Towards more efficient monodimensional zeolite catalysts: n-alkane hydro-isomerisation on hierarchical ZSM-22
Danny
Verboekend
a,
Karine
Thomas
b,
Maria
Milina
a,
Sharon
Mitchell
a,
Javier
Pérez-Ramírez
*a and
Jean-Pierre
Gilson
*b
aInstitute for Chemical and Bioengineering, Department of Chemistry and Applied Biosciences, ETH Zurich, Wolfgang-Pauli-Strasse 10, HCI E 125, CH-8093, Zurich, Switzerland. E-mail: jpr@chem.ethz.ch; Fax: +41 44 633 7120
bLaboratoire Catalyse et Spectrochimie, ENSICAEN, Université de Caen, CNRS, 6 Bd du Maréchal Juin, 14050 Caen, France. E-mail: jean-pierre.gilson@ensicaen.fr; Fax: +33 2 31 45 28 22
First published on 24th August 2011
Abstract
A hierarchical (mesoporous) ZSM-22 zeolite displays a greatly enhanced sorption capacity for n-octane, compared to its purely microporous parent. In n-octane hydro-isomerisation, the mesoporous bi-functional Pt/ZSM-22 catalyst clearly outperforms its microporous parent, judged by the higher monobranched isomer yield. This is attributed to an increased number of accessible micropore mouths in the mesoporous zeolite.
1. Introduction
Shape-selective zeolites brought breakthroughs in oil refining and petrochemicals processing1 and have already demonstrated their great potential in fine chemicals production.2 Shape selectivity occurs when the size of a reactant, product, or transition state matches closely the pore dimensions of a particular zeolite framework. The most well-known and widely-used shape-selective zeolite catalyst is undoubtedly the 3-dimensional, 10-membered ring ZSM-5 (pores: 0.51 × 0.55 nm, 0.53 × 0.56 nm).3 It is applied with great commercial success in aromatics processing and its potential for methanol upgrading is well established.4,5 Superior performances of hierarchical ZSM-5 (e.g. prepared by desilication) in a wide range of access- and diffusion-limited reactions have also been reported.6–8ZSM-22 (TON framework) is another interesting medium-pore zeolite of commercial relevance, used to dewax top tier luboils (high viscosity index for performances combined with good cold flow properties) by isomerisation of normal to branched paraffins, especially to monomethyls.9 This process is progressively replacing dewaxing by cracking (bi-functional mordenite and ZSM-5 catalysts) and by solvent extraction. The very high monobranching selectivity of ZSM-22 is elegantly described by the pore-mouth selectivity concept,10 albeit still a matter of debate.11 Recently, in the conversion of methanol to olefins, ZSM-22 displayed interesting performances, again due to its unique selectivity.4,5 However, the monodimensional (1D) 10-membered ring pores (0.46 × 0.57 nm) of ZSM-22 lead to reduced accessibility and increased mass-transport constraints and greater susceptibility to deactivation compared to ZSM-5.
The combination of post-synthetic treatments is increasingly applied to tune the catalytic performance of zeolites by tailoring their accessibility and acid site speciation.8,12 We recently reported a two-step post-synthetic approach to prepare hierarchical ZSM-22.13 In particular, we emphasised the need for a mild acid treatment of the alkaline-treated zeolite to restore access to its 1D micropores, easily blocked by residual aluminium-containing species. Meanwhile, the isomerisation of 1-butene over hierarchical Nu-10 (TON framework), prepared by desilication in aqueous NaOH, was reported.14 The modified zeolite showed an increased initial activity compared to its parent, attributed to enhanced active site accessibility, while an influence on product selectivity was not apparent. Moreover, the reaction suffered from rapid micropore blockage and severe deactivation by coking.
Herein, we report the hydro-isomerisation of n-octane (n-C8) on bi-functional catalysts derived from microporous and hierarchical ZSM-22. This reaction captures most of the chemical and mass-transport aspects of the longer chain hydrocarbons (n-C20+) found in waxy luboils.15 It therefore meets the requirements to indicate whether desilication, combined with a selective removal of aluminium-rich debris by acid treatment,13 improves the performance of ZSM-22 in a key catalytic reaction of oil refining. This communication presents the enhanced adsorptive properties of mesoporous ZSM-22 compared to its purely microporous parent, and the higher yields of monobranched isomers in the hydro-isomerisation of n-octane over a derived bi-functional Pt/ZSM-22 catalyst.
