz, P-OCH2CH3), 17.8 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.52 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 262.14; anal. calcd for C11H16O4PF (262): C, 50.39; H, 6.15%. Found: C, 50.35; H, 6.10%.
O); ESI-MS: 262.14; anal. calcd for C11H16O4PF (262): C, 50.39; H, 6.15%. Found: C, 50.35; H, 6.10%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1026 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 9.38 (s, 1H, imidazole NH), 7.32–7.39 (m, 2Ar-H), 5.12 (d, 2JP-H = 10.3 Hz, P-C-H), 3.52–3.78 (m, 4H, P(O)CH2CH3), 1.23 (t, 3JH-H = 7.3 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 149.3 (C-1), 125.2 (C-3), 124.6 (C-4), 72.8 (P-C), 60.1 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 15.6 (d, 3JP-C = 5.7 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.72 (P
O, phosphonate), 1026 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 9.38 (s, 1H, imidazole NH), 7.32–7.39 (m, 2Ar-H), 5.12 (d, 2JP-H = 10.3 Hz, P-C-H), 3.52–3.78 (m, 4H, P(O)CH2CH3), 1.23 (t, 3JH-H = 7.3 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 149.3 (C-1), 125.2 (C-3), 124.6 (C-4), 72.8 (P-C), 60.1 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 15.6 (d, 3JP-C = 5.7 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 21.72 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 235.21; anal. calcd for C8H16N2O4P (235): C, 40.85; H, 6.86%. Found: C, 40.82; H, 6.81%.
O); ESI-MS: 235.21; anal. calcd for C8H16N2O4P (235): C, 40.85; H, 6.86%. Found: C, 40.82; H, 6.81%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1005 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 9.94 (s, 1H, indole NH), 7.02–8.66 (m, 5Ar-H), 4.76 (d, 2JP-H = 10.2 Hz, P-C-H), 3.61–3.77 (m, 4H, P(O)CH2CH3), 1.18 (t, 3JH-H = 7.3 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 138.36 (C-4), 135.52 (C-9), 121.06 (C-2), 120.64 (C-6), 119.04 (C-7), 117.35 (C-8), 116.38 (C-1), 115.15 (C-5), 76.26 (P-C), 59.96 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 16.12 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 20.69 (P
O, phosphonate), 1005 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 9.94 (s, 1H, indole NH), 7.02–8.66 (m, 5Ar-H), 4.76 (d, 2JP-H = 10.2 Hz, P-C-H), 3.61–3.77 (m, 4H, P(O)CH2CH3), 1.18 (t, 3JH-H = 7.3 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 138.36 (C-4), 135.52 (C-9), 121.06 (C-2), 120.64 (C-6), 119.04 (C-7), 117.35 (C-8), 116.38 (C-1), 115.15 (C-5), 76.26 (P-C), 59.96 (d, 2JP-C = 7.6 Hz, P-OCH2CH3), 16.12 (d, 3JP-C = 5.8 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 20.69 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 283.18; anal. calcd for C13H18NO4P (283): C, 55.12; H, 6.41%. Found: C, 55.09; H, 6.38%.
O); ESI-MS: 283.18; anal. calcd for C13H18NO4P (283): C, 55.12; H, 6.41%. Found: C, 55.09; H, 6.38%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1011 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.13–8.09 (m, 3Ar-H), 5.06 (s, 2H), 4.94 (d, 2JP-H = 10.0 Hz, P-C-H), 3.92–4.09 (m, 4H, P(O)CH2CH3), 1.27 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 158.8 (C-6), 128.7 (C-2), 128.6 (C-4), 128.0 (C-1), 125.3 (C-3), 124.1 (C-5), 76.1 (P-C), 63.0 (d, 2JP-C = 7.5 Hz, P-OCH2CH3), 16.3 (d, 3JP-C = 5.7 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.57 (P
O, phosphonate), 1011 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 7.13–8.09 (m, 3Ar-H), 5.06 (s, 2H), 4.94 (d, 2JP-H = 10.0 Hz, P-C-H), 3.92–4.09 (m, 4H, P(O)CH2CH3), 1.27 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 13C-NMR (CDCl3, 75.4 MHz): δ 158.8 (C-6), 128.7 (C-2), 128.6 (C-4), 128.0 (C-1), 125.3 (C-3), 124.1 (C-5), 76.1 (P-C), 63.0 (d, 2JP-C = 7.5 Hz, P-OCH2CH3), 16.3 (d, 3JP-C = 5.7 Hz, P-OCH2-CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.57 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 313.05; anal. calcd for C11H15O4PCl2 (313): C, 42.19; H, 4.83%. Found: C, 42.14; H, 4.81%.
