DOI:
10.1039/C1CY00271F
(Perspective)
Catal. Sci. Technol., 2011,
1, 1298-1310
Asymmetric organocatalytic reactions by bifunctional amine-thioureas
Received
14th July 2011
, Accepted 24th August 2011
First published on 12th September 2011
Abstract
The development of organocatalysts has greatly changed the art of organic transformation in the chemical synthesis community for the past decades. Nature's work underpins the success of hydrogen-bonding catalysis were obviously shown in a myriad of enzymatic reactions. More recently, the emergence of bifunctional organocatalysts which work complementarily in activating two components of a chemical reaction has emerged as a frontier of research in asymmetric synthesis. In this particular review, works on asymmetric reactions catalyzed by bifunctional amine-thioureas are examined.

Woon-Yew Siau
| Woon-Yew Siau was born in Johor Bahru, Malaysia, in 1988. He earned his BASc (Honours) from National University of Singapore (NUS) in 2011. In the same year, he joined NUS chemistry department as a PhD student. His research interests lie in the discovery of new catalytic reactions. |

Jian Wang
| Jian Wang obtained his BSc from An Hui Normal University in China and completed in PhD study in University of New Mexico in the United States. He carried out his postdoctorial studies at Scripps Research Institute and joined National University of Singapore (NUS) as an assistant professor to embark on his career on asymmetric organocatalysis, novel synthetic method, and natural product synthesis. |
Introduction
Synthetic methodologies that manipulate the stereochemical outcome of the organic molecules have progressively gained awareness in organic synthesis. Hence, asymmetric synthesis is desirable, and if possible, a catalytic method. Organocatalysis is considered to be the most convenient and easy way to tackle this problem.1 In particular, hydrogen-bonding mediated catalysis has gained recognition and is widely employed in enantioselective transformations.2 Small organic molecules with hydrogen-bond donor capability were known to activate substrates through LUMO lowering and thiourea-based organocatalyst evolved as a prominent class due to its dual hydrogen-bonding donor characteristic. Various research groups have demonstrated that catalysts with bifunctional motif could promote enantioselective reactions in an excellent manner. In this context, the term “bifunctionality” was realised when a Lewis basic functionality was incorporated into the catalytic system in addition to hydrogen bond donors (Fig. 1).3 These two functional groups work synergistically to bring about the activation of both nucleophilic and electrophilic components in a reaction. In addition, cinchona alkaloids and their thiourea-modified frameworks are also widely seen in asymmetric reactions as the basic tertiary amine group present in the quinuclidine ring is a versatile nucleophilic base in the context of asymmetric reactions.4
 |
| | Fig. 1 A bifunctional amine thiourea catalyst (Takemoto's catalyst, left), and general scaffold of bifunctional amine thioureas (right). | |
With the emergence of this new catalytic system, bifunctional mode catalysis with hydrogen bonding capability has broadened the scope of work in asymmetric reactions in recent year. To the benefit of reading and ease of understanding, asymmetric reactions catalyzed by bifunctional amine thioureas will be discussed between 2003 and 2011.
2. Hydrogen bonding catalysis: underlying principle of biological processes5
Activation of electrophiles by hydrogen bonding is commonly seen in biological pathway, especially enzymatic hydrolysis of amide bonds.6 The most notable example is serine protease.7 The mechanistic study has shed light on the importance of hydrogen bonding moiety embedded in the catalytic site and suggested that amide hydrolysis takes place when both electrophile and nucleophile are activated (Scheme 1). In 2003, Takemoto and co-workers has successfully elaborated the fundamental principle of hydrogen bonding catalysis through the design of a bifunctional thiourea: thiourea moiety mimicks “oxyanion hole” and Lewis basic site (usually a tertiary amine group) functions as histidine/aspartate proton shuttle system (Fig. 2).8a
 |
| | Scheme 1 Serine protease: Biological process of amide hydrolysis with the assistance of hydrogen bonding and bifunctional catalysis. | |
 |
| | Fig. 2 Comparison between H-bond biocatalysis and chemical catalysis. | |
3. Michael addition
Michael addition is a highly versatile synthetic tool to join two chemical entities together during the course of reaction. Asymmetric Michael addition is particular useful as selective C–C or C–X bonds could be formed in a stereocontrolled manner with the use of bifunctional amine-thioureas.
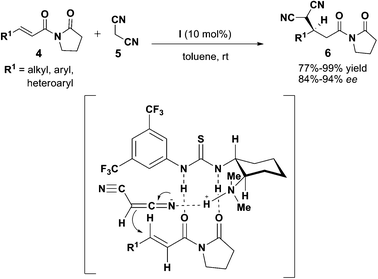
First enantioselective Michael addition was reported by Takemoto's group in 2003 with the use of a bifunctional amine thiourea-organocatalyst (Scheme 2).8 The bifunctional organocatalyst I which was shown to promote enantioselective Michael reaction between malonates 2 and nitroolefins 1 has marked the breakthrough in organocatalysis as excellent stereochemical information was observed in the study.9 The mechanistic insight of the reaction was later elucidated by Pápai et al. using in silico study.10 Despite different substrate activation mode (Fig. 3) was proposed, same stereochemistry was predicted for the reaction. To further expand the scope of work on bifunctional thiourea catalyst, Takemoto and co-workers demonstrated the first asymmetric Michael addition reaction of malononitrile 5 to α,β-unsaturated imides 4 without the use of metal catalysis11 (Scheme 3).
 |
| | Scheme 2 Takemoto's bifunctional organocatalyst-catalyzed Michael reaction of malonates to nitroolefins. | |
Cinchona alkaloids are example of naturally occurring bifunctional organocatalysts. However, no much attention was paid to their application for asymmetric induction, and it was until 1980, Wynberg and co-workers reported a detailed investigation of base-catalyzed Michael addition of thiols to α,β-unsaturated ketones in the presence of a catalytic amount of cinchona alkaloids.12 Later on, given that they are readily available and tunable, transformation of the initially existing hydroxyl group to “privileged” thiourea moiety has made them better hydrogen bond donor (Fig. 4). In view of the great synthetic applications of 1,4-addition Michael adducts of nitroalkanes,13 Soós et al. had reported an enantioselective reaction between chalcones 7 and nitromethane 8 using a cinchona alkaloid derived thiourea organocatalyst (Table 1).14 In the particular study, (R)-Baclofen was obtained in high enantioselectivity (Table 1, entry 1) and thereby, offering a better route to other biologically important γ-amino acids.15 The conformation adopted by cinchona derivatives was important for the observed catalytic activity.
 |
| | Fig. 4 Bifunctional cinchona organocatalysts. | |
| Entry |
|
R1 |
R2 |
Yield (%) |
ee (%) |
|
Reactions were carried out in toluene in capped vials.
|
| 1 |
7a
|
H |
p-Cl |
94 |
95 (R) |
| 2 |
7b
|
H |
p-F |
94 |
98a |
| 3 |
7c
|
H |
o-Me |
93 |
89a |
| 4 |
7d
|
p-OMe |
H |
80 |
96a |
| 5 |
7e
|
H |
H |
94 |
96 |
Inspired by the binapthyl moiety, Wang et al. developed a novel bifunctional amine-thiourea VIII for asymmetric reaction between 1,3-diketone 10 and nitroolefins 1 (Scheme 4).16 Due to poor performance of Takemoto's catalyst I and cinchona modified thiourea IV, catalyst VIII turned out to be a superior bifunctional bifunctional organocatalyst in the reaction. Subsequently, synthesis of valuable α-substituted-β-amino acids17 was also highlighted in the study using Michael adduct 12. Research has been on going to explore the synthesis of enantiopure β-amino acids as these are important chemical entity in both academia and industry.18 As a result, synthesis of β-amino acids becomes a synthetic challenging issue for organic chemists. To address this issue, Chen et al. conducted an asymmetric Michael reaction between α-substituted cyanoacetates 15 and vinyl ketones 16 in a non-polar solvent (Scheme 5).19 Unlike the 1,3-dicarbonyl analogues, α-substituted cyanoacetates20 are good starting material for the conversion to β2,2-amino acid in spite of their incapability of two-point binding with the catalyst.