2. Experimental
Zeolite and catalyst preparation
The as-synthesised ZSM-22 zeolite (kindly supplied by RIPP, Beijing, China) still contained the template, 1,6-diaminohexane, which was carefully removed by calcination in static air (823 K, 15 h, a ramp rate of 3 K min−1) to obtain the parent zeolite (code P). The mesoporous zeolite (code NaOH–HCl) was obtained by alkaline treatment of the parent sample in aqueous NaOH (0.6 M, 358 K, 120 min, 3.3 g of solid per 100 cm3 of solution), followed by acid treatment in aqueous HCl (0.1 M, 338 K, 6 h, 1 g of solid per 100 cm3 of solution). Both the P and NaOH–HCl zeolites were converted to the protonic form by three consecutive ion exchanges in NH4NO3 (0.1 M, 298 K, 12 h, 1 g of solid per 100 cm3 of solution) followed by calcination as stated above. Platinum was loaded on the zeolites by incipient wetness impregnation with aqueous Pt(NH3)4(NO3)2, followed by drying at 393 K for 12 h, and calcination at 673 K for 3 h in an air flow of 100 cm3 min−1.Characterisation methods
X-Ray diffraction (XRD) patterns were recorded using a PANalytical X’Pert PRO-MPD diffractometer equipped with Bragg-Brentano geometry and Ni-filtered Cu Kα radiation (λ = 0.1541 nm). Data were collected in the range of 5–50° 2θ, with an angular step size of 0.05° and a counting time of 8 s per step. Si, Al, and Pt contents were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES, Horiba Ultra 2). N2 isotherms were measured in a Quantachrome Quadrasorb-SI analyser at 77 K. The samples were degassed under vacuum at 573 K for 10 h prior to the measurement. Transmission electron microscopy (TEM) was performed with a Phillips CM12 instrument operated at 100 kV. Scanning transmission electron microscopy (STEM) was carried out with an FEI Tecnai F30 microscope operated at 300 kV. Adsorption of n-C8 was measured in an Intelligent Gravimetric Analyser (IGA-002, Hiden Analytical Ltd.) at 298 K. The samples (10 mg) were outgassed at 583 K for 10 h prior to measurement. The isotherms were measured by increasing the n-C8 vapour pressure in the range of 0–18 mbar. The upper limit was determined by the saturated vapour pressure of n-octane (ca. 19 mbar at 298 K). CO pulse chemisorption at 303 K was carried out in a Thermo TPDRO 1100 unit. Prior to the CO pulses, the samples (100 mg) were pre-treated at 393 K in He flow (20 cm3 min−1) for 1 h, followed by reduction in 5 vol% H2 in He (20 cm3 min−1) at 548 K for 1 h, and subsequent purging for 1 h with He at the same temperature. The interval between successive pulses (loop volume = 344 μl, 5 vol% CO/He) was kept as short as possible to avoid desorption. A Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO stoichiometry of 1
CO stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was assumed to calculate the active metal surface area. Infrared spectra were recorded at room temperature with a Nicolet Nexus FTIR spectrometer equipped with a DTGS detector. The zeolite powders were pressed into self-supporting wafers (ca. 10 mg) and activated under vacuum (10−4 Pa) for 1 h at 673 K. Adsorption of pyridine (Py) and 2,6-lutidine (Lu) was performed at 393 K for 15 min, followed by desorption for 15 min at 473 K to remove weakly adsorbed molecules. All spectra were normalised to the weight of the wafers.
1 was assumed to calculate the active metal surface area. Infrared spectra were recorded at room temperature with a Nicolet Nexus FTIR spectrometer equipped with a DTGS detector. The zeolite powders were pressed into self-supporting wafers (ca. 10 mg) and activated under vacuum (10−4 Pa) for 1 h at 673 K. Adsorption of pyridine (Py) and 2,6-lutidine (Lu) was performed at 393 K for 15 min, followed by desorption for 15 min at 473 K to remove weakly adsorbed molecules. All spectra were normalised to the weight of the wafers.
