Retracted Article: Synthesis of a novel type of chiral salen Mn(III) complex immobilized on crystalline zinc poly(styrene-phenylvinylphosphonate)-phosphate (ZnPS-PVPP) as effective catalysts for asymmetric epoxidation of unfunctionalized olefins†
Jing
Huang
,
Xiangkai
Fu
* and
Qiang
Miao
College of Chemistry and Chemical Engineering Southwest University, Research Institute of Applied Chemistry Southwest University, The Key Laboratory of Applied Chemistry of Chongqing Municipality, The Key Laboratory of Eco-environments in Three Gorges Reservoir Region Ministry of Education, Chongqing 400715, China. E-mail: fxk@swu.edu.cn; Fax: +86 2368254000; Tel: +86 2368253704
First published on 31st August 2011
Abstract
A novel type of organic polymer–inorganic hybrid material layered crystalline ZnPS-PVPP was designed and prepared. Notably, the layered crystalline ZnPS-PVPP were obtained in the absence of any templated agents. An array of new heterogeneous catalysts were gained by grafting chiral salen Mn(III) onto aryldiamine modified ZnPS-PVPP. The immobilized catalysts exhibited higher chiral induction in the asymmetric epoxidation of α-methylstyrene and indene with m-CPBA and NaIO4 as oxidants than those of the corresponding homogeneous catalyst (ee, >99% vs. 54% and >99% vs. 65%) and could be reused nine times without significant loss of activity and enantioselectivity. Furthermore, this novel type of catalyst can also be efficiently used in large-scale reactions with the enantioselectivity being maintained at the same level, which provided the potentiality for application in industry.
1. Introduction
Epoxides of non-functionalized olefins are very important intermediates in the manufacture of drugs, vitamins, fragrances, and optical materials.1–5 Consequently, there has been considerable interest in the development of highly efficient epoxidation catalysts.6,7 Chiral Mn(III) salen complexes have proven to be highly efficient homogeneous catalysts for the asymmetric epoxidation of unfunctionalized olefins.8–10 However, the catalysts generally present problems in separation and recycling under a homogeneous catalytic system. Just as this, the heterogenization of chiral Mn(III) salen complexes have received great attention in the last decades11–13 due to efficient product purification and easy catalyst recovery.In the previous works, many efforts have also been devoted to the immobilization of homogeneous chiral salen Mn(III) complexes in our group in the last decades. The immobilized chiral salen Mn(III) catalysts on modified zirconium oligostyrenylphosphonate-phosphate (ZSPP) and zirconium poly(styrene-phenylvinyl phosphonate)-phosphate (ZPS-PVPA) showed higher enantioselectivity and reusability than that of the homogeneous chiral catalyst in asymmetric epoxidation of unfunctionalized olefins under the same conditions.14–17
Nevertheless, few efforts have focused on the research in organic–inorganic hybrid zinc phosphonate-phosphate as catalyst supports, even less on organic polymer–inorganic hybrid zinc phosphonate-phosphate used for immobilization of chiral salen Mn(III). In this work, a series of new type of layered crystalline inorganic–organic polymer hybrid materials ZnPS-PVPP are prepared and aryldiamine modified ZnPS-PVPP were applied to immobilize the chiral salen Mn(III) complexes through axial coordination. The immobilized catalysts (3a–3c) effectively catalyzed epoxidation of α-methylstyrene with m-CPBA as the oxidant. These results are obviously better than those achieved with the homogeneous catalyst 2. Notably, the immobilized catalysts could be reused at least nine times without significant loss of activity and enantioselectivity and also can be applied in large-scale reactions with the conversion and enantioselectivity being maintained at the same level. Furthermore, the immobilized catalysts showed high reactivity in the absence of NMO, which was entirely different from most results reported.
2. Experiment
2.1 Materials and instruments
(1R, 2R)-(−)-1,2-Diaminocyclohexane, chloromethyl methyl ether (toxic compound), α-methylstyrene, n-nonane, N-methylmorpholine N-oxide (NMO) and m-chloroperbenzoic acid (m-CPBA) were supplied by Alfa Aesar. Other commercially available chemicals were laboratory-grade reagents from local suppliers. Chiral salen ligand and chiral homogeneous catalyst salen Mn(III) were synthesized according to the standard literature procedures,8 and further identified by analysis and comparison of IR spectra with literature.18FT-IR spectra were recorded from KBr pellets using a Bruker RFS100/S spectrophotometer (USA) and diffuse reflectance UV-vis spectra of the solid samples were recorded in the spectrophotometer with an integrating sphere using BaSO4 as a standard. 1H NMR and 31P NMR were performed on an AV-300 NMR instrument at ambient temperature at 300 and 121 MHz, respectively. All of the chemical shifts were reported downfield in ppm relative to the hydrogen and phosphorus resonance of TMS and 85% H3PO4, respectively. Number- and weight-average molecular weights (Mn and Mw) and polydispersity (Mw/Mn) were estimated by a Waters1515 gel permeation chromatograph (GPC; against polystyrene standards) using THF as an eluent (1.0 mL min−1) at 35 °C. X-Ray photoelectron spectrum was recorded on an ESCALab250 instrument. The interlayer spacings were obtained on a DX-1000 automated X-ray power diffractometer, using Cu Kα radiation and internal silicon powder standard with all samples. The patterns were generally measured between 3.00° and 80.00° with a step size of 0.02° per min and X-ray tube settings of 36 kV and 20 mA. C, H and N elemental analysis was obtained from an EATM 1112 automatic elemental analyzer instrument (Thermo, USA). TG analyses were performed on a SBTQ600 thermal analyzer (USA) with the heating rate of 20 °C min−1 from 25 to 1000 °C under flowing N2 (100 mL min−1). The Mn contents of the catalysts were determined by TAS-986G (Pgeneral, China) atomic absorption spectroscopy. SEM were performed by KYKY-EM 3200 (KYKY, China) microscopy. TEM were obtained on a TECNAI10 (PHILIPS, Holland) apparatus. Nitrogen adsorption isotherms were measured at 77 K on a 3H-2000I (Huihaihong, China) volumetric adsorption analyzer with the BET method. The racemic epoxides were prepared by epoxidation of the corresponding olefins by 3-chloroperbenzoic acid in CH2Cl2 and confirmed by NMR (Bruker AV-300), and the gas chromatography (GC) was calibrated with the samples of n-nonane, olefins and corresponding racemic epoxides. The conversions (with n-nonane as internal standard) and the ee values were analyzed by gas chromatography (GC) with a Shimadzu GC2010 (Japan) instrument equipped using a chiral column (HP19 091G-B213, 30 m × 30 m × 0.32 mm × 0.25 μm) and FID detector, injector 230 °C, detector 230 °C. Ultrapure N2 was the carrier gas (rate 34 mL min−1) with a carrier pressure 39.1 kPa and the injection pore temperature was set at 230 °C. The column temperature for indene and α-methylstyrene was programmed in the range of 80–180 °C. The oven temperature program was initially started at 80 °C, held for 3 min; then raised to 150 °C at 7 °C min−1 and held for 5 min; raised to 220 °C at 7 °C min−1, and was finally set at 220 °C constant for 3 min. The total run time of the GC program for α-methylstyrene was 30 min. The appearance time of n-nonane, α-methylstyrene, and two racemic epoxides was at 5.29, 9.2, 12.9, 13.0 min, respectively. The similar run program was set for indene.