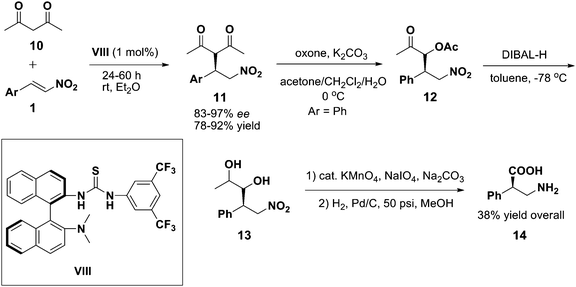 |
| | Scheme 4 Michael addition of 1,3-diketone 10 to nitroolefins 1 catalyzed by binaphthyl-derived thiourea organocatalyst VIII and the synthesis of α-phenyl-β-alanine 12. | |
 |
| | Scheme 5 Organocatalytic approach to multifunctional compounds with an all-carbon-substituted quaternary stereocenter and its possible active intermediate calculated by PM3 (semi-empirical method). | |
3.2 Michael addition for C–O, C–N and C–S bond formation
Oxo-, aza- and sulfa-Michael addition are particular useful for the construction of heteroatom compounds. β-hydroxylation reaction21 employing oxime as the oxygen source was illustrated by Jørgensen's group (Table 2).22 This enantioselective oxa-Michael addition23 was the first documented protocol to access optically active aliphatic nitro- or aminoalcohols 18 with the use of nitroalkenes 16 and ethyl glyoxylate oximes 17. Subsequently, selective cleavage of O–N bond was also shown using adduct 18d under hydrogenation conditions (H2/Pd/C; (Boc)2O) or using ZrCl4 and NaBH4.24
Table 2 Enantioselective β-hydroxylation of nitroalkenes 1 catalyzed by cinchona alkolide amine-thiourea IV
Great attention has also been focused on the enantioselective synthesis of nitrogen-containing compounds due to their broad utility and application in organic synthesis and medicinal chemistry.25,26 Aza-Michael addition is the most direct and easiest way to access such valuable organic molecules. Using N-heterocycle 20 as a nucleophilic source, the enantioselective conjugate addition was accomplished in the presence of a bifuntional cinchona alkaloid based thiourea IV (Table 3).27 This protocol was applicable to a broad scope of α,β-unsaturated ketones, furnishing the desired products 22 in good yields and with moderate to good enantioselectivities. On the other hand, organosulfur compounds are sparsely reported in asymmetric Michael reaction, particular with the use of bifunctional amine-thioureas.28,29 In this instance, Wu et al. envisioned an asymmetric reaction between arylthiols 23 and α,β-unsaturated carbonyl compounds (24 and 25, Scheme 6).30 Stabilization of the thiolate and activation of the electrophilic α,β-unsaturated system via hydrogen bonding is the fundamental aspect of this conjugate reaction.
| Entry |
Product |
Ar |
R
|
Yield (%) |
ee (%) |
| 1 |
22a
|
p-ClC6H4 |
Ph |
79 |
61 |
| 2 |
22b
|
o-ClC6H4 |
Ph |
69 |
63 |
| 3 |
22c
|
p-NO2C6H4 |
Ph |
85 |
63 |
| 4 |
22d
|
p-MeOC6H4 |
Ph |
62 |
57 |
| 5 |
22e
|
Ph |
Ph |
67 |
64 |
| 6 |
22f
|
Ph |
p-ClC6H4 |
78 |
62 |
| 7 |
22g
|
2-Thiophenyl |
2-Thiophenyl |
69 |
56 |
Besides, a weakly nucleophilic thioacetic acid 28 was reported to react with α,β-unsaturated ketones 21 to afford the oxo-thiolate products 29 in diethyl ether at room temperature. The reaction was facilitated in the presence of catalyst I and lower enantioselectivities were observed with electron-withdrawing groups and alkyl substituents. After all, the products could be obtained in an excellent yields (95–100%) and with moderate enantioselectivities (up to 65% ee, Scheme 7).31
4. 1,2-Addition
1,2-addition reaction is not uncommon in organic synthesis and it often provides a better route to nitrogen-containing compounds. Electrophilic acceptors like imines play an important role for this kind of organic transformation and could be easily generated through the formation of Schiff base.32 Often, electron-withdrawing groups like tert-butyl-carbonate group (t-Boc), carbobenzyloxy group (Cbz), and fluorenylmethyloxycarbonyl group (Fmoc) are installed in the molecular scaffold for the purpose of establishing hydrogen bonding with the thiourea moiety, thus activating imine moiety.
Although much work has been done for nitro-Mannich (or aza-Henry) reactions,33 it was not until Wang et al. documented a highly enantioselective and diastereoselective protocol for the synthesis of β-nitro amines 31 (Scheme 8). The bifunctional amine-thiourea IX bearing an extra hydrogen bonding donor at the sulphonamide part34 (NH2SO2Ar) appeared to exhibit excellent catalytic performance for the transformation at low temperature. To further expand the work on cinchona alkaloid derived amine-thiourea, Deng's group has studied an 1,2-addition approach towards biological interesting β-amino acids under a rather mild, moisture and air-compatible condition (Scheme 9).35 The generality of the reaction was subsequently investigated. A wide range of alkyl and aryl substituted malonates 2 were employed as nucleophiles, and good yields (55–99%) and excellent enantioselectivities (88–99% ee) were attainable. Around the same time, Dixon and co-workers published a similar work on Mannich reaction regarding the synthesis of β-amino esters 36 (Scheme 10).36 The synthesis was catalyzed by a cinchonine modified bifunctional thiourea and the decarboxylation reaction was carried out in a simple procedure.
 |
| | Scheme 8 Highly enantioselective and diastereoselective nitro-Mannich reaction and activation of imines via hydrogen bonding with thiourea IX (left). | |
 |
| | Scheme 10 A simple organocatalytic route to β-amino esters 36. | |
In 2006, Deng's group made an advance leap in organocatalysis by demonstrating an asymmetric version of Friedel–Crafts reaction37 with the use of a cinchona thiourea-derived organocatalyst (Scheme 11). The indoles 37 reacted with the imines 30 in 1,2-fashion to give rise to the product 38 in both excellent yields and enantioselective controls.38 It is noteworthy that both enantiomeric pair of 38 could be obtained in highly enantioselective manner via this synthetic methodology with the use of catalyst VII or quinine derived amine-thiourea. The conversion of N-Ts to N-Bs was also highlighted in the work without affecting the stereochemical information.
 |
| | Scheme 11 Asymmetric Friedel–Crafts alkylation reaction catalyzed by bifunctional amine-thiourea VII. | |
Last but not least, the author would also want to highlight another 1,2-reaction contributed by Jacobsen and co-workers.39 Valuable chiral building blocks for instance α-hydroxy acids, β-amino alcohols could be easily derived from cyanohydrins40 from the standpoint of synthetic organic chemistry. As a result, development of cyanation chemistry is highly acknowledged.41 Jacobsen et al. presented an enantioselective cyanosilylation of ketones 39 with trimethylsilanecarbonitrile (TMSCN, 40) in the presence of a catalyst XId (Scheme 12).42
 |
| | Scheme 12 1,2-cyanosilylation of ketones by Jacobsen's bifunctional thiourea catalyst | |
Despite the challenging issue arise from the formation of a quaternary carbon center,43 such synthetic protocol is of great interest of synthetic organic chemists. To probe the viability of the cyclohexane-based amine thiourea (catalyst I, namely Takemoto's catalyst), Takemoto and co-workers has identified a reaction between N-Boc benzaldimine 30 with prochiral 1,3-dicarbonyl compounds 33, furnishing quaternary-carbon containing compounds in good yield (Table 4).44
Table 4 Stereoselective Mannich reaction for the construction of quaternary stereocenter
5. Bifunctional mode on cascade reaction
Unlike classical chemical synthesis, cascade reaction offers a rather quick and efficient route to assemble complex molecular architecture in one-pot process.45 The strategy underscored the fundamental principles of biosynthesis has enabled the assembly of small organic molecules in a chemical reaction without isolating the intermediates or changing the reaction conditions. As a result, cascade reaction is considered as an atom economic pathway as it requires no protecting-group chemistry during the course of reaction. Moreover, this also greatly simplifies the synthetic steps and eventually, leads to step and cost economic if it meets the need of industrial application one day. Furthermore, owing to its operationally simple and environmentally benign approach, organocatalytic cascade reaction has evolved as a powerful tool for the total synthesis of natural product-like scaffolds as well as biologically interesting compounds in recent year.46
Primary and secondary amines are shown to activate electrophiles (LUMO-lowering) and nucleophiles (HOMO-raising) in a sequential manner through the iminium and enamine catalytic pathway respectively.47 However, the scope of reactions is bound to carbonyl-containing compounds. In view of the demand for a new template for activating non-carbonyl systems, such catalytic system is hoped to trigger off the cascade reaction between the reacting species at the same time. In contrast to enamine-iminium pathway, a bifunctional system with hydrogen-bonding capability could elso induce the electrophilicity of reagent through noncovalent interactions.48