Catalytic testing
Isomerisation of n-C8 was carried out in a continuous-flow fixed-bed micro-reactor containing 1 g of the catalyst diluted with 1 g of silicon carbide. The catalysts were evaluated at 573 K under a total pressure of 5 MPa, and space times in the range of 14–90 kg s mol−1. The feed was delivered through a liquid chromatography pump (Gilson 302). The gaseous products at the reactor outlet were analysed online with a Varian 3800 gas chromatograph, fitted with a fused silica capillary column (Chrompack CP-SIL 5 CB WCOT) and a flame ionisation detector (FID). Prior to testing, the catalyst was calcined in situ at atmospheric pressure for 2 h with flowing air at 673 K and cooled to 523 K under a He flow. The sample was then reduced at 523 K in flowing H2 for 1 h prior to pressurisation. All gases were purified from water and oxygen contaminants using 3A zeolite and BTS (Fluka) traps. The n-C8 conversion was calculated as X (%) = (1 − An-C8/Atotal) × 100, where An-C8 and Atotal designate the peak areas of n-C8 and all observed products, respectively. The weight yield of lumped products, YL, was expressed as YL (%) = (AL/Atotal) × 100, with AL and Atotal denoting the peak areas of lumped products (mbC8 = monobranched C8 isomers, dbC8 = dibranched C8 isomers, cracking) and all observed products, respectively.3. Results and discussion
The parent, P, ZSM-22 (molar Si/Al ratio = 38) is crystalline and pure, since only sharp reflections of the TON framework are observed by XRD (Fig. 1a, inset). The predominantly microporous character of P is confirmed by N2 adsorption, evidencing significant uptake only at low relative pressures (Fig. 1a). TEM shows the typical agglomerates of needle-like ZSM-22 crystals (Fig. 2).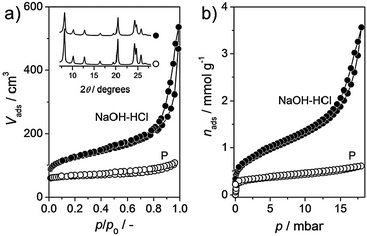 | ||
| Fig. 1 (a) N2 isotherms of the parent (P) and hierarchical (NaOH–HCl) ZSM-22 zeolites. Inset: corresponding X-ray diffraction patterns. (b) Sorption curves of n-octane in the ZSM-22 samples at 298 K. | ||
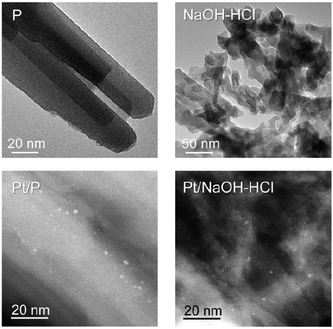 | ||
| Fig. 2 TEM images of the parent (P) and hierarchical (NaOH–HCl) ZSM-22 zeolites (top). STEM images of Pt/P and Pt/NaOH–HCl bi-functional catalysts show the platinum nanoparticles as bright spots (bottom). | ||
The sequential alkaline and acid treatments, performed as described in previous work,13 produce sample NaOH–HCl. We have not included the unwashed alkaline-treated sample, i.e., coded ‘NaOH’, in this communication on the basis of the extensive presence of debris,13 leading to severe blockage of the pore mouths and micropore volume. After the post-synthetic modifications, the Si/Al ratio of NaOH–HCl increases to 50. This is a consequence of the mild acid treatment, known to remove Al-rich debris from the zeolite. The N2 adsorption isotherms of this sample display a markedly increased uptake in the range of p/p0 = 0.1–0.8 (Fig. 1a). As discussed in ref. 13, and clearly discerned by TEM, the secondary porosity induced by the alkaline treatment is ascribed to the creation of intra-crystalline mesopores and inter-crystalline voids (Fig. 2). The latter originate from the fragmentation of the needle-like agglomerates. Moreover, a slightly enhanced uptake at p/p0 < 0.1 in the N2 isotherm confirms the preservation of microporosity. Table 1 indicates that the mesopore surface area (Smeso) increases roughly 5-fold from 41 (P) to 203 m2 g−1 (NaOH–HCl), whereas the micropore volume (Vmicro) is equivalent for both samples (0.08 cm3 g−1). The latter value is characteristic of the TON framework. Deposition of ca. 0.25 wt% of Pt on the ZSM-22 zeolites leads to only minor changes in porosity, the microporosity dropping to 0.07 cm3 g−1, while the mesoporosity remains mostly unchanged. The Pt dispersion is relatively high (ca. 45%) and similar for both samples; this ensures that the catalysts are well-balanced, i.e. that the rate-determining step is the acid-catalysed rearrangement of the carbocations on the zeolite surface.16 The STEM images show small particles in the nm-size range (Fig. 2), in good agreement with the dispersion measured by CO chemisorption.17 The XRD pattern of the treated sample confirms that the long-range crystallinity is also retained (Fig. 1a, inset). Both porosity and the Si/Al ratio of NaOH–HCl differ slightly from the values reported previously.13 This result can be tentatively explained by a more homogeneous Al distribution in the parent zeolite crystals of this study.