2.2 Synthesis of the support (Scheme 1)
 | ||
| Scheme 1 Synthesis of the support. | ||
1-Phenylvinyl phosphonic acid (4 g, 21.7 mmol), styrene (20 mL, 173.9 mmol), ethyl acetate (150 mL) and benzoyl peroxide (BPO, 1.0 g, 4.7 mmol) were used for preparation of PS-PVPA copolymer according to the literature.16 Yield 7.52 g. GPC: Mn = 38608, m = 38, n = 8, Mw/Mn = 2.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Found: C, 58.08; H, 4.97%. Calc. for C72H73O11P3Na2Zn3: C, 59.71; H, 5.04%.
O). Found: C, 58.08; H, 4.97%. Calc. for C72H73O11P3Na2Zn3: C, 59.71; H, 5.04%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P–OH), 1650, 1542, 1510, 1493 (−C6H5), 1267 (P
P–OH), 1650, 1542, 1510, 1493 (−C6H5), 1267 (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 700 (C–Cl) cm−1. Found: C, 51.16; H, 4.09%. Calc. for C80H81O11P3Cl8Na2Zn3: C, 52.31; H, 4.41%.
O), 700 (C–Cl) cm−1. Found: C, 51.16; H, 4.09%. Calc. for C80H81O11P3Cl8Na2Zn3: C, 52.31; H, 4.41%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the mixture was stirred and kept at 70 °C for 12 h. Subsequently, the mixture was neutralized and the solvent was vaporized. Then, the product was filtered, washed and dried in vacuo. Reaction yield always exceeded 90%. Correspondingly, the sharp C–Cl peak (owing to −CH2Cl groups) at 700 cm−1 in the ZnCMPS-PVPA actually vanished or was seen as a weak band after introduction of aryldiamines. The products were abbreviated as 1a, 1b and 1c in turn. 1a, found: C, 62.16; H, 5.21; N, 9.16%. Calc. for C128H137N16O11P3Na2Zn3: C, 63.81; H, 5.69; N, 9.31%. 1b, found: C, 61.15; H, 5.02; N, 9.05%. Calc. for C128H137N16O11P3Na2Zn3: C, 63.81; H, 5.69; N, 9.31%. 1c, found: C, 69.16; H, 5.45; N, 7.26%. Calc. for C176H169N16O11P3Na2Zn3: C, 70.05; H, 5.61; N, 7.43%.
1), and the mixture was stirred and kept at 70 °C for 12 h. Subsequently, the mixture was neutralized and the solvent was vaporized. Then, the product was filtered, washed and dried in vacuo. Reaction yield always exceeded 90%. Correspondingly, the sharp C–Cl peak (owing to −CH2Cl groups) at 700 cm−1 in the ZnCMPS-PVPA actually vanished or was seen as a weak band after introduction of aryldiamines. The products were abbreviated as 1a, 1b and 1c in turn. 1a, found: C, 62.16; H, 5.21; N, 9.16%. Calc. for C128H137N16O11P3Na2Zn3: C, 63.81; H, 5.69; N, 9.31%. 1b, found: C, 61.15; H, 5.02; N, 9.05%. Calc. for C128H137N16O11P3Na2Zn3: C, 63.81; H, 5.69; N, 9.31%. 1c, found: C, 69.16; H, 5.45; N, 7.26%. Calc. for C176H169N16O11P3Na2Zn3: C, 70.05; H, 5.61; N, 7.43%.
2.3 Synthesis of grafting chiral salen Mn(III) catalyst onto ZnAMPS-PVPP (Scheme 2)
 | ||
| Scheme 2 Synthetic route of the catalysts. | ||
2.4 Synthesis of homogeneous chiral salen Mn(III)
Chiral salen Mn(III) (4 mmol) in 10 mL of THF was added dropwise to the solution of a proportional amount of aryldiamine (such as a: m-phenylenediamine, b: p-phenylenediamine, c: benzidine) pre-swelled in THF for 30 min and Et3N (5 mmol) (the mol ratio of aryldiamine to chiral salen Mn(III) is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The homogeneous chiral salen Mn(III) were prepared according to a similar procedure to heterogeneous catalysts (in Section 2.3). IR (KBr):νmax/cm−1 1650, 1542, 1510, 1493 (−C6H5), 1140 (−NH2), 3415, 1617(−NH−), 1639(−C
1). The homogeneous chiral salen Mn(III) were prepared according to a similar procedure to heterogeneous catalysts (in Section 2.3). IR (KBr):νmax/cm−1 1650, 1542, 1510, 1493 (−C6H5), 1140 (−NH2), 3415, 1617(−NH−), 1639(−C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) cm−1. 4a, found: C, 69.86; H, 8.06; N, 7.12%. Calc. for C42H59N4O2Mn: C, 71.39; H, 8.36; N, 7.93%. 4b, found: C, 70.12; H, 8.12; N, 7.28%. Calc. for C42H59N4O2Mn: C, 71.39; H, 8.36; N, 7.93%. 4c, found: C, 72.15; H, 7.86; N, 7.01%. Calc. for C42H59N4O2Mn: C, 73.66; H, 8.06; N, 7.16%.