Wang et al. has disclosed a new organocatalyzed enantioselective cascade Michael-aldol reaction towards stereochemically, highly enantio- (91–99% ee) and diastereoselective (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) complex benzothiopyrans 45 (Scheme 13).49 The reaction was accomplished with the use of 2-mercaptobenzaldehyde 43 and α,β-unsaturated oxazolidinone 44 in the presence of a cinchona-derived thiourea IV (1 mol% of catalyst loading). It is noteworthy that the desired compounds were furnished with three chiral centres in one-pot process. Highly functionalized 4-chromanones are important scaffold in medicinal chemistry.50 Our group has made an effort to undertake the development of the asymmetric synthesis of a variety of spiro-(thio)chromans 47. In the particular work, we were envisioned that dihedral angle between amine and thiourea groups is crucial for asymmetric induction and hence, a series of C1-symmetric bifunctional amine-thiourea catalysts was synthesized for investigation (Fig. 5). The bifunctional indane amine-thiourea XIV was shown to successfully promote asymmetric cyclization reaction between 2-mercaptobenzaldehyde 43 and (E)-3-benzylidenechroman-4-one 46 in a highly concise manner (Scheme 14).51
1 dr) complex benzothiopyrans 45 (Scheme 13).49 The reaction was accomplished with the use of 2-mercaptobenzaldehyde 43 and α,β-unsaturated oxazolidinone 44 in the presence of a cinchona-derived thiourea IV (1 mol% of catalyst loading). It is noteworthy that the desired compounds were furnished with three chiral centres in one-pot process. Highly functionalized 4-chromanones are important scaffold in medicinal chemistry.50 Our group has made an effort to undertake the development of the asymmetric synthesis of a variety of spiro-(thio)chromans 47. In the particular work, we were envisioned that dihedral angle between amine and thiourea groups is crucial for asymmetric induction and hence, a series of C1-symmetric bifunctional amine-thiourea catalysts was synthesized for investigation (Fig. 5). The bifunctional indane amine-thiourea XIV was shown to successfully promote asymmetric cyclization reaction between 2-mercaptobenzaldehyde 43 and (E)-3-benzylidenechroman-4-one 46 in a highly concise manner (Scheme 14).51
 |
| | Scheme 13 Hydrogen-bonding promoted cascade thio-Michael-aldol reaction. | |
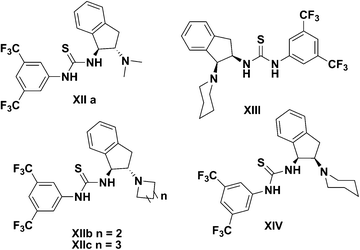 |
| | Fig. 5 Wang's group developed bifunctional indane amine-thioureas. | |
 |
| | Scheme 14 Enantioselective heterocyclic synthesis of spiro-(thio)chromans 47. | |
Malononitrile 5 serves as an important building block due to its two versatile nitrile groups in organic synthesis.52 Recently, our group has uncovered the synthetic utility of malononitrile in a Michael-oxa-Michael-tautomerization reaction by demonstrating its capability as both nucleophile and electrophile, giving rise to pyranochromenes 49 with the use of Michael acceptor 46 and malononitrile 5. It was shown that instead of the formation of simple Michael adduct 48, the reaction would favour the cascade product in the presence of an indane catalyst XIV (Scheme 15).53 Further in our recent progress, β,γ-unsaturated ketoesters 51 was used to react with α-nitroketones 50 in the presence of a rigid bifunctional thiourea-organocatalyst XIII (Scheme 16).54 The Michael adduct B was proposed to undergo hemiketalization and retro-Henry reaction to afford 5-nitro-pent-2-enoates 52, a precursor to α-ketolactams or γ-amino-α-keto acids.55
 |
| | Scheme 15 Asymmetric organocatalytic approach towards pyranochromene moiety. | |
 |
| | Scheme 16 Organocatalytic asymmetric approach to α-ketolactams or γ-amino-α-keto acids. | |
Despite the aforementioned synthetic advantages, cascade reaction is also useful for the preparation of cyclised product. Organocatalytic transformation towards medicinally useful and biologically important compounds has tremendously transformed the art of organic synthesis in the field of medicinal chemistry in recent year.56 2-Amino-4H-chromene-3-carbonitrile is an important molecular framework due to its broad application in the field of medicinal chemistry.57 To our delight, we successfully described an asymmetric version to synthesize this chromene system via an organocatalytic approach using α-amido sulfone amines 53 (Scheme 17).58
 |
| | Scheme 17 Enantioselective Mannich intramolecular ring cylization-tautomerization. | |
6. Miscellaneous reactions
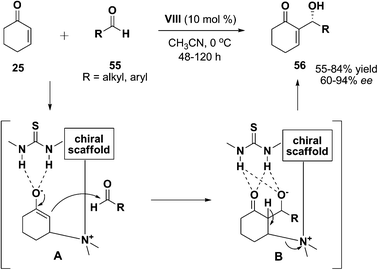
Nucleophilic unhindered tertiary amine is well-known for its performance for the Morita–Baylis–Hillman (MBH) reaction.59 By forming the double hydrogen bonding with the carbonyl compounds, bifunctional amine-thioureas could even act as suitable catalyst for the enantioselective Morita–Baylis–Hillman (MBH) reaction in organic synthesis. First bifunctional amine-thiourea catalyzed Morita–Baylis–Hillman reaction was described by Wang et al. (Scheme 18).60 The reaction was carried out with the use of cylic enones 25 as α,β-unsaturated system and aldehydes 55 in the presence of a binaphthyl-derived thiourea organocatalyst VIII. The reaction was well-tolerated with a broad range of linear aliphatic and aromatic aldehydes, furnishing the allylic alcohols in moderate to good yields (55–84%) and with moderate to excellent enantioselectivities (60–94% ee). It was proposed that the reaction proceeded via the formation of enolate, which could be stabilized by the thiourea moiety (A). In the last step, deprotonation of the α-hydrogen leaded to the elimination of the catalyst for next catalytic cycle (B).
Dynamic kinetic resolution (DKR) is a common strategy to access highly enantiopure compounds.61 This is particularly useful if the achiral starting materials are cheap and easily available. In addition, tedious and elaborative synthetic steps could be avoided without compromising the stereochemical outcome of the desired products. To probe the feasibility of bifunctional amine-thiourea on resolution, Berkessel and co-workers employed catalyst XV in a highly enantioselective DKR process of azlactones.62N- and O-protected amino acids were obtained in good to excellent yields and with excellent enantioselectivities in the presence of allylic alcohols (Table 5).
Table 5 Substrate screening using catalyst XV
| Entry |
R
|
Time/h |
Conversion (%) |
ee (%) |
|
Yield using 1.7-fold concentration of the substrates.
|
| 1 |
Me |
24 |
94 |
80 |
| 2 |
i-Pr |
48 |
89a |
90a |
| 3 |
PhCH2 |
48 |
98 |
77 |
| 4 |
i-Bu |
48 |
77 |
91 |
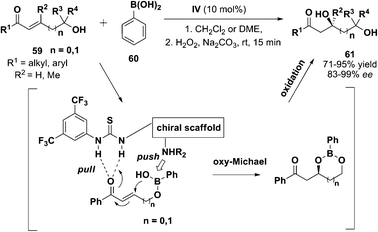
Furthermore, inspired by the bifunctionally activated push/pull system, researchers deduced that boronic esters63 could be activated to facilitate organic transformation in a stereocontrolled manner. A research group lead by Flack applied amine-thiourea to catalyze an unprecedented intramolecular oxy-Michael addition reaction (Scheme 19).64 Under the optimized condition, various γ-hydroxy-α,β-enones 59 could be converted to the desired adduct 61 in good to excellent yields (62–95%) and enantioselectivities (87–99% ee).
Another common technique used in asymmetric synthesis is desymmetrization reaction.65 Desymmetrization is an easy and quick method to tackle enantioselective synthesis when chiral center is required to be derived from a symmetrical molecule. Quinine-derived bifunctional organocatalyst IV was demonstrated to catalyze bicyclic meso-anhydrides 62 accomplishing chiral hemiester 63 at room temperature (Scheme 20).66
 |
| | Scheme 20 Enantioselective desymmetrization of bicyclic meso-anhydrides in dioxane at room temperature. | |
Diels–Alder reaction is common route to the synthesis of valuable cyclic building blocks.67 Orientation of both nucleophiles and electrophiles presents a challenging issue for Diels–Alder reaction, in particular with the use of organocatalyst.68 Amine and thiourea groups are not only responsible for HOMO–LUMO activation, induction of the rearrangement of both nucleophiles and electrophiles also play a crucial part so that other undesired isomers could be eliminated in the reaction. Intrigued by this hypothesis, Deng and co-workers described an organocatalytic asymmetric Diels–Alder reaction of 2-pyrones 74 in the presence of a cinchona-derived amine-thiourea IV and VI (Table 6).69 The reaction was successfully conducted at low temperature as well as ambient temperature within 20 h.