| Sample | Si/Ala, mol mol−1 | Pta (wt%) | D Pt b (%) | V micro c/cm3 g−1 | V pore d/cm3 g−1 | S meso c/m2 g−1 |
|---|---|---|---|---|---|---|
| a ICP-OES. b Platinum dispersion measured by pulse CO chemisorption. c t-Plot method. d Volume adsorbed at p/p0 = 0.99. | ||||||
| P | 38 | — | — | 0.08 | 0.17 | 41 |
| NaOH–HCl | 50 | — | — | 0.08 | 0.70 | 203 |
| Pt/P | 38 | 0.3 | 47 | 0.07 | 0.23 | 49 |
| Pt/NaOH–HCl | 50 | 0.2 | 40 | 0.07 | 0.74 | 199 |
The sorption curves of n-C8 in the ZSM-22 samples are shown in Fig. 1b. The similarity between these isotherms and those of N2 confirms the accessibility of the mesoporosity introduced. The total uptakes of n-C8 (0.10 cm3 g−1 for P, 0.58 cm3 g−1 for NaOH–HCl) are slightly lower compared to the pore volumes mentioned in Table 1. This could indicate an incomplete accessibility of the TON micropores, but this is difficult to determine as the density of n-C8 in the micropores is unknown.18
The acid site speciation and accessibility are determined by infrared spectroscopy of adsorbed alkyl pyridines of different sizes, i.e. pyridine (0.57 nm) and lutidine (0.67 nm).19Fig. 3a shows the IR spectra of the zeolites prior to adsorption, indicating a decrease of the bridged hydroxyls (3600 cm−1), i.e. the Brønsted acid sites, and a substantial increase of the silanols (3745 cm−1), upon sequential treatments.13Figs. 3b and c show the IR spectra of the surfaces of the two samples in strong interaction with pyridine and lutidine, respectively. Pyridine provides the speciation of the acid sites (Brønsted at 1455 cm−1 and Lewis at 1557 cm−1) while lutidine reveals only the Brønsted sites (1640 cm−1) on the outer surface.19 The spectra are quantified in Table 2. Using pyridine, predominately Brønsted (BPy = 208 μmol g−1) and fewer Lewis acid sites (LPy = 35 μmol g−1) are measured on the parent zeolite. The corresponding accessibility index (ACIPy = 0.58) is determined by dividing the total number of acid sites by the number of Al in the samples. This is lower than that reported previously (ACIPy ≈ 1),13 indicating that the intrinsic features of a parent (ZSM-22) zeolite can differ substantially depending on its origin. Moreover, it emphasises the usefulness of the ACI in comparing zeolites of identical structures prepared by different routes.19 In the present case, supporting the more homogenous distribution of Al (vide supra) in the current sample, since a higher number of Brønsted acid sites are present inside the relatively inaccessible micropores. Because of its moderately-sized and unidirectional 10 MR micropores, the TON structure is probably very sensitive to such changes. The number of acid sites probed by lutidine (BLu = 16 μmol g−1) is about one-tenth of those accessible to pyridine. This further stresses the hampered accessibility of probe hydrocarbon molecules to the catalytically active acid sites in the ZSM-22 crystals. After the post-synthetic treatments (NaOH–HCl), the number of Brønsted sites drops slightly, in line with the reduced Al content and a similar ACIPy is estimated. However, the number of Brønsted acid sites accessible to lutidine quadruples, which is attributed to an increased pore mouth density following the introduction of mesoporosity.