N) cm−1. 4a, found: C, 69.86; H, 8.06; N, 7.12%. Calc. for C42H59N4O2Mn: C, 71.39; H, 8.36; N, 7.93%. 4b, found: C, 70.12; H, 8.12; N, 7.28%. Calc. for C42H59N4O2Mn: C, 71.39; H, 8.36; N, 7.93%. 4c, found: C, 72.15; H, 7.86; N, 7.01%. Calc. for C42H59N4O2Mn: C, 73.66; H, 8.06; N, 7.16%.
2.5 Asymmetric epoxidation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile
1 mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water at room temperature for 2.5 h and with alkene (1 mmol) and NaIO4 (2 mmol) in the presence of 5 mol% catalysts.
water at room temperature for 2.5 h and with alkene (1 mmol) and NaIO4 (2 mmol) in the presence of 5 mol% catalysts.
2.6 The reusability of the catalyst
In a typical recirculation, the equal volume of hexane was added to the reaction mixture after the reactions. Thereafter, the organic phase was separated, and the catalyst was washed with hexane and deionized water, and dried over vacuum at 60 °C. The recovered dried solid catalyst was weighed and reused in the next run. In every run the same proportion of the substrate-to-catalyst and solvent-to-catalyst was retained.2.7 Characterization of copolymer PS-PVPA
To a solution of PS-PVPA (0.1 g) in 20 mL of THF, sodium hydroxide standard solution in water was added titrated slowly with vigorous magnetic stirring. According to the consumed volume of sodium hydroxide standard solution originating in the place of sudden change of pH in the pH − V NaOH titration curve, the content of phosphonic acid in the co-polymer PS-PVPA could be calculated using the formula.2.8 Chemical analysis
In a white porcelain crucible, a sample of 50 mg ZnPS-PVPP was put in it and was heated up to 700 °C for 5 h in a Muffle furnace. Due to the high temperature, ZnPS-PVPP decomposed. Then 20 mL of hydrochloric acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the porcelain crucible and was heated to boiling for 30 min on the electric furnace. In the resulting solution, the sodium content was determined by AAS.
1) was added to the porcelain crucible and was heated to boiling for 30 min on the electric furnace. In the resulting solution, the sodium content was determined by AAS.
2.9 General procedure for a large-scale asymmetric epoxidation reaction
A solution of catalyst 3b (2.5 mmol), n-nonane (50 mmol) and α-methylstyrene (50 mmol) in CH2Cl2 (150 mL) at –40 °C was stirred for 30 min. Then, m-CPBA (100 mmol) was added to the solution step by step. After 5 h, Na2CO3 (100 mL, 1.0 M) was added to quench the reaction. And the organic layer was dried over sodium sulfate, and the catalyst was precipitated out from the solution by adding hexane and kept for subsequent use without further purification. The conversion and ee values of the epoxide were determined by GC.3. Results and discussion
3.1 Characterizations of the supports and the heterogeneous chiral catalysts
where W is the mass of the copolymer; n is the number of styrene in the unit of the copolymer; C0 is the concentration of sodium hydroxide standard solution and V is the consumed volume of sodium hydroxide standard solution corresponding to the place of sudden change of pH in the pH − V NaOH titration curve.
Using this formula, it could be inferred that on average every segment of the molecule chain—(St)m1–(PVPA)n–(St)m2–(PVPA)n(St)m3—in the copolymer contained 8 organic phosphonates (n = 8). Subsequently, it could be deduced that the copolymer on average was comprised of 38 units (m = 38).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching band of complex 2 appeared at 1630 cm−1 (5 in Fig. S2, ESI†). While for the supported catalysts this band was also observed at the vicinity of 1632 cm−1. All the samples (3a–3c) and complex 2 had shown the same band at 1638 cm−1 attributed to the vibration of the imine group. The stretching vibration at 1030 cm−1 which was assigned to characteristic vibrations of the phosphonic acid group in the support was obviously weakened due to the electronic structure changes for the host–guest interaction. Moreover, an additional band around 3408 cm−1 was observed for the samples, which was assigned to the stretching vibration of N–H groups.
N) stretching band of complex 2 appeared at 1630 cm−1 (5 in Fig. S2, ESI†). While for the supported catalysts this band was also observed at the vicinity of 1632 cm−1. All the samples (3a–3c) and complex 2 had shown the same band at 1638 cm−1 attributed to the vibration of the imine group. The stretching vibration at 1030 cm−1 which was assigned to characteristic vibrations of the phosphonic acid group in the support was obviously weakened due to the electronic structure changes for the host–guest interaction. Moreover, an additional band around 3408 cm−1 was observed for the samples, which was assigned to the stretching vibration of N–H groups.
Diffuse reflectance UV-vis spectra (Fig. S3, ESI†) also gave obvious evidence for the successful immobilization. The spectra of the supported catalysts displayed features similar to those of the neat chiral salen Mn(III) complex 2. According to complex 2, the bands at 334 nm could be attributed to the charge transfer transition of salen ligand. The band at 435 nm was due to the ligand-to-metal charge transfer transition, and the band at 510 nm was assigned to the d–d transition of the Mn(III) salen system. While for the heterogeneous catalysts, all the characteristic bands appeared in their spectra but the immobilized salen Mn(III) catalysts exhibited a blue shift from 334, 435 and 510 nm to 330, 427 and 503 nm, which indicated that an interaction existed between the salen Mn(III) complex and the aryldiamine modified ZnPS-PVPP.