Table 6 Cinchona alkoid amine-thioures IV- and VI-catalyzed stereoselective Diels–Alder reactions
7. Flexible bifunctional thiourea catalysts and their application
Vinyl sulfones are synthetically useful and unique acceptors in conjugate addition.70 As a result, they are applicable in catalytic asymmetric Michael addition reactions. Chen et al. has described an enantioselective synthesis towards various sulfones or sulfonyl compounds 70 with a quaternary carbon centre using α-substituted cyanoacetates 15 and vinyl sulfones 69 in the presence of a flexible bifunctional amine-thiourea XVIa (Scheme 21).71 This was the first reaction which suggested a doubly hydrogen bonding interaction between the NH of the thiourea and the sulfone functionality.
 |
| | Scheme 21 Enantioselective construction of quaternary carbon compounds 70. | |
Functionalized coumarins exhibited a wide range of biological properties.72 For instance, warfarin, bromodialone and phenprocoumon are coumarin-based anticoagulant drug that are commonly found in medicinal chemistry. However, they are formulated as racemate. Synthesis to afford one of the enantiomers is highly desirable as this could avoid potential side-effect arising from the metabolism of another enantiomer.73 To address this issue, our group was trying to make an effort to it. Delightfully, a flexible catalyst like XVII was developed in our group and was shown to catalyze an enantioselective reaction between 4-hydroxy-2H-chromen-2-one 71 and β,γ-unsaturated ketoesters 51 (Scheme 22).74 Dual characteristics possessed by the reacting molecules allowed the cascade reaction to take place smoothly, affording the final product 72 in the one-pot process.
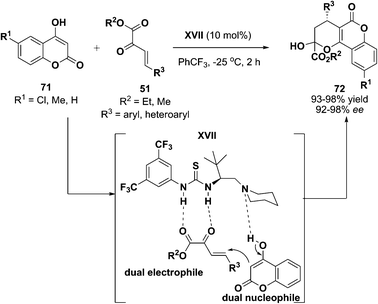 |
| | Scheme 22 Enantioselective synthesis of coumarins. | |
8. Immobilized bifunctional amine-thiourea catalysts
In 2006, Takemoto and co-workers reported case studies towards the immobilization of bifunctional thiourea on polymer support using an ester moiety as the linker group.75a It was shown that soluble ester-functionalized thiourea derivative XVIII (10 mol% loading) turned out to catalyze the model Michael addition of diethyl malonate 2 to tran-β-nitrostyrene 1 in 88% yield and in 91% enantioselectivity after 48 h in toluene. The insoluble crosslinked polystyrene-bound thiourea derivatives XIX-1 and XIX-2 exhibited a drastically reduced catalytic activity and gave the desired (S)-configured Michael adduct after 6 d at room temperature in 37% and 4% yield (87% and 88% ee, respectively, Scheme 23). On the other hand, soluble poly(ethylene glycol) (PEG)-bound thiourea XIX-3 could be used in dichloromethane under homogeneous condition and able to afford Michael adduct in 71% yield and 86% ee after 6 d at room temperature. The catalyst was readily recovered by filtration after addition of diethyl ether to the reaction mixture and could be reused without further purification to give the same adduct in comparable yield (74%) and ee value (90%) in second run. PEG-bound thiourea XIX-3 also catalyzed the enantioselective double Michael addition of a γ,δ-unsaturated β-ketoester 73 to trans-β-nitrostyrene 1, resulting in the desired 4-nitrocyclohexanone derivative 82 (63% yield; 76% ee; rt, 6 d). No impact on catalytic activity of the catalyst (second run: 64% yield; 76% ee third run: 63% yield; 79% ee) even though it was reused in this reaction.75b,c
9. Summary
Asymmetric organocatalysis using bifunctional amine-thioureas has been presented in this review. The success of bifunctional catalysis which works cooperatively between double hydrogen bonding character of thiourea moiety and basic amine group has underscored the fundamental principle of biocatalysis in which both nucleophile and electrophile are activated to bring about a chemical reaction. These catalysts could also be derived from readily available privileged scaffolds. Another highlight of using bifunctional system to catalyze a chemical reaction is the cascade reaction. Complex molecules could be generated in an efficient way and environmentally friendly manner. Last but not least, case study pertaining to the use of immobilization technique of the bifunctional amine-thiourea onto polymer-support surface was also reviewed. Much has already been understood, however, other breakthrough using bifunctional amine-thioureas is anticipated in the near future.
Acknowledgements
We gratefully acknowledge the National University of Singapore for financial support of our work (Academic Research Grant: R143000408133, R143000408733 and R143000443112).
References
- For reviews and books: see:
(a) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2001, 40, 3726 CrossRef CAS;
(b) M. Benaglia, A. Puglisi and F. Cozzi, Chem. Rev., 2003, 103, 3401 CrossRef CAS;
(c)
A. Berkessel and H. GrMger, Metal-Free Organic Catalysts in Asymmetric Synthesis, Wiley-VCH, Weinheim, 2004 Search PubMed;
(d) “Special Issue: Asymmetric Organocatalysis”, Acc. Chem. Res. 2004, 37, 487;
(e) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS;
(f) J. T. Mohr, M. R. Krout and B. M. Stoltz, Nature, 2008, 455, 323 CrossRef CAS;
(g) D. W. C. MacMillan, Nature, 2008, 455, 304 CrossRef CAS;
(h)
A. Berkessel and H. Groger, Asymmetric Organocatalysis, Wiley, Weinheim, 2005 Search PubMed;
(i)
P. I. Dalko, Enantioselective Organocatalysis, Wiley, Weinheim, 2007 Search PubMed;
(j)
M. T. Reetz, B. List, S. Jaroch and H. Weinmann, Organocatalysis, Springer, 2007 Search PubMed;
(k)
B. List, Asymmetric Organocatalysis, Springer, 2009 Search PubMed.
-
(a) M. Sigman and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 4901 CrossRef CAS;
(b) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289 RSC;
(c) P. M. Pihko, Angew. Chem., Int. Ed., 2004, 43, 2062 CrossRef CAS;
(d) M. Oestreich, Nachr. Chem., 2004, 52, 35 Search PubMed;
(e) C. Bolm, T. Rantanen, I. Schiffers and L. Zani, Angew. Chem., Int. Ed., 2005, 44, 1758 CrossRef CAS.
- For selected examples of other bifunctional systems, see:
(a) H. Gröger, Chem.–Eur. J., 2001, 7, 5246 CrossRef CAS;
(b) M. Shibasaki and N. Yoshikawa, Chem. Rev., 2002, 102, 2187 CrossRef CAS;
(c) J.-A. Ma and D. Cahard, Angew. Chem., Int. Ed., 2004, 43, 4566 CrossRef CAS;
(d) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS;
(e) H. Yamamoto and K. Futatsugi, Angew. Chem., Int. Ed., 2005, 44, 1924 CrossRef CAS;
(f) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS.
- For selected examples of cinchona-derivatives as an organocatalyst, see:
(a) Y. Iwabuchi, M. Nakatani, N. Yokoyama and S. Hatakeyama, J. Am. Chem. Soc., 1999, 121, 10219 CrossRef CAS;
(b) K. Kacprzak and J. Gawronski, Synthesis, 2001, 961 CrossRef CAS;
(c) S. France, D. J. Guerin, S. J. Miller and T. Lectka, Chem. Rev., 2003, 103, 2985 CrossRef CAS;
(d) S.-K. Tian, Y. Chen, J. Hang, L. Tang, P. McDaid and L. Deng, Acc. Chem. Res., 2004, 37, 621 CrossRef CAS;
(e) H. Li, Y. Wang, L. Tang and L. Deng, J. Am. Chem. Soc., 2004, 126, 9906 CrossRef CAS;
(f) X. Liu, H. Li and L. Deng, Org. Lett., 2005, 7, 167 CrossRef CAS;
(g) T. Marcelli, J.-H. Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2006, 45, 7496 CrossRef CAS.
- Detailed discussion on hydrogen-bonding, see:
(a)
G. C. Pimentel and A. L. McClellan, The Hydrogen Bond, Freeman, San Francisco, 1960 Search PubMed;
(b)
L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, 3rd edn, 1968 Search PubMed;
(c)
G. A. Jeffrey and W. Saenger, Hydrogen Bonding in Biological Structures, Springer, Berlin, 1991 Search PubMed;
(d) Y.-M. Chook, J. V. Gray, H. Ke and W. N. Lipscomb, J. Mol. Biol., 1994, 240, 476 CrossRef CAS;
(e) A. Y. Lee, P. A. Karplus, B. Ganem and J. Clardy, J. Am. Chem. Soc., 1995, 117, 3627 CrossRef CAS;
(f) C.-H. Wong, R. H. Halcomb, Y. Ichikawa and T. Kajimoto, Angew. Chem., Int. Ed. Engl., 1995, 34, 412 CrossRef CAS;
(g)
G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, Oxford, 1999 Search PubMed;
(h) T. D. Machajewski and C.-H. Wong, Angew. Chem., Int. Ed., 2000, 39, 1352 CrossRef CAS.
-
(a) A. J. White and C. W. Wharton, Biochem. J., 1990, 270, 627 CAS;
(b) P. Bryan, W. Pantoliano, S. G. Quill, H.-Y. Hsiao and T. Poulos, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 3743 CAS.
-
K. Brocklehurst, A. B. Watts, M. Patel, C. S. Verma and E. W. Thomas, Cysteine proteinases, in Comprehensive Biological Catalysis, ed. M. L. Sinnott, London, 1998, vol. 1 Search PubMed.