O); ESI-MS: 313.05; anal. calcd for C11H15O4PCl2 (313): C, 42.19; H, 4.83%. Found: C, 42.14; H, 4.81%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1028 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.84–7.17 (m, 4Ar-H), 4.74 (d, 2JP-H = 10.2 Hz, P-C-H), 3.87–4.16 (m, 4H, P(O)CH2CH3), 1.33 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 23.71 (P
O, phosphonate), 1028 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.84–7.17 (m, 4Ar-H), 4.74 (d, 2JP-H = 10.2 Hz, P-C-H), 3.87–4.16 (m, 4H, P(O)CH2CH3), 1.33 (t, 3JH-H = 7.0 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 23.71 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 278.19; anal. calcd for C11H16O4PCl (278): C, 47.41; H, 5.79%. Found: C, 47.37; H, 5.74%.
O); ESI-MS: 278.19; anal. calcd for C11H16O4PCl (278): C, 47.41; H, 5.79%. Found: C, 47.37; H, 5.74%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1025 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.56–6.94 (m, 4Ar-H), 4.80 (d, 2JP-H = 10.4 Hz, P-C-H), 3.65–3.84 (m, 4H, P(O)CH2CH3), 1.13 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.63 (P
O, phosphonate), 1025 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.56–6.94 (m, 4Ar-H), 4.80 (d, 2JP-H = 10.4 Hz, P-C-H), 3.65–3.84 (m, 4H, P(O)CH2CH3), 1.13 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.63 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 260.04; anal. calcd for C11H17O5P (260): C, 50.77; H, 6.58%. Found: C, 50.72; H, 6.51%.
O); ESI-MS: 260.04; anal. calcd for C11H17O5P (260): C, 50.77; H, 6.58%. Found: C, 50.72; H, 6.51%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1042 (P-O-C); 1H NMR (CDCl3, 500 MHz): δ 7.19–7.38 (m, 4Ar-H), 4.94 (d, 2JP-H = 10.4 Hz, 1H), 3.92–4.08 (m, 4H, P(O)CH2CH3), 2.85–2.92 (m, 1H), 1.17–1.26 (m, 6H), 1.165 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 31P NMR (CDCl3, 121.4 MHz): δ 22.83 (P
O, phosphonate), 1042 (P-O-C); 1H NMR (CDCl3, 500 MHz): δ 7.19–7.38 (m, 4Ar-H), 4.94 (d, 2JP-H = 10.4 Hz, 1H), 3.92–4.08 (m, 4H, P(O)CH2CH3), 2.85–2.92 (m, 1H), 1.17–1.26 (m, 6H), 1.165 (t, 3JH-H = 7.2 Hz, 6H, P(O)CH2CH3); 31P NMR (CDCl3, 121.4 MHz): δ 22.83 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 286.15; anal. calcd for C14H23O4P (286): C, 58.73; H, 8.10%. Found: C, 58.69; H, 8.07%.
O); ESI-MS: 286.15; anal. calcd for C14H23O4P (286): C, 58.73; H, 8.10%. Found: C, 58.69; H, 8.07%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1061 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.87–7.01 (m, 4Ar-H), 5.18 (d, 2JP-H = 10.3 Hz, P-C-H), 4.02–4.29 (m, 4H, P(O)CH2CH3), 1.19 (t, 3J = 7.3 Hz, 6H, P(O)CH2CH3), 2.82 (s, 6H); 31P-NMR (CDCl3, 121.4 MHz): δ 21.40 (P
O, phosphonate), 1061 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.87–7.01 (m, 4Ar-H), 5.18 (d, 2JP-H = 10.3 Hz, P-C-H), 4.02–4.29 (m, 4H, P(O)CH2CH3), 1.19 (t, 3J = 7.3 Hz, 6H, P(O)CH2CH3), 2.82 (s, 6H); 31P-NMR (CDCl3, 121.4 MHz): δ 21.40 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 287.21; anal. calcd for C13H22NO4P (287): C, 54.35; H, 7.72%. Found: C, 54.31; H, 7.69%.