 | ||
| Fig. 1 TG curves of ZnPS-PVPP(A) and 3b (B) | ||
As could be seen from Fig. 2, the XRD patterns of ZnPS-PVPP displayed a broad 001 peak (the lowest-angle diffraction peak in the pattern), accompanied with other peaks at higher-order 00n peaks at larger angles and lower intensities such as at 38.04°. Therefore, it could be deduced that ZnPS-PVPP could be applied as mesoporous materials. After the immobilization treatment, some chiral salen Mn(III) complexes were immobilized on the external surface of ZnPS-PVPP and other chiral salen Mn(III) complexes were present inside the nanopores according to N2 adsorption–desorption isotherms presented in Table 1. That is to say, the morphology of ZnPS-PVPP displayed amorphous in some extent and then many crystalline peaks of support disappeared for heterogeneous catalysts as observed in Fig. 2. The intensities of the peaks at 2.02°, 25.4°and 42.16° reflections decreased with a small shift toward lower 2θ values, which showed that the mesoporous structure of ZnPS-PVPP remained a layered structure. Meanwhile, some peaks in the pattern of the catalyst relatively were reinforced, leading to other peaks in the pattern of the catalyst weakened. On the other hand, the interlayer distance which could be calculated (via the Bragg equation, nλ = 2d sinθ) of the immobilized catalyst 3b (43.7 Å) was nearly twice as much as that of ZnPS-PVPP (21.12 Å), owing to the chiral salen Mn(III) introduced in ZnPS-PVPP making the zinc layer stretched and broader. The peculiar appearance was entirely different to most of the results reported.14–16 The amount of Mn(salen) anchored onto ZnPS-PVPP is in the range of 0.66–0.78 mmol g−1 ascertained by AAS based on the Mn element. In view of these facts, it could be inferred that the chiral salen Mn had been attached.
| Sample | Surface area/m2 g−1 | Pore volume (×10−2 cm3 g−1) | Average pore diameter/nm | Mn content /mmol g−1 |
|---|---|---|---|---|
| ZnPS-PVPP | 4.9 | 1.3 | 3.5 | — |
| ZnCMPS-PVPP | 36.9 | 18.82 | 10.21 | — |
| 1b | 42.5 | 24.2 | 11.39 | — |
| 3b | 31.66 | 5.43 | 1.56 | 0.72 |
| 3c | 35.97 | 7.33 | 1.68 | 0.67 |
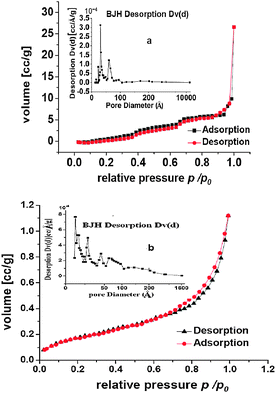 | ||
| Fig. 3 The nitrogen adsorption–desorption isotherm and pore distribution of (a) ZnPS-PVPA; (b) the supported catalyst 3b. | ||
The corresponding textural parameters calculated by N2 adsorption–desorption isotherms were presented in Table 1. As described in Table 1, after the support ZnPS-PVPP was chloromethylated and arylaminated, an obvious increase in BET surface area was observed (from 4.9 to 36.9 and to 42.5 m2 g−1), represented as 1b in Scheme 1, as well as increase in the pore volume (from 1.3 to 18.82 and to 24.2 × 10−2 cm3 g−1) and in average pore diameter (from 3.5 to 10.21 and to 11.39 nm). In contrast with this phenomenon, a decrease in BET surface area from 42.5 to 31.66 m2 g−1, in pore volume from 24.2 to 5.43 × 10−2 cm3 g−1 and in average pore diameter from 11.39 to 1.56 nm was observed upon immobilization of complex 2 onto ZnPS-PVPP modified by arylaminomethyl (1b). On the basis of this, it could be deduced that some chiral salen Mn(III) complexes were immobilized on the external surface of ZnPS-PVPP and other chiral salen Mn(III) complexes were present inside the nanopores. In other words, there were two forms of immobilization of ligand: inner type and outer type.
 | ||
| Fig. 4 XPS spectra of the heterogeneous catalyst 3b. | ||
3.1.8.1 Structure of the copolymer. The potentiality for ZnPS-PVPP as excellent catalyst supports was correlated with the structure of the copolymer. The possible structure of copolymer is shown in Fig. 5. In the experiment, the content of PVPA in the copolymer was controlled by means of adjusting the amount of initiator and ratio of PVPA to St. Generally speaking, the main segments of the molecule chain are –(St)m1–(PVPA)n–(St)m2–(PVPA)n(St)m3– in the copolymer, here the n is usually 1 and seldom more than 2, because the ratio of PVPA to St in the experiment is 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and the relative reactivities in the copolymerization for PVPA and St are comparable, thus the content ratio of PVPA to St in the copolymers prepared is found to be in the range of 1
8 and the relative reactivities in the copolymerization for PVPA and St are comparable, thus the content ratio of PVPA to St in the copolymers prepared is found to be in the range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–9. On the basis of the data, it can be deduced that a great number of hydrophobic segments of polystyrene can form half or part of holes and cavums with different volume and shapes for that the content of hydrophobic segments of polystyrene in the copolymer is much more than that of hydrophilic segments of PVPA in the copolymer, thus many hydrophilic –PO3H2 groups might gather together. The biggest surface area with the ratio of organic phosphonate to phosphate is 1
6–9. On the basis of the data, it can be deduced that a great number of hydrophobic segments of polystyrene can form half or part of holes and cavums with different volume and shapes for that the content of hydrophobic segments of polystyrene in the copolymer is much more than that of hydrophilic segments of PVPA in the copolymer, thus many hydrophilic –PO3H2 groups might gather together. The biggest surface area with the ratio of organic phosphonate to phosphate is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.16 When aqueous solution of hydrated zinc acetate is added, the phosphate, the phosphonic acid in the copolymer and water react with Zn2+ immediately to form white colloidal precipitation of ZnPS-PVPP. During the process, almost all phosphate in the reaction solution participate in forming the white colloidal precipitate of ZnPS-PVPP, while most of the organic phosphoric acid in the copolymer participate in forming or entering the white colloidal precipitation and little free phosphoric acid in the copolymer covers on the surface of white colloidal precipitation of ZnPS-PVPP. Simultaneously, hydrophobic segments of polystyrene in the copolymer which exist half or part of holes and cavums with different volume and shapes are also covered on the surface of the white colloidal precipitation of ZnPS-PVPP. The two neighboring –PO3H2 groups in the copolymer may be same or in different white colloidal precipitation which will aggregate into bigger colloidal precipitation particles of ZnPS-PVPP. Therefore, it is the hydrophobic of polystyrene parts and the hydrophilic of phosphate parts in ZnPS-PVPP that make ZnPS-PVPP possess dual properties which are hydrophobic and hydrophilic. Meanwhile, ZnPS-PVPP could be reacted with other complexes either in aqueous solution or in organic solvent even in mixed solvent which is the character that many other materials couldn't compare with. According to the surface morphology, there are probably two kinds of gathering model.