-
(a) T. Okino, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2003, 125, 12672 CrossRef CAS;
(b) T. Okino, Y. Hoashi, T. Furukawa, X.-N. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119 CrossRef CAS.
- For metal catalysis, see:
(a) J. Ji, D. M. Barnes, J. Zhang, S. A. King, S. J. Wittenberger and H. E. Morton, J. Am. Chem. Soc., 1999, 121, 10215 CrossRef CAS;
(b) C. A. Luchaco-Cullis and A. H. Hoveyda, J. Am. Chem. Soc., 2002, 124, 8192 CrossRef CAS;
(c) A. Alexakis, C. Benhaim, S. Rosset and M. Humam, J. Am. Chem. Soc., 2002, 124, 5262 CrossRef CAS;
(d) D. M. Barnes, J. Ji, M. G. Fickes, M. A. Fitzgerald, S. A. King, H. E. Morton, F. A. Plagge, M. Preskill, S. H. Wagaw, S. J. Wittenberger and J. Zhang, J. Am. Chem. Soc., 2002, 124, 13097 CrossRef CAS;
(e) M. Watanabe, A. Ikagawa, H. Wang, K. Murata and T. Ikariya, J. Am. Chem. Soc., 2004, 126, 11148 CrossRef CAS.
- A. Hamza, G. Schubert, T. Soós and I. Pápai, J. Am. Chem. Soc., 2006, 128, 13151 CrossRef CAS.
- For metal-catalyzed Michael addition of malononitriles, see:
(a) K. Itoh and S. Kanemasa, J. Am. Chem. Soc., 2002, 124, 13394 CrossRef CAS;
(b) K. Itoh, Y. Oderaotoshi and S. Kanemasa, Tetrahedron: Asymmetry, 2003, 14, 635 CrossRef CAS;
(c) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2003, 125, 11204 CrossRef CAS;
(d) I. T. Raheem, S. N. Goodman and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 706 CrossRef;
(e) S. Kanemasa and K. Ito, Eur. J. Org. Chem., 2004, 4741 CrossRef CAS.
-
(a) R. Helder, R. Arends, W. Bolt, H. Hiemstra and H. Wynberg, Tetrahedron Lett., 1977, 18, 2181 CrossRef;
(b) H. Hiemstra and H. Wynberg, J. Am. Chem. Soc., 1981, 103, 417 CrossRef CAS.
- For the works on nitroalkanes, see:
(a)
N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001 Search PubMed;
(b) R. Ballini, G. Bosica, D. Fiorini, A. Palmieri and M. Petrini, Chem. Rev., 2005, 105, 933 CrossRef CAS.
- B. Vakulya, S. Varga, A. Csámpai and T. Soós, Org. Lett., 2005, 7, 1967 CrossRef CAS.
- For Corey's work on (R)-Baclofen synthesis: E. J. Corey and F.-Y. Zhang, Org. Lett., 2000, 2, 4257 Search PubMed.
- J. Wang, H. Li, L. Zu and W. Wang, Org. Lett., 2005, 7, 4713 CrossRef CAS.
- Organocatalytic, enantioselective Mannich-type reactions for the preparation of α- and β-amino acids, see:
(a) A. Córdova, W. Notz, G. Zhong, J. M. Betancort and C. F. Barbas III, J. Am. Chem. Soc., 2002, 124, 1842 CrossRef CAS;
(b) A. Córdova, S. Watanabe, F. Tanaka, W. Notz and C. F. Barbas III, J. Am. Chem. Soc., 2002, 124, 1866 CrossRef CAS;
(c) W. Wang, J. Wang and H. Li, Tetrahedron Lett., 2004, 45, 7243 CrossRef CAS;
(d) J. M. M. Verkade, L. J. C. van Hemert, P. J. M. Quaedflieg and F. P. J. T. Rutjes, Chem. Soc. Rev., 2008, 37, 29 RSC;
(e) R. G. Arrayás and J. C. Carretero, Chem. Soc. Rev., 2009, 38, 1940 RSC.
- For works on β-amino acids, see:
(a) S. Abele and D. Seebach, Eur. J. Org. Chem., 2000, 1 CrossRef CAS;
(b) A. G. Wenzel and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 12964 CrossRef CAS;
(c) M. Liu and M. P. Sibi, Tetrahedron, 2002, 58, 7991 CrossRef CAS;
(d) D. J. Guerin and S. J. Miller, J. Am. Chem. Soc., 2002, 124, 2134 CrossRef CAS;
(e) J.-A. Ma, Angew. Chem., Int. Ed., 2003, 42, 4290 CrossRef CAS;
(f) N. Sewald, Angew. Chem., Int. Ed., 2003, 42, 5794 CrossRef CAS;
(g)
A. G. Wenzel and E. N. Jacobsen, Asymmetric Catalysis in Enantioselective Synthesis of b-Amino Acids, Harvard Univ., Cambridge, MA 02138, USA Search PubMed;
(h) M. P. Sibi and K. Patil, Angew. Chem., Int. Ed., 2004, 43, 1235 CrossRef CAS;
(i) M. P. Sibi, H. Tatamidani and K. Patil, Org. Lett., 2005, 7, 2571 CrossRef CAS;
(j)
E. Juaristi, Enantioselective Synthesis of β-Amino Acids, Wiley, New York, 2005 Search PubMed.
- T.-Y. Li, Q. Chai, J. Long, B.-J. Li, Y. Wu, L.-S. Ding and Y.-C. Chen, Chem.–Eur. J., 2007, 13, 319 CrossRef.
- For examples by using chiral auxiliary strategies see:
(a) C. Cativiela, M. D. Dĺaz-de-Villegas and J. A. Gálvez, J. Org. Chem., 1994, 59, 2497 CrossRef CAS;
(b) R. Badorrey, C. Cativiela, M. D. Dĺaz-de-Villegas, J. A. Gálvez and Y. Lapea, Tetrahedron: Asymmetry, 1997, 8, 311 CrossRef CAS;
(c) A. Avenoza, C. Cativiela, M. Paris and J. M. Peregrina, Tetrahedron: Asymmetry, 1995, 6, 1409 CrossRef CAS.
- Diastereoselective hydroxylation: N. J. Adderly, D. J. Buchanan, D. J. Dixon and D. I. Lainé, Angew. Chem., Int. Ed., 2003, 42, 4241 Search PubMed.
- S. Bertelsen, P. Dinér, R. L. Johansen and K. A. Jørgensen, J. Am. Chem. Soc., 2007, 129, 1536 CrossRef CAS.
- Recent reviews on oxa-Michael reactions:
(a) C. F. Nising and S. Bräse, Chem. Soc. Rev., 2012 10.1039/C1CS15167C;
(b) C. F. Nising and S. Bräse, Chem. Soc. Rev., 2008, 37, 1218 RSC.
- P. Dinér, M. Nielsen, S. Bertelsen, B. Niess and K. A. Jørgensen, Chem. Commun., 2007, 3646 RSC.
- For synthesis of N-heterocycles, see:
(a)
A. R. Katritzky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, Pergamon, Oxford, UK, 2nd edn, 2002 Search PubMed;
(b)
J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell Science, Oxford, UK, 4nd edn, 2000 Search PubMed;
(c)
T. Eicher and S. Hauptmann, The Chemistry of Heterocylces, Wiley-VCH, New York, 2nd edn, 2003 Search PubMed;
(d) M. G. M. Purwanto and K. Weisz, Curr. Org. Chem., 2003, 7, 427 CAS.
- For asymmetric works on N-heterocycles, see
(a) J. K. Myers and E. N. Jacobsen, J. Am. Chem. Soc., 1999, 121, 8959 CrossRef CAS;
(b) T. E. Hortsmann, D. J. Guerin and S. J. Miller, Angew. Chem., Int. Ed., 2000, 39, 3635 CrossRef;
(c) G. M. Sammis and E. N. Jacobsen, J. Am. Chem. Soc., 2003, 125, 4442 CrossRef CAS;
(d) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2003, 125, 11204 CrossRef CAS;
(e) C. D. Vanderwal and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 14724 CrossRef CAS;
(f) S. J. Miller, Acc. Chem. Res., 2004, 37, 601 CrossRef CAS;
(g) M. Gandelman and E. N. Jacobsen, Angew. Chem., Int. Ed., 2005, 44, 2393 CrossRef CAS.
- J. Wang, L. Zu, H. Li, H. Xie and W. Wang, Synthesis, 2007, 2576 CrossRef CAS.
- For organo-sulfur chemistry, see
(a)
A. Nudelman, The Chemistry of Optically Active Sulfur Compounds, Gordon and Breach, New York, 1984 Search PubMed;
(b)
C. Chatgilialoglu and K.-D. Asmus, Sulfur-Centered Reactive Intermediates in Chemistry and Biology, Springer, New York, 1991 Search PubMed;
(c)
P. Metzner and A. Thuillier, Sulfur Reagents in Organic Synthesis, Academic Press, New York, 1994 Search PubMed;
(d) D. Enders, K. Lüttgen and A. A. Narine, Synthesis, 2007, 959 CrossRef CAS.