O); ESI-MS: 287.21; anal. calcd for C13H22NO4P (287): C, 54.35; H, 7.72%. Found: C, 54.31; H, 7.69%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, phosphonate), 1032 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.78–7.07 (m, 4Ar-H), 4.87 (d, 2JP-H = 10.2 Hz, P-C-H), 3.90–4.19 (m, 4H, P(O)CH2CH3), 3.86 (s, 3H), 3.57 (s, 3H), 1.28 (t, 3J = 7.2 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.20 (P
O, phosphonate), 1032 (P-O-C); 1H-NMR (CDCl3, 500 MHz): δ 6.78–7.07 (m, 4Ar-H), 4.87 (d, 2JP-H = 10.2 Hz, P-C-H), 3.90–4.19 (m, 4H, P(O)CH2CH3), 3.86 (s, 3H), 3.57 (s, 3H), 1.28 (t, 3J = 7.2 Hz, 6H, P(O)CH2CH3); 31P-NMR (CDCl3, 121.4 MHz): δ 22.20 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); ESI-MS: 304.08; anal. calcd for C13H21O6P (304): C 51.31, H 6.96%. Found: C 51.29, H 6.92%.
O); ESI-MS: 304.08; anal. calcd for C13H21O6P (304): C 51.31, H 6.96%. Found: C 51.29, H 6.92%.
Conclusions
In conclusion, low molecular weight PEG in water has been utilized for the first time as a new solution medium for the efficient one-pot synthesis of α-amino and α-hydroxy phosphonates. The advantages of this procedure are operational simplicity, reusability, wide substrate scope, and high yields. We believe that this method serves as a better alternative to the existing one for the synthesis of α-amino and α-hydroxy phosphonates.Acknowledgements
The authors express their grateful thanks to Prof. C. D. Reddy, Department of Chemistry, Sri Venkateswara University, Tirupati, for his helpful discussions and also thank BRNS, BARC, Mumbai, for providing financial assistance (2010/37C/26/BRNS/1424).Notes and references
- (a) R. Engel, Chem. Rev., 1977, 77, 349–367 CrossRef CAS; (b) J. Hiratake and J. Oda, Biosci., Biotechnol., Biochem., 1997, 61, 211–218 CrossRef CAS; (c) K. A. Schug and W. Lindner, Chem. Rev., 2005, 105, 67–113 CrossRef CAS; (d) K. Moonen, I. Laureyn and C. V. Stevens, Chem. Rev., 2004, 104, 6177–6215 CrossRef CAS; (e) F. Palacios, C. Alonso and J. M. de los Santos, Curr. Org. Chem., 2004, 8, 1481–1496 CrossRef CAS.
- (a) P. Kafarski, B. Lejczak, R. Tyka, L. Koba, E. Pliszczak and P. Wieczorek, J. Plant Growth Regul., 1995, 14, 199–203 CrossRef CAS; (b) Y. Ishiguri, Y. Yamada, T. Kato, M. Sasaki and K. Mukai, Eur. Pat. Appl., EP 82-301905, 1982, Chem. Abstr., 1983, 98, 102686u Search PubMed.
- (a) F. R. Atherton, M. J. Hall, C. H. Hassall, S. W. Holmes, R. W. Lambert, J. Lloyd and P. S. Ringrose, Antimicrob. Agents Chemother., 1980, 18, 897–905 CAS; (b) I. A. Natchev, Liebigs Ann. Chem., 1988, 1988, 861–867 CrossRef; (c) J. Emsley and D. Hall, The Chemistry of Phosphorus, Harper & Row, London, 1976, pp. 494–498 Search PubMed; (d) L. Maier and H. Spörri, Phosphorus, Sulfur Silicon Relat. Elem., 1991, 61, 69–75 CrossRef CAS; (e) J. Huang and R. Chen, Heteroat. Chem., 2000, 11, 480–492 CrossRef CAS; (f) A. Paymen, K. H. Budt, J. Spanig, B. Stowasser and D. Ruppert, Tetrahedron Lett., 1992, 33, 4549–4552 CrossRef.
- (a) P. Kafarski and B. Lejczak, Curr. Med. Chem., 2001, 1, 301–312 CAS; (b) L. Berlicki and P. Kafarski, Curr. Org. Chem., 2005, 9, 1829–1850 CrossRef CAS.