2.16 When aqueous solution of hydrated zinc acetate is added, the phosphate, the phosphonic acid in the copolymer and water react with Zn2+ immediately to form white colloidal precipitation of ZnPS-PVPP. During the process, almost all phosphate in the reaction solution participate in forming the white colloidal precipitate of ZnPS-PVPP, while most of the organic phosphoric acid in the copolymer participate in forming or entering the white colloidal precipitation and little free phosphoric acid in the copolymer covers on the surface of white colloidal precipitation of ZnPS-PVPP. Simultaneously, hydrophobic segments of polystyrene in the copolymer which exist half or part of holes and cavums with different volume and shapes are also covered on the surface of the white colloidal precipitation of ZnPS-PVPP. The two neighboring –PO3H2 groups in the copolymer may be same or in different white colloidal precipitation which will aggregate into bigger colloidal precipitation particles of ZnPS-PVPP. Therefore, it is the hydrophobic of polystyrene parts and the hydrophilic of phosphate parts in ZnPS-PVPP that make ZnPS-PVPP possess dual properties which are hydrophobic and hydrophilic. Meanwhile, ZnPS-PVPP could be reacted with other complexes either in aqueous solution or in organic solvent even in mixed solvent which is the character that many other materials couldn't compare with. According to the surface morphology, there are probably two kinds of gathering model.
 | ||
| Fig. 5 Possible ideal structure of copolymer. | ||
Fig. 6a signifies that the two or more neighboring –PO3H2 groups (there is a polystyrene chain between and connected them) are in the same colloidal precipitation; while Fig. 6b signifies that the two neighboring –PO3H2 groups (there is a polystyrene segment between which linked them) are in different colloidal precipitation. In the aggregation process of the colloidal precipitation into bigger particles, many precipitation particles link each other to form even bigger hybrid ZnPS-PVPP precipitation particles, a great deal of channels, holes and cavums with different volume and shapes are gradually formed. Therefore, if precipitation particles link in the form of model b, different lengths and shapes of channels, holes and cavums will come into being. And if the particles link in the form of model a, the channels, holes and cavums with smaller volume will be formed and cover on the surface of the small precipitation particles.20
 | ||
| Fig. 6 Possible ideal structure of ZnPS-PVPP. | ||
3.1.8.2 The hypothesized layered structure of ZnPS-PVPP. In the hypothesized models deduced for ZnPS-PVPP (Fig. 7), some oxygen atoms of the hydroxyl groups or hydroxy sodium in the segments of the inorganic phosphate groups were coordinated with zinc atoms, making the zinc atoms self-assemble in the same plane, while the other oxygen atoms of the portion of the inorganic phosphate groups in the ZnPS-PVPP stretched over the surface of the zinc layer. On the other side, there are several types of organic polymer phosphonate-PO3H2 (opp-PO3H2) formed in ZnPS-PVPP: opp-PO3H2 ③ was located on the interlayer surface of one zinc layer, and was connected to other particle of ZnPS-PVPP by a polystyrene segment, in other words, opp-PO3H2 ③ and its one neighboring opp-PO3H2 were located not in the same but different particle of ZnPS-PVPP. The same to the opp-PO3H2 group
 . Opp-PO3H2 group ② and ④ were linked to each other by a polystyrene chain and situated on the interlayer surface of the two adjacent zinc layers respectively. Opp-PO3H2 group ① was perched on the interlayer surface of one zinc layer and joined to the opp-PO3H2 group ⑦ by a polystyrene chain which lied on the surface of another contiguous zinc interlayer space. Both opp-PO3H2 group ⑤ and opp-PO3H2 group ⑥ which were conjunct to one another were located on the interlayer surface of the same zinc layer, similar to the opp-PO3H2 group ⑧ and opp-PO3H2 group ⑨. In summary, there were at least three imaginable structures: (i) two adjacent opp-PO3H2 groups (there is a polystyrene chain between which connected them) were situated on the same layer in the same crystalline grain; (ii) two neighboring opp-PO3H2 groups (there is also a polystyrene chain among them which linked them) were located on the different layer in the same crystalline grain; (iii) two contiguous opp-PO3H2 groups (there is a polystyrene segment between which joined them) in the uniform particle were perched on diverse crystalline grains. So pores or channels of various sizes and shapes by appropriate modification of the styrene-phenylvinylphosphonic acid copolymer chain were formed that consequentially give birth to significant impact on the excellent catalytic activity.20
. Opp-PO3H2 group ② and ④ were linked to each other by a polystyrene chain and situated on the interlayer surface of the two adjacent zinc layers respectively. Opp-PO3H2 group ① was perched on the interlayer surface of one zinc layer and joined to the opp-PO3H2 group ⑦ by a polystyrene chain which lied on the surface of another contiguous zinc interlayer space. Both opp-PO3H2 group ⑤ and opp-PO3H2 group ⑥ which were conjunct to one another were located on the interlayer surface of the same zinc layer, similar to the opp-PO3H2 group ⑧ and opp-PO3H2 group ⑨. In summary, there were at least three imaginable structures: (i) two adjacent opp-PO3H2 groups (there is a polystyrene chain between which connected them) were situated on the same layer in the same crystalline grain; (ii) two neighboring opp-PO3H2 groups (there is also a polystyrene chain among them which linked them) were located on the different layer in the same crystalline grain; (iii) two contiguous opp-PO3H2 groups (there is a polystyrene segment between which joined them) in the uniform particle were perched on diverse crystalline grains. So pores or channels of various sizes and shapes by appropriate modification of the styrene-phenylvinylphosphonic acid copolymer chain were formed that consequentially give birth to significant impact on the excellent catalytic activity.20
 | ||
| Fig. 7 The hypothesized layered structure of ZnPS-PVPP. | ||
 | ||
| Fig. 8 SEM images of ZnPS-PVPP (1) and 3b (2). | ||
The as-synthesized sample of the layered mesoporous ZnPS-PVPP was washed in deionized water, ultrasounded for 2 h and their morphology determined by transmission electron microscopy. The TEM photography of ZnPS-PVPP (Fig. 9) manifested that the structure of the support was spheroid, its channels, holes and cavums could be discerned clearly, and their sizes were about 70–80 nm. Allowing for the interlayer spacing (2.12 nm) and the average diameter of these secondary channels (50–60 nm), it could be deduced that each particle consisted of stacks of 25–30 layers of ZnPS-PVPP, resulting in few mesopores with the average dimensions of 3.5 nm. While for the heterogeneous catalyst 3b, the configuration was filiform and loose, and its channels, holes and cavums also existed in it. Just for this, substrates would have more chance to transfer to the internal catalytic active sites in solution.