- For examples of organometallics catalyzed Michael addition of thiols, see:
(a) M. Zielinska-Błajet, R. Kowalczyk and J. Skarzewski, Tetrahedron, 2005, 61, 5235 CrossRef CAS;
(b) E. Emori, T. Arai, H. Sasai and M. Shibasaki, J. Am. Chem. Soc., 1998, 120, 4043 CrossRef CAS;
(c) K. Nishimura, M. Ono, Y. Nagaoka and K. Tomioka, J. Am. Chem. Soc., 1997, 119, 12974 CrossRef CAS;
(d) S. K. Garg, R. Kumar and A. K. Chakraborti, Tetrahedron Lett., 2005, 46, 1721 CrossRef CAS.
- Y. Chen, B. Li, L. Jiang, M. Liu, L. Ding and Y. Wu, Synlett, 2005, 4, 603.
- H. Li, L. Zu, J. Wang and W. Wang, Tetrahedron Lett., 2006, 47, 3145 CrossRef CAS.
- For metal-catalyzed Mannich-type reactions, see:
(a) D. Ferraris, B. Young, C. Cox, T. Dudding, W. J. III. Drury, L. Ryzhkov, T. Taggi and J. Lectka, J. Am. Chem. Soc., 2002, 124, 67 CrossRef CAS;
(b) B. M. Trost and L. M. Terrell, J. Am. Chem. Soc., 2003, 125, 4712 CrossRef CAS;
(c) B. Westermann, Angew. Chem., Int. Ed., 2003, 42, 151 CrossRef CAS;
(d) A. Ting and S. E. Schaus, Eur. J. Org. Chem., 2007, 5797 CrossRef CAS for organocatalytic Mannich, see: ;
(e) B. J. List, J. Am. Chem. Soc., 2000, 122, 9336 CrossRef CAS.
- For selected nitro-Mannich reactions:
(a) J. C. Anderson, A. J. Blake, G. P. Howell and C. Wilson, J. Org. Chem., 2005, 70, 549 CrossRef CAS for catalytic nitro-Mannich, see: ;
(b) K. Yamada, S. J. Harwood, H. Groger and M. Shibasaki, Angew. Chem., Int. Ed., 1999, 38, 3504 CrossRef CAS;
(c) K. Y. Yamada, G. Moll and M. Shibasaki, Synlett, 2001, 980 CrossRef CAS;
(d) B. Westermann, Angew. Chem., Int. Ed., 2003, 42, 151 CrossRef CAS.
- Previous work:
(a) C.-J. Wang, Z.-H. Zhang, X.-Q. Dong and X.-J. Wu, Chem. Commun., 2008, 1431 RSC;
(b) C.-J. Wang, X.-Q. Dong, Z.-H. Zhang, Z. Y. Xue and X.-L. Teng, J. Am. Chem. Soc., 2008, 130, 8606 CrossRef CAS.
- J. Song, Y. Wang and L. Deng, J. Am. Chem. Soc., 2006, 128, 6048 CrossRef CAS.
- A. L. Tillman, J. Yeb and D. J. Dixon, Chem. Commun., 2006, 1191 RSC.
- Copper-catalyzed Friedel–Crafts reactions, see:
(a) M. Johannsen, Chem. Commun., 1999, 2233 RSC;
(b) J. Hao, S. Taktak, K. Aikawa, Y. Yusa, M. Hatano and K. Mikami, Synlett, 2001, 1443 CrossRef CAS;
(c) X.-L. Mi, S.-Z. Luo, J.-Q. He and J.-P. Cheng, Tetrahedron Lett., 2004, 45, 4567 CrossRef CAS;
(d) J. Esquivias, R. M. Arrayas and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 629 CrossRef CAS;
(e) Y. Jia, J. Xie, H. Duan, L. Wang and Q. Zhou, Org. Lett., 2006, 8, 1621 CrossRef CAS . For organo-catalyzed Friedel–Crafts reactions, see: ;
(f) Y. Sohtome, B. Shin, N. Horitsugi, R. Takagi and K. Noguchi, Angew. Chem., Int. Ed., 2010, 49, 7299 CrossRef CAS;
(g) D.-P. Li, Y. C. Guo, Y. Ding and W.-J. Xiao, Chem. Commun., 2006, 799 RSC;
(h) E. M. Fleming, T. McCabe and S. J. Connon, Tetrahedron: Asymmetry, 2006, 17, 3153;
(i) G. Bartoli, M. Bosco, A. Carlone, F. Pesciaioli and L. Sambri, Org. Lett., 2007, 9, 1403 CrossRef CAS;
(j) D. B. Ramachary, G. B. Reddy and R. Mondal, Tetrahedron Lett., 2007, 48, 7618 CrossRef CAS;
(k) S. Lee and D. W. C. MacMillan, J. Am. Chem. Soc., 2007, 129, 15438 CrossRef CAS;
(l) J. A. McCubbin and O. V. Krokhin, Tetrahedron Lett., 2010, 51, 2447 CrossRef CAS;
(m) V. Terrasson, R. Marcia de Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 14, 2635;
(n) D. Enders, M. Seppelt and T. Beck, Adv. Synth. Catal., 2010, 352, 1413 CrossRef CAS;
(o) G. Liu, S. Zhang, H. Li, T. Zhang and W. Wang, Org. Lett., 2011, 13, 828 CrossRef CAS;
(p) P. Chauhan and S. S. Chimni, Eur. J. Org. Chem., 2011, 1636 CrossRef CAS.
- Y.-Q. Wang, J. Song, R. Hong, H. Li and L. Deng, J. Am. Chem. Soc., 2006, 128, 8156 CrossRef CAS.
- For Jacobsen's selected works:
(a) M. S. Sigman and E. N. Jacobsen, J. Am. Chem. Soc., 1998, 120, 4901 CrossRef CAS;
(b) M. S. Sigman, P. Vachal and E. N. Jacobsen, Angew. Chem., Int. Ed., 2000, 39, 1279 CrossRef CAS;
(c) P. Vachal and E. N. Jacobsen, Org. Lett., 2000, 2, 867 CrossRef CAS;
(d) P. Vachal and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 10012 CrossRef CAS;
(e) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 10558 CrossRef CAS.
- For synthetic utility of cyanohydrins: R. J. H. Gregory, Chem. Rev., 1999, 99, 3649 Search PubMed.
- For development of cyanation chemistry, see
(a) J.-M. Brunel and I. P. Holmes, Angew. Chem., Int. Ed., 2004, 43, 2752 CrossRef CAS;
(b) Y. Hamashima, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2000, 122, 7412 CrossRef CAS;
(c) H. Deng, M. P. Isler, M. L. Snapper and A. M. Hoveyda, Angew. Chem., Int. Ed., 2002, 41, 1009 CrossRef CAS;
(d) F. Chen, F. X. Feng, B. Qin, B. G. Zhang and Y. Jiang, Org. Lett., 2003, 5, 949 CrossRef CAS;
(e) S.-K. Tian, R. Hong and L. Deng, J. Am. Chem. Soc., 2003, 125, 9900 CrossRef CAS;
(f) D. H. Riu and E. J. Corey, J. Am. Chem. Soc., 2005, 127, 5384 CrossRef CAS.
- E. F. Douglas and E. N. Jacobsen, J. Am. Chem. Soc., 2005, 127, 8964 CrossRef CAS.
- For recent reviews on catalytic enantioselective construction of all carbon quaternary chiral centers see:
(a) J. Christoffers and A. Baro, Adv. Synth. Catal., 2005, 347, 1473 CrossRef CAS;
(b) E. A. Peterson and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101(11), 943;
(c) C. J. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363 CrossRef CAS;
(d) D. J. Ramon and M. Yus, Curr. Org. Chem., 2004, 8, 149 CrossRef CAS.
- Y. Yamaoka, H. Miyabe, Y. Yasui and Y. Takemoto, Synthesis, 2007, 16, 2571.