- N. Strater and W. N. Lipscomb, Biochemistry, 1995, 34, 9200–9210 CrossRef CAS.
- (a) V. P. Kukhar and V. A. Solodenko, Russ. Chem. Rev. (Engl. Transl.), 1987, 56, 859–874 CrossRef; (b) T. Yokomatsu, Y. Yoshida and S. Shibuya, J. Org. Chem., 1994, 59, 7930–7933 CrossRef CAS.
- P. Merino, E. Marqués López and R. P. Herrera, Adv. Synth. Catal., 2008, 350, 1195–1208 CrossRef CAS.
- (a) S. Laschat and H. Kunz, Synthesis, 1992, 90–94 CrossRef CAS; (b) H. J. Ha and G. S. Nam, Synth. Commun., 1992, 22, 1143–1148 CrossRef CAS; (c) J. S. Yadav, B. V. S. Reddy, S. Raj, K. B. Reddy and A. R. Prasad, Synthesis, 2001, 2277–2280 CrossRef CAS; (d) F. R. Atherton, C. H. Hassal and R. W. Lambert, J. Med. Chem., 1986, 29, 29–40 CrossRef CAS.
- (a) J. Zon, Pol. J. Chem., 1981, 55, 643–646 CAS; (b) C. Qian and T. Huang, J. Org. Chem., 1998, 63, 4125–4128 CrossRef CAS; (c) S. Chandrasekhar, S. J. Prakash, V. Jagadeshwar and C. Narsihmulu, Tetrahedron Lett., 2001, 42, 5561–5563 CrossRef CAS; (d) A. K. Bhattacharya and T. Kaur, Synlett, 2007, 745–748 CrossRef CAS; (e) S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2007, 72, 1263–1270 CrossRef CAS; (f) M. H. Sarvari, Tetrahedron, 2008, 64, 5459–5466 CrossRef; (g) A. K. Bhattacharya and K. C. Rana, Tetrahedron Lett., 2008, 49, 2598–2601 CrossRef CAS; (h) S. D. Mitragotri, D. M. Pore, U. V. Desai and P. P. Wadgaonkar, Catal. Commun., 2008, 9, 1822–1826 CrossRef CAS; (i) A. Heydari, H. Hamadi and M. Pourayoubi, Catal. Commun., 2007, 8, 1224–1226 CrossRef CAS; (j) A. Heydari and A. Arefi, Catal. Commun., 2007, 8, 1023–1026 CrossRef CAS; (k) A. Heydari, A. Karimian and J. Ipaktschi, Tetrahedron Lett., 1998, 39, 6729–6732 CrossRef CAS; (l) M. Tajbakhsh, A. Heydari, H. Alinezhad, M. Ghanei and S. Khaksar, Synthesis, 2008, 352–354 CrossRef CAS; (m) S. Bhagat and A. K. Chakraborti, J. Org. Chem., 2008, 73, 6029–6030 CrossRef CAS.
- (a) G. C. S. Reddy, M. V. N. Reddy, N. B. Reddy and C. S. Reddy, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 74–80 CrossRef CAS; (b) K. R. K. K. Reddy, M. A. Kumar, M. V. N. Reddy, C. D. Reddy and C. S. Reddy, Tetrahedron Lett., 2008, 49, 6337–6340 CrossRef CAS.
- (a) V. N. Vasudevan and S. V. Rajender, Green Chem., 2001, 3, 146–148 RSC; (b) A. Haimov and R. Neumann, Chem. Commun., 2002, 876–877 RSC; (c) L. Heiss and H. J. Gais, Tetrahedron Lett., 1995, 36, 3833–3866 CrossRef CAS; (d) S. Chandrasekar, Ch. Narsihmulu, S. S. Shameem and N. R. Reddy, Chem. Commun., 2003, 1716–1717 RSC; (e) K. Tanemura, T. Suzuki, Y. Nishida and T. Horaguchi, Chem. Lett., 2005, 576–577 CrossRef CAS; (f) T. J. Dickerson, N. N. Reed and K. D. Janda, Chem. Rev., 2002, 102, 3325–3344 CrossRef CAS; (g) A. Kamal, D. R. Reddy and Rajendar, Tetrahedron Lett., 2006, 47, 2261–2264 CrossRef CAS.