3.2 Enantioselective epoxidation of unfunctionalized olefins
| Entry | Substrateb | Catalyst | Oxidant system | Time/h | T/°C | Conv.% | eec | TOFd × 10−4 (s−1) |
|---|---|---|---|---|---|---|---|---|
| a Reactions were carried out in CH2Cl2 (4 mL) with alkene (1 mmol), n-nonane (internal standard, 1 mmol), NMO (5 mmol), homogeneous (5 mol%) or heterogeneous salen Mn(III) catalysts (5 mol%) and m-CPBA (2 mmol). The conversion and the ee values were determined by GC with chiral capillary columns HP19091G-B 213, 30 m × 0.32 mm × 0.25 μm. b A = α-methylstyrene, B = indene. c (S)-form. d Turnover frequency (TOF) is calculated by the expression of [product]/[catalyst] × time (s−1). | ||||||||
| 1 | A | 2 | m-CPBA/NMO | 5 | −40 | >99 | 54 | 11.11 |
| 2 | 4b | m-CPBA | 5 | −40 | 64 | 90.8 | 7.11 | |
| 3 | 3a | m-CPBA | 5 | −40 | 98.6 | >99 | 10.96 | |
| 4 | 3b | m-CPBA | 5 | −40 | >99 | >99 | 11.11 | |
| 5 | 3c | m-CPBA | 5 | −40 | 96.8 | >99 | 10.76 | |
| 6 | B | 2 | m-CPBA/NMO | 1 | 0 | 92 | 65 | 51.11 |
| 7 | 4b | m-CPBA | 1 | 0 | 98.7 | 83.7 | 54.83 | |
| 8 | 3a | m-CPBA | 1 | 0 | 80.5 | 94.5 | 44.72 | |
| 9 | 3b | m-CPBA | 1 | 0 | >99 | >99 | 55.55 | |
| 10 | 3c | m-CPBA | 1 | 0 | 86.4 | 92.3 | 48.00 | |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O was altered. It was steric hindrance that made olefins approaching salen Mn(V)
O was altered. It was steric hindrance that made olefins approaching salen Mn(V)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O difficult and lower ee values were obtained. Obviously, there is a lot of work to be done on mechanistic and structural aspects of our reaction and catalyst.
O difficult and lower ee values were obtained. Obviously, there is a lot of work to be done on mechanistic and structural aspects of our reaction and catalyst.
| Entry | Substratec | Catalyst | Oxidant system | Time/h | T/°C | Conv.% | eed | TOFe × 10−4 (s−1) |
|---|---|---|---|---|---|---|---|---|
| a Reactions were carried out in CH2Cl2 (4 mL) with alkene (1 mmol), n-nonane (internal standard, 1 mmol), NMO (5 mmol), homogeneous (5 mol%) or heterogeneous salen Mn(III) catalysts (5 mol%) and m-CPBA (2 mmol). The conversion and the ee values were determined by GC with chiral capillary columns HP19091G-B 213, 30 m × 0.32 mm × 0.25 μm. b Reaction conditions: alkene (1 mmol), NaIO4 (2 mmol), catalyst (0.03 mmol), CH3CN/H2O (10 mL/5 mL). c A = α-methylstyrene. d Same as in Table 2. e Same as in Table 2. | ||||||||
| 1 | A | 2 | m-CPBA/NMO | 5 | −40 | >99 | 54 | 11.11 |
| 2 | A | 4b | m-CPBA/NMO | 5 | −40 | 88 | 86 | 9.78 |
| 3 | A | 4b | m-CPBA | 5 | −40 | 64 | 90.8 | 7.11 |
| 4 | A | 3b | m-CPBA/NMO | 5 | −40 | 6.6 | 3.1 | 0.73 |
| 5 | A | 3b | m-CPBA | 5 | −40 | >99 | >99 | 11.11 |
| 6 | A | 2 | NaIO4/imidazole | 7 | 25 | >99 | >99 | 7.94 |
| 7 | A | 4b | NaIO4/imidazole | 7 | 25 | >99 | >99 | 7.94 |
| 8 | A | 4b | NaIO4 | 7 | 25 | >99 | >99 | 7.94 |
| 9 | A | 3b | NaIO4/imidazole | 7 | 25 | >99 | >99 | 7.94 |
| 10 | A | 3b | NaIO4 | 7 | 25 | >99 | >99 | 7.94 |
On the other hand, the heterogeneous catalysts 3a–3c were also applied in the epoxidation of α-methylstyrene in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile
1 mixture of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water with NaIO4 as the oxidative system. Jacobsen's catalyst 2 and homogeneous catalyst 4b were tested for comparable purposes. As described in Table 3, the supported catalyst 3b showed comparable catalytic activity to that of the homogeneous catalyst 4b and Jacobsen's catalyst 2. The conversion and enantioselectivity of catalyst 3b exceeded 95%, even >99%. It could be deduced that the heterogeneous catalysts 3b possessed efficient catalytic abilities whether the axial ligand imidazole existed or not. Meanwhile, the axial ligand imidazole made little impact on the catalytic activities that the conversion and enantioselectivity increased a little in the presence of imidazole (entry 9 vs. 10: conv%, from >99 to >99; ee%, >99 vs. >99).
water with NaIO4 as the oxidative system. Jacobsen's catalyst 2 and homogeneous catalyst 4b were tested for comparable purposes. As described in Table 3, the supported catalyst 3b showed comparable catalytic activity to that of the homogeneous catalyst 4b and Jacobsen's catalyst 2. The conversion and enantioselectivity of catalyst 3b exceeded 95%, even >99%. It could be deduced that the heterogeneous catalysts 3b possessed efficient catalytic abilities whether the axial ligand imidazole existed or not. Meanwhile, the axial ligand imidazole made little impact on the catalytic activities that the conversion and enantioselectivity increased a little in the presence of imidazole (entry 9 vs. 10: conv%, from >99 to >99; ee%, >99 vs. >99).