- For selected reviews of organocatalytic cascade reactions, see:
(a) T. Dudding, A. M. Hafez, A. E. Taggi, T. R. Wagerle and T. Lectka, Org. Lett., 2002, 4, 387 CrossRef CAS;
(b) Y. Yamamoto, N. Momiyama and H. Yamamoto, J. Am. Chem. Soc., 2004, 126, 5962 CrossRef CAS;
(c) J. W. Yang, M. T. Hechavarria Fonseca and B. List, J. Am. Chem. Soc., 2005, 127, 15036 CrossRef CAS;
(d) M. Marigo, T. Schulte, J. Franzen and K. A. Jørgensen, J. Am. Chem. Soc., 2005, 127, 15710 CrossRef CAS;
(e) D. Enders, M. R. M. Huttl, C. Grondal and G. Raabe, Nature, 2006, 441, 861 CrossRef CAS;
(f) Y. Wang, X.-F. Liu and L. Deng, J. Am. Chem. Soc., 2006, 128, 3928 CrossRef CAS;
(g) H. Sundén, I. Ibrahem, G.-L. Zhao and L. Eriksson, Chem.–Eur. J., 2007, 13, 574 CrossRef CAS;
(h) W. Wang, H. Li, J. Wang and L. Zu, J. Am. Chem. Soc., 2006, 128, 10345;
(i) P. Galzerano, F. Pesciaioli, A. Mazzanti, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 7892 CrossRef CAS;
(j) L.-Q. Li, Y.-J. Cao, X.-P. Liu, J. An, C.-J. Yao, Z.-H. Ming and W.-J. Xiao, J. Am. Chem. Soc., 2008, 130, 6946 CrossRef CAS;
(k) L. Albrecht, H. Jiang, G. Dickmeiss, B. Gschwend, S. G. Hansen and K. A. Jørgensen, J. Am. Chem. Soc., 2010, 132, 9188 CrossRef CAS.
-
(a) G. Luppi, M. Monari, R. J. Correa, F. de A. Violante, A. C. Pinto, B. Kaptein, Q. B. Broxterman, S. J. Garden and C. Tomasini, Tetrahedron, 2006, 62, 12017 CrossRef CAS;
(b) T. Itoh, M. Yokoya, K. Miyauchi, K. Nagata and A. Ohsawa, Org. Lett., 2006, 8, 1533 CrossRef CAS;
(c) A. S. Paraskar and A. Sudulai, Tetrahedron, 2006, 62, 5756 CrossRef CAS;
(d) K. C. Nicolaou, D. Sarlah and D. M. Shaw, Angew. Chem., Int. Ed., 2007, 46, 4708 CrossRef CAS;
(e) J. W. Yang, M. T. Hechavarria Fonseca and B. List, Angew. Chem., Int. Ed., 2004, 43, 6660 CrossRef;
(f) J. W. Yang, M. T. Hechavarria Fonseca, N. Vignola and B. List, Angew. Chem., Int. Ed., 2005, 44, 108 CrossRef CAS;
(g) S. Mayer and B. List, Angew. Chem., Int. Ed., 2006, 45, 4193 CrossRef CAS;
(h) C. Grondal and D. Enders, Adv. Synth. Catal., 2007, 349, 694 CrossRef CAS;
(i) D. Enders and T. Gaspari, Chem. Commun., 2007, 88 RSC;
(j) X. Xu, T. Furukawa, T. Okino, H. Miyabe and Y. Takemoto, Chem.–Eur. J., 2006, 12, 466 CrossRef;
(k) X.-H. Chen, X.-Y. Xu, H. Liu, L.-F. Cun and L.-Z. Gong, J. Am. Chem. Soc., 2006, 128, 14802 CrossRef CAS.
- For the reviews on the work of amine-based organocatalysts, see:
(a) P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef CAS;
(b) L.-W. Xu, J. Luo and Y. Lu, Chem. Commun., 2009, 1807 RSC;
(c) L. Jiang and Y.-C. Chen, Catal. Sci. Technol., 2011, 1, 354 RSC.
- For the review on carbonyl compounds activation: P. M. Pihko, Angew. Chem., Int. Ed., 2004, 43, 2062 Search PubMed.
- L. Zu, J. Wang, H. Xie and W. Wang, J. Am. Chem. Soc., 2007, 129, 1036 CrossRef CAS.
- Books and selected examples of applications of chromanone:
(a)
F. M. Dean, Naturally Occurring Oxygen Ring Compounds, Butterworths, London, 1963 Search PubMed;
(b)
G. P. Ellis, Chromans and Tocopherols, Wiley, New York, 1977 Search PubMed;
(c) S. T. Saengchantara and T. W. Wallace, Nat. Prod. Rep., 1986, 3, 465 RSC.
- Y. Gao, Q. Ren, H. Wu, M. Li and J. Wang, Chem. Commun., 2010, 46, 9232 RSC.
- For reactions using malononitrile, see:
(a) I. T. Raheem, S. N. Goodman and E. N. Jaconsen, J. Am. Chem. Soc., 2004, 126, 706 CrossRef;
(b) M. S. Taylor and E. N. Jacobsen, J. Am. Chem. Soc., 2003, 125, 11204 CrossRef CAS;
(c) K. Itoh and S. Kanemasa, J. Am. Chem. Soc., 2002, 124, 13394 CrossRef CAS;
(d) S. Kanemasa and K. Itoh, Eur. J. Org. Chem., 2004, 4741 CrossRef CAS.
- Q. Ren, Y. Gao and J. Wang, Chem.–Eur. J., 2010, 16, 13594 CrossRef CAS.
- Y. Gao, Q. Ren, W.-Y. Siau and J. Wang, Chem. Commun., 2011, 47, 5819 RSC.
- Review on ketoacids, see: A. J. L. Cooper, J. Z. Ginos and A. Meister, Chem. Rev., 1983, 83, 321 Search PubMed.
- For works on cascade reactions,
(a) B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. G. Lotti, D. J. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfield, J. Med. Chem., 1988, 31, 2235 CrossRef CAS;
(b) J. Poupaert, P. Carato and E. Colacino, Curr. Med. Chem., 2005, 12, 877 CrossRef CAS;
(c) D. J. Triggle, Cell. Mol. Neurobiol., 2003, 23, 293 CrossRef CAS;
(d) A. A. Patchett and R. P. Nargund, Annu. Rep. Med. Chem., 35, 289 Search PubMed;
(e) L. A. Thompson, Curr. Opin. Chem. Biol., 2000, 4, 324 CrossRef CAS;
(f) A. Nefzi, J. M. Ostresh and R. A. Houghten, Chem. Rev., 1997, 97, 449 CrossRef CAS.
-
(a) J. L. Wang, D. Liu, Z. Zhang, S. Shan, X. Han, S. M. Srinvasula, C. M. Croce, E. S. Alnemeri and Z. Huang, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7124 CrossRef CAS;
(b) W. Kemnitzer, J. Drewe, S. Jiang, H. Zhang, Y. Wang, J. Zhao, S. Jia, J. Herich, D. Labreque, R. Storer, K. Meerovitch, D. Bouffard, R. Rej, R. Denis, C. Blais, S. Lamothe, G. Attardo, H. Gourdeau, B. Tseng, S. Kasibhatla and S. X. Cai, J. Med. Chem., 2004, 47, 6299 CrossRef CAS;
(c) D. R. Anderson, S. Hegde, E. Reinhard, L. Gomez, W. F. Vernier, L. Lee, S. Liu, A. Sambandam, P. A. Snider and L. Masih, Bioorg. Med. Chem. Lett., 2005, 15, 1587 CrossRef CAS;
(d) W. Kemnitzer, S. Kasibhatla, S. Jiang, H. Zhang, J. Zhao, S. Jia, L. Xu, C. Crogan-Grundy, R. Denis, N. Barriault, L. Vaillancourt, S. Charron, J. Dodd, G. Attardo, D. Labrecque, S. Lamothe, H. Gourdeau, B. Tseng, J. Drewe and S. X. Cai, Bioorg. Med. Chem. Lett., 2005, 15, 4745 CrossRef CAS.
- Q. Ren, W.-Y. Siau, Z. Y. Du, K. Zhang and J. Wang, Chem.–Eur. J., 2011, 17, 7781 CrossRef CAS.
- For recent reviews on MBH reaction:
(a) G.-N. Ma, J.-J. Jiang, M. Shi and Y. Wei, Chem. Commun., 2009, 5496 RSC;
(b) G. Masson, C. Housseman and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 4614 CrossRef CAS;
(c) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS;
(d) P. Langer, Angew. Chem., Int. Ed., 2000, 39, 3049 CrossRef CAS.
- J. Wang, H. Li, X. Yu, L. Zu and W. Wang, Org. Lett., 2005, 7, 4293 CrossRef CAS.
-
(a) H. B. Kagan and J. C. Fiaud, Top. Stereochem., 18, 249 Search PubMed;
(b) K. Faber, Chem.–Eur. J., 2001, 7, 5004 CrossRef CAS;
(c) H. Pellissier, Tetrahedron, 2003, 59, 8291 CrossRef CAS.
- A. Berkessel, S. Mukherjee, F. Cleemann, T. N. Muller and J. Lex, Chem. Commun., 2005, 1898 RSC.
- For enantioselective works using boronate, see:
(a) Y. Yamaoka, H. Miyabe and Y. Takemoto, J. Am. Chem. Soc., 2007, 129, 6686 CrossRef CAS;
(b) J. A. Bishop, S. Lou and S. E. Schaus, Angew. Chem., Int. Ed., 2009, 48, 4337 CrossRef CAS;
(c) P. N. Moquist, T. Kodama and S. E. Schaus, Angew. Chem., Int. Ed., 2010, 49, 7096 CrossRef CAS.