- (a) G. Sharma, R. Kumar and A. K. Chakraborti, Tetrahedron Lett., 2008, 49, 4269–4271 CrossRef CAS; (b) S. V. Chankeshwara and A. K. Chakraborti, Org. Lett., 2006, 8, 3259–3262 CrossRef CAS.
- M. Anil Kumar, M. F. Stephen Babu, K. Srinivasulu, Y. B. Kiran and C. S. Reddy, J. Mol. Catal. A: Chem., 2007, 265, 268–271 CrossRef.
- K. Babak and S. Mina, Tetrahedron Lett., 2007, 48, 9015–9017 CrossRef.
- (a) R. Bihovsky, M. Tao, G. J. Wells and J. P. Mallamo, J. Med. Chem., 1998, 41, 3912–3916 CrossRef; (b) J. A. Sikorski, M. J. Miller, D. S. Braccolino, D. G. Cleary, S. D. Corey, J. L. Font, K. J. Gruys, C. Y. Han, K. C. Lin, P. D. Pansegrau, J. E. Ream, D. Schnur, A. Shah and M. C. Walker, Phosphorus, Sulfur Silicon Relat. Elem., 1993, 76, 375–378 CrossRef CAS; (c) D. L. Pompliano, E. Rands, M. D. Schaber, S. D. Mosser, N. J. Anthony and J. B. Gibbs, Biochemistry, 1992, 31, 3800–3807 CrossRef CAS; (d) B. Stowasser, K. H. Budt, J. Q. Li, A. Peyman and D. Ruppert, Tetrahedron Lett., 1992, 33, 6625–6628 CrossRef CAS; (e) R. Snoeck, A. Holy, C. Dewolf-Peeters, J. Van Den Oord, E. De Clercq and G. Andrei, Antimicrob. Agents Chemother., 2002, 46, 3356–3361 CrossRef CAS; (f) M. L. Peters, M. Leonard and A. A. Licata, Cleveland Clin. J. Med., 2001, 68, 945–951 CrossRef CAS.
- (a) B. Kaboudin, Tetrahedron Lett., 2003, 44, 1051–1053 CrossRef CAS; (b) H. Firouzabadi, N. Iranpoor and S. Sobhani, Synth. Commun., 2004, 34, 1463–1471 CrossRef CAS; (c) F. Eymery, B. Lorga and P. Savignac, Tetrahedron, 1999, 55, 2671–2686 CrossRef; (d) H. Firouzabadi, N. Iranpoor, S. Sobhani and Z. Amoozgar, Synthesis, 2004, 1771–1774 CrossRef CAS.
- (a) P. Kafarski and B. Lecjzak, J. Mol. Catal. B: Enzym., 2004, 29, 99–104 CrossRef CAS; (b) A. A. Smaardijk, S. Noorda, F. Van Bolhuis and H. Wynberg, Tetrahedron Lett., 1985, 26, 493–496 CrossRef CAS; (c) S. Samanta and C. G. Zhao, J. Am. Chem. Soc., 2006, 128, 7442–7443 CrossRef CAS; (d) M. D. Groaning, B. J. Rowe and C. D. Spilling, Tetrahedron Lett., 1998, 39, 5485–5488 CrossRef CAS; (e) X. Zhou, X. Liu, D. Shang, J. Xin and X. Feng, Angew. Chem., Int. Ed., 2008, 47, 392–394 CrossRef CAS; (f) J. P. Duxbury, J. N. D. Warne, R. Mushtaq, W. Ward, M. Pett Thornton, M. Jiang, R. Greatrex and T. P. Kee, Organometallics, 2000, 19, 4445–4457 CrossRef CAS; (g) T. Arai, M. Bougauchi, H. Sasai and M. Shibaski, J. Org. Chem., 1996, 61, 2926–2927 CrossRef CAS; (h) F. Texier Boullet and M. Lequitte, Tetrahedron Lett., 1986, 27, 3515–3516 CrossRef CAS; (i) S. S. Swapnil, H. K. Amol, N. W. Madhav, H. G. Charansingh, B. S. Bapurao and S. S. Murlidhar, ARKIVOC, 2009, ii, 138–148 Search PubMed; (j) D. Simoni, R. Rondanin, M. Morini, R. Baruchello and F. P. Lnvidiata, Tetrahedron Lett., 2000, 41, 1607–1610 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