In general, the additives N-methylmorpholine N-oxide (NMO) and imidazole, which are used to improve epoxidation yields and enantioselection, bind to the Mn(III) center prior to the epoxidation reaction, as evidenced by the alteration of the Mn(III) parallel mode EPR signal.24 Additives to the Mn(III) salen reaction mixture, such as NMO and imidazole, generally facilitate faster reaction rates, higher epoxide yields, and improved enantioselectivity. However, the additives in this text played such different roles that the catalytic activities did not increase but decrease with the addition of NMO in m-CPBA as the oxidative system or slightly increase in the presence of imidazole with NaIO4 as the oxidative system. In addition, the additives were expensive commonly and the superior catalytic activities were still obtained in their absence. From the commercial viewpoint, the heterogeneous catalyst 3b had the potential application in industry.
3.3 The reusability of the catalyst
The reusability of a heterogeneous catalyst is of great importance from synthetic and economical points of view. The homogeneous catalysts could not recover even one time, in contrast, the supported catalysts 3a–3c could be filtered and reused several times without significant loss of their activity.To assess the long-term stability and reusability of the supported chiral salen Mn(III) catalysts, α-methylstyrene was used as a mode substrate, and recycling experiments were carried out with catalyst 3b. At the end of each reaction, the catalyst was separated by adding hexane, washed with deionized water and dried carefully before using it in the next run. Above 95% recycling of the catalyst was achieved in every run. The recovered dried solid catalyst was weighed and reused in the next run. In every run the same ratio of the substrate-to-catalyst and solvent-to-catalyst was kept. The filtrates were collected for determination of Mn leaching. After using catalyst 3b for twelve consecutive times, the results were listed in Table 4.
| Run | Time/h | Conversion (%) | eeb (%) | TOFc × 10−4 (s−1) |
|---|---|---|---|---|
| a Reactions were carried out at −40 °C in CH2Cl2 (2 mL) with α-methylstyrene (1 mmol), n-nonane (internal standard, 1 mmol), m-CPBA (2 mmol), heterogeneous salen Mn(III) catalysts (5 mol%). The conversion and the nonane (50 mmol), heterogeneous catalyst 3b (2.5 mmol), m-CPBA(100 mmol), respectively. b Same as in Table 2. c Same as in Table 2. | ||||
| 1 | 5 | >99 | >99 | 11.11 |
| 2 | 5 | >99 | >99 | 11.11 |
| 3 | 5 | >99 | >99 | 11.11 |
| 4 | 5 | >99 | >99 | 11.11 |
| 5 | 5 | 97.8 | >99 | 10.87 |
| 6 | 5 | 96 | >99 | 10.67 |
| 7 | 5 | 92 | 97 | 10.22 |
| 8 | 5 | 90 | 93 | 9.99 |
| 9 | 5 | 88 | 86.1 | 9.78 |
| 10 | 5 | 85.2 | 78.9 | 9.47 |
| 11 | 5 | 80.1 | 65.4 | 8.90 |
| 12 | 5 | 73 | 29.4 | 8.11 |
Obviously, the yield and enantioselectivity decreased slightly after recycling nine times and still gave yield (88%) and enantioselectivity (86.1%). The effective separation the chiral Mn(III) salen complexes by the solid support ZnPS-PVPP contributed to the good stability of the heterogeneous chiral Mn(III) salen catalyst in case that they would dimerize to inactive μ-oxo-Mn(IV) species. The decrease of the yield can be attributed to the decomposition of the chiral Mn(III) salen complex under epoxidation conditions25 and the loss of the hyperfine granules of the heterogeneous chiral Mn(III) salen catalysts (formed in the reaction due to stirring). The Mn content of the heterogeneous catalyst 3b is 0.46 mmol g−1 compared with the total amount (around 0.72 mmol g−1) when the heterogeneous catalyst was recycled for 9 times.
The nature of the recovered catalyst 3b was followed by IR (Fig. 10). The result indicated that characteristic bands of the catalyst at 2954, 2864 and 1630 cm−1 disappeared or weakened after recycling ten times. These revealed that the active sites of the salen Mn(III) complex and the ZnPS-PVPP support under acid reaction conditions were partly destroyed (Fig. 11). Moreover, other effects can be used to explain these results: (i) leaching of metal complexes from the materials or (ii) blocking of the pores and secondary channels either by inactive Mn(IV)-oxo species was believed to be generated during the catalytic mechanism26 or by some other insoluble degraded product obtained by side reactions, which after several washing could not be removed from the materials or (iii) collapsing of some of the pillars during the catalysis experiments.