- D.-R. Li, A. Murugan and J. R. Falck, J. Am. Chem. Soc., 2008, 130, 46 CrossRef CAS.
- Organocatalytc asymmetric desymmetrization reactions, see:
(a) C. E. Müller and P. R. Schreiner, Angew. Chem., Int. Ed., 2011, 50, 6012 CrossRef;
(b) G. Dickmeiss, V. De Sio, J. Udmark, T. B. Poulsen, V. Marcos and K. A. Jørgensen, Angew. Chem., Int. Ed., 2009, 48, 6650 CrossRef CAS;
(c) Q. Gu, Z.-Q. Rong, C. Zheng and S.-L. You, J. Am. Chem. Soc., 2010, 132, 4056 CrossRef CAS;
(d) Q. Liu and T. Rovis, J. Am. Chem. Soc., 2006, 128, 2552 CrossRef CAS;
(e) S. Mizuta, M. Sadamori, T. Fujimoto and I. Yamamoto, Angew. Chem., Int. Ed., 2003, 42, 3383 CrossRef CAS;
(f) K. Mori, T. Katoh, T. Suzuki, T. Noji, M. Yamanaka and T. Akiyama, Angew. Chem., Int. Ed., 2009, 48, 9652 CAS;
(g) K. Mikami, S. Narisawa, M. Shimizu and M. Terada, J. Am. Chem. Soc., 1992, 114, 6566 CrossRef CAS.
- H. S. Rho, S. H. Oh, J. W. Lee, J. Y. Lee, J. Chin and C. E. Song, Chem. Commun., 2008, 1208 RSC.
- For reviews, see:
(a) K. Afarinkia, V. Vinader, T. D. Nelson and G. H. Posner, Tetrahedron, 1992, 48, 9111 CrossRef CAS;
(b) B. T. Woodard and G. H. Posner, Adv. Cycloaddit., 1999, 5, 47 Search PubMed.
- For studies on hydrogen-bond-promoted asymmetric Diels–Alder reaction, see:
(a) Y. Huang, A. K. Unni, A. N. Thadani and V. H. Rawal, Nature, 2003, 424, 146 CrossRef CAS;
(b) A. K. Unni, N. Takenaka, H. Yamamoto and V. H. Rawal, J. Am. Chem. Soc., 2005, 127, 1336 CrossRef CAS . For a review of hydrogen bond-based asymmetric catalysis, see: ;
(c) M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS . For other asymmetric Diels–Alder reactions by organocatalysts, see: ;
(d) K. A. Ahrendt, C. J. Borths and D. W. C. MacMillan, J. Am. Chem. Soc., 2000, 122, 4243 CrossRef CAS;
(e) K. Juhl and K. A. Jørgensen, Angew. Chem., Int. Ed., 2003, 42, 1498 CrossRef CAS;
(f) D. B. Ramachary, N. S. Chowdari and C. F. Barbas III, Angew. Chem., Int. Ed., 2003, 42, 4233 CrossRef CAS;
(g) M. He, J. R. Struble and J. W. Bode, J. Am. Chem. Soc., 2006, 128, 8418 CrossRef CAS;
(h) J. Shen, T. T. Nguyen, Y.-P. Goh, W. Ye, X. Fu, J. Xu and C.-H. Tan, J. Am. Chem. Soc., 2006, 128, 13692 CrossRef CAS;
(i) J. Wolfer, T. Bekele, C. J. Abraham, C. Dogo-Isonagie and T. Lectka, Angew. Chem., Int. Ed., 2006, 45, 7398 CrossRef CAS.
- Y. Wang, H. Li, Y.-Q. Wang, Y. Liu, B. M. Foxman and L. Deng, J. Am. Chem. Soc., 2007, 129, 6364 CrossRef CAS.
- For reviews, see:
(a) N. S. Simpkins, Tetrahedron, 1990, 46, 6951 CrossRef CAS;
(b) C. Najera and M. Yus, Tetrahedron, 1999, 55, 10547 CrossRef CAS;
(c) M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixao and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 2668 CAS;
(d) Q. Zhu and Y. Lu, Angew. Chem., Int. Ed., 2010, 49, 7753 CrossRef CAS.
- T. Y. Liu, J. Long, B. J. Li, L. Jiang, R. Li, Y. Wu, L. S. Ding and Y. C. Chen, Org. Biomol. Chem., 2006, 4, 2097 RSC.
- For selected reviews, see:
(a)
R. D. H. Murray, J. Mendez and S. A. Brown, The Natural Coumarins, Wiley, New York, 1982 Search PubMed;
(b)
The Handbook of Natural Flavonoids, ed. J. B. Harborne and H. Baxter, Wiley, Chichester (UK), 1999 Search PubMed;
(c) I. Manolov and N. D. Danchev, Eur. J. Med. Chem., 1995, 30, 531 CrossRef CAS;
(d) C. Mouli, T. Giridhar, R. D. Mohan and R. B. Reddy, J. Heterocycl. Chem., 1996, 33, 5 CrossRef;
(e) R. A. O_Reilly, N. Engl. J. Med., 1976, 295, 354 CrossRef CAS;
(f) H. Y. Li, A. J. Robinson and J. Feaster, Tetrahedron Lett., 1996, 37, 1551 CrossRef CAS;
(g) G. Cravotto, G. M. Nano, G. Palmisano and S. Tagliapietra, Tetrahedron: Asymmetry, 2001, 12, 707 CrossRef CAS;
(h) K. C. Fylaktakidou, D. J. Hadjipavlou-Litina, K. E. Litinas and D. N. Nicolaides, Curr. Pharm. Des., 2004, 10, 3813 CrossRef CAS;
(i) J. R. S. Hoult and M. Paya, Gen. Pharmacol., 1996, 27, 713 CrossRef CAS.
-
(a)
H. P. Rang, M. M. Dale, J. M. Ritter and P. Gardner, Pharmacology, Churchill Livingston, Philadephia, 4th edn, 2001 Search PubMed;
(b)
H. J. Bardsley and A. K. Daly, WO 00/43003, 2000 Search PubMed.
-
(a) Q. Ren, Y. Gao and J. Wang, Chem.–Eur. J., 2010, 16, 13068 CrossRef . For other indane amine-thiourea catalyzed reactions, see: ;
(b) Y. J. Gao, Q. Ren, S.-M. Ang and J. Wang, Org. Biomol. Chem., 2011, 9, 3691 RSC;
(c) Q. Ren, Y. J. Gao and J. Wang, Org. Biomol. Chem., 2011, 9, 5297 RSC.
-
(a) H. Miyabe, S. Tuchida, M. Yamauchi and Y. Takemoto, Synthesis, 2006, 3295 CAS;
(b) F. Cozzi, Adv. Synth. Catal., 2006, 348, 1367 CrossRef CAS;
(c) M. Benaglia, New J. Chem., 2006, 30, 1525 RSC.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 

















![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) complex benzothiopyrans 45 (Scheme 13).49 The reaction was accomplished with the use of 2-mercaptobenzaldehyde 43 and α,β-unsaturated oxazolidinone 44 in the presence of a cinchona-derived thiourea IV (1 mol% of catalyst loading). It is noteworthy that the desired compounds were furnished with three chiral centres in one-pot process. Highly functionalized 4-chromanones are important scaffold in medicinal chemistry.50 Our group has made an effort to undertake the development of the asymmetric synthesis of a variety of spiro-(thio)chromans 47. In the particular work, we were envisioned that dihedral angle between amine and thiourea groups is crucial for asymmetric induction and hence, a series of C1-symmetric bifunctional amine-thiourea catalysts was synthesized for investigation (Fig. 5). The bifunctional indane amine-thiourea XIV was shown to successfully promote asymmetric cyclization reaction between 2-mercaptobenzaldehyde 43 and (E)-3-benzylidenechroman-4-one 46 in a highly concise manner (Scheme 14).51
1 dr) complex benzothiopyrans 45 (Scheme 13).49 The reaction was accomplished with the use of 2-mercaptobenzaldehyde 43 and α,β-unsaturated oxazolidinone 44 in the presence of a cinchona-derived thiourea IV (1 mol% of catalyst loading). It is noteworthy that the desired compounds were furnished with three chiral centres in one-pot process. Highly functionalized 4-chromanones are important scaffold in medicinal chemistry.50 Our group has made an effort to undertake the development of the asymmetric synthesis of a variety of spiro-(thio)chromans 47. In the particular work, we were envisioned that dihedral angle between amine and thiourea groups is crucial for asymmetric induction and hence, a series of C1-symmetric bifunctional amine-thiourea catalysts was synthesized for investigation (Fig. 5). The bifunctional indane amine-thiourea XIV was shown to successfully promote asymmetric cyclization reaction between 2-mercaptobenzaldehyde 43 and (E)-3-benzylidenechroman-4-one 46 in a highly concise manner (Scheme 14).51