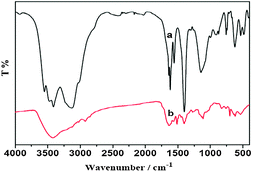 | ||
| Fig. 10 FT-IR spectra of (a) the fresh catalyst 3b and (b) the ten times used catalyst 3b. | ||
 | ||
| Fig. 11 The theoretic changing progress of catalyst 5c in acid solution. | ||
3.4 Large-scale asymmetric epoxidation reaction
We further performed different proportions of large-scale asymmetric epoxidation reactions with n-nonane and α-methylstyrene and m-CPBA. The same catalyst loading of 5 mol% as in the experimental scale was used. The large-scale experiments can be facilely carried out using the same procedure as for the experimental scale reactions. As can be seen from the results summarized in Table 5, delightfully, the conversion and enantioselectivity maintained at the same level for the large-scale reactions under whichever condition that the large scale is 50 times or 100 times (Fig. S4, ESI†) as much as the experimental scale.| Entry | Time/h | Conversion (%) | eef (%) | TOFg × 10−4 (s−1) |
|---|---|---|---|---|
| a Reactions were carried out at −40 °C in CH2Cl2 with α-methylstyrene, n-nonane, m-CPBA, heterogeneous salen Mn(III) catalysts (5 mol%). The conversion and the ee values were determined by GC with chiral capillary columns HP19091G-B213, 30 m × 0.32 mm × 0.25 μm. b The usage amounts of reagents were α-methylstyrene (1 mmol), n-nonane (1 mmol), heterogeneous catalyst 3b (0.05 mmol), m-CPBA (2 mmol), respectively. c The usage amounts of reagents were α-methylstyrene (50 mmol), n-nonane (50 mmol), heterogeneous catalyst 3b (2.5 mmol), m-CPBA (100 mmol), respectively. d The usage amounts of reagents were α-methylstyrene (50 mmol), n-nonane (50 mmol), heterogeneous catalyst 3b (0.5 mmol), m-CPBA (100 mmol), respectively. e The usage amounts of reagents were α-methylstyrene (100 mmol), n-nonane (100 mmol), heterogeneous catalyst 3b (5 mmol), m-CPBA (200 mmol), respectively. f Same as in Table 2. g Same as in Table 2. | ||||
| 1b | 5 | >99 | >99 | 11.11 |
| 2c | 5 | >99 | >99 | 11.11 |
| 3d | 5 | >99 | >99 | 11.11 |
| 4e | 5 | >99 | >99 | 11.11 |
4. Conclusions
In summary, novel type of immobilized chiral salen Mn(III) complexes onto ZnAMPS-PVPP were synthesized and applied as catalysts in the asymmetric epoxidation of unfunctionalized olefins. The new heterogeneous catalysts indicated the comparable or even higher conversions and enantioselectivities than those of corresponding homogeneous catalysts under the same conditions. Furthermore, the immobilized catalysts exhibited remarkable high catalytic activity in the absence of NMO. This was mainly attributed to the similar structure of the heterogeneous catalysts to NMO and the spatial constraint effects of the special structure of ZnPS-PVPP. Moreover, the supported chiral catalysts were relatively stable and could be reused nine times in the asymmetric epoxidation of α-methylstyrene. Remarkably, this organocatalyzed asymmetric epoxidation reaction can be performed on a large-scale with the catalytic ability being maintained at the same level, which offers a great possibility for applications in industry.Acknowledgements
This work was financially supported by National Ministry of Science and Technology Innovation Fund for High-tech Small and Medium Enterprise Technology (NO.09C26215112399) and National Ministry of Human Resources and Social Security Start-up Support Projects for Students Returned to Business, Office of Human Resources and Social Security Issued 2009 (143).References
- E. M. McGarrigle and D. G. Gilheany, Chem. Rev., 2005, 105, 1563 CrossRef CAS.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor and N. Rasouli, Inorg. Chem. Commun., 2007, 10, 1537 CrossRef CAS.
- E. Angelescu, O. D. Pavel, R. Bıirjega, M. Florea and R. Zavoianu, Appl. Catal., A, 2008, 341, 50 CrossRef CAS.
- S. Zhong, Y. Tan, Z. Fu, Q. Xie, F. Xie, X. Zhou, Z. Ye, G. Peng and D. Yin, J. Catal., 2008, 256, 154 CrossRef CAS.
- L. Feng, E. Urnezius and R. L. Luck, J. Organomet. Chem., 2008, 693, 1564 CrossRef CAS.
- Y. Jin, P. Wang, D. Yin, J. Liu, H. Qiu and N. Yu, Microporous Mesoporous Mater., 2008, 111, 569 CrossRef CAS.
- R. Luque, S. K. Badamali, J. H. Clark, M. Fleming and D. J. Macquarrie, Appl. Catal., A, 2008, 341, 154 CrossRef CAS.
- W. Zhang, J. L. Loebach, S. R. Wilson and E. N. Jacobsen, J. Am. Chem. Soc., 1990, 112, 2801 CrossRef CAS.
- K. Srinivasan, P. Michaud and J. K. Kochi, J. Am. Chem. Soc., 1986, 108, 2309 CrossRef CAS.
- W. Zhang, N. H. Lee and E. N. Jacobsen, J. Am. Chem. Soc., 1994, 116, 425 CrossRef CAS.
- Q. H. Xia, H. Q. Ge, C. P. Ye, Z. M. Liu and K. X. Su, Chem. Rev., 2005, 105, 1603 CrossRef CAS.
- C. Li, H. Zhang, D. Jiang and Q. Yang, Chem. Commun., 2007, 547–558 RSC.
- H. Zhang, Y. Zhang and C. Li, J. Catal., 2006, 238, 369 CrossRef CAS.
- W. S. Ren, X. K. Fu, H. B. Bao, R. F. Bai, P. P. Ding and B. L. Sui, Catal. Commun., 2009, 10, 788 CrossRef CAS.
- W. S. Ren and X. K. Fu, J. Mol. Catal. A: Chem., 2009, 312, 40 CrossRef CAS.
- B. W. Gong, X. K. Fu, J. X. Chen, Y. D. Li, X. C. Zou, X. B. Tu, P. P. Ding and L. P. Ma, J. Catal., 2009, 262, 9 CrossRef CAS.
- X. C. Zou, X. K. Fu, Y. D. Li, X. B. Tu, S. D. Fu, Y. F. Luo and X. J. Wu, Adv. Synth. Catal., 2010, 352, 163 CrossRef CAS.
- M. Palncki, P. J. Pospisil, W. Zhang and E. N. Jacobsen, J. Am. Chem. Soc., 1994, 116, 9333 CrossRef.
- B. W. Gong, X. K. Fu, H. S. Shen, L. H. Ma and Y. J. Xia, Fine Chem., 2008, 25, 603 CAS.
- J. Huang, X. K. Fu, G. Wang, C. Li and X. Y. Hu, Dalton Trans., 2011, 40, 3631 RSC.
- S. Liao, X. Z. Tian, W. W. Wu, X. Chen, T. S. Wang and J. T. Li, Chin. J. Chem., 2008, 26, 1837 CrossRef CAS.
- A. R. Silva, J. L. Figueiredo, C. Freire and B. Castro, Microporous Mesoporous Mater., 2004, 68, 83 CrossRef CAS.
- C. Li, Catal. Rev., 2004, 46, 419 CAS.
- K. A. Campbell, M. R. Lashley, J. K. Wyatt, M. H. Nantz and R. D. Britt, J. Am. Chem. Soc., 2001, 123, 5710 CrossRef CAS.
- C. E. Song, E. J. Roh, B. M. Yu, D. Y. Chi, S. C. Kim and K. J. Lee, Chem. Commun., 2000, 615–616 RSC.
- P. McMorn and G. J. Hutchings, Chem. Soc. Rev., 2004, 33, 108 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, FT-IR, UV-vis. See DOI: 10.1039/c1cy00285f |
| This journal is © The Royal Society of Chemistry 2011 |