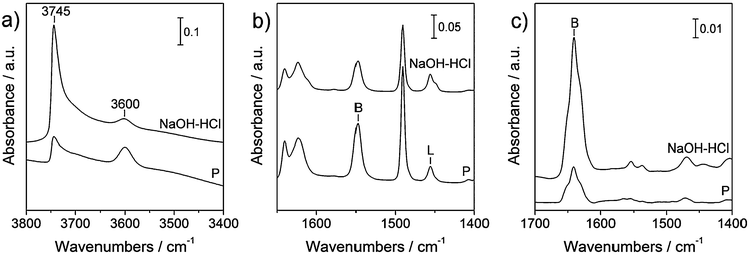 | ||
| Fig. 3 FTIR spectra of the parent (P) and mesoporous ZSM-22 zeolites (NaOH–HCl) after (a) activation, (b) adsorption of pyridine, and (c) adsorption of 2,6-lutidine. Bands related to the adsorption of (alkyl) pyridines on Brønsted and Lewis acid sites are indicated by B or L labels, respectively. | ||
The bi-functional catalysts derived from the parent (Pt/P) and mesoporous (Pt/NaOH–HCl) zeolites are both very active in hydro-isomerisation (Fig. 4a). However, at relatively low space time (W/F = 36 kg s mol−1), the hierarchical zeolite displays a higher conversion (78%) than its parent (67%). A precise quantification of the benefits of the introduction of mesoporosity on catalytic activity will be better evidenced at shorter space times, where intrinsic activities of both samples can be measured. An in-depth kinetic study, as outlined elsewhere,15 is outside the scope of this communication.
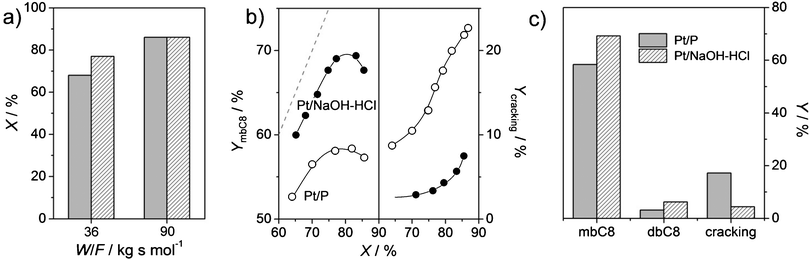 | ||
| Fig. 4 Hydro-isomerisation of n-C8 over Pt/ZSM-22 at T = 573 K, P = 5 MPa, and W/F = 14–90 kg s mol−1. (a) Influence of space time (W/F) on conversion at PH2 = 0.5 MPa for Pt/P and Pt/NaOH–HCl. The legend in (c) also applies to (a). (b) Selectivities of monobranched (mbC8) and cracking as a function of conversion. The dotted line illustrates the maximum attainable yield (at 100% selectivity). (c) Overview of selectivities at 80% conversion. Dibranched octane isomers are represented by dbC8. | ||
Fig. 4b and c display the expected features of well-balanced bi-functional ZSM-22 catalysts, i.e., a high monobranching selectivity at high conversion coupled to a late onset of cracking.15 At 80% conversion, the purely microporous Pt/ZSM-22 yields 60% of monobranched isomers, while the dibranched do not exceed 5%, no tribranched are detected, but cracking is significant (ca. 15%). Such a yield of monobranched isomers is comparable to earlier results,15 although those were obtained under somewhat different operating conditions (lower temperature, lower total pressure and higher molar H2/n-C8 ratio). The hierarchical Pt/NaOH–HCl zeolite catalyst displays a substantially increased yield of monobranched isomers (70%), a similar yield of dibranched (5%) and a much reduced (ca. 5%) cracking. This latter yield pattern is highly attractive, since 95% of the products either meet the required specifications (mono- and, to a lesser extent, di-branched isomers) or could meet them if the unconverted feed is recycled to extinction. Such a remarkably high monobranched yield and low cracking of the mesoporous zeolite are related to the increased number of pore mouths and the associated reduced length of the micropores. Both minimise the average residence time of molecules inside the ZSM-22 micropores, maximising the primary products and reducing the extent of undesired consecutive reactions such as cracking.
4. Conclusions
The introduction of mesoporosity coupled to a preserved microporosity increases the activity and significantly improves the monobranching selectivity of a hierarchical ZSM-22 catalyst in n-alkane isomerisation. This is attributed to an increased number of pore mouths and the associated decreased diffusion length for reactants and products. Our results point out that desilication, in combination with a mild acid treatment, is a very effective method to maximise the catalytic potential of unidirectional zeolites.Acknowledgements
ETH Zürich, the Swiss National Science Foundation (Project number: 200021-134572), and Région Basse-Normandie are acknowledged for funding.Notes and references
- W. Vermeiren and J.-P. Gilson, Top. Catal., 2009, 52, 1131 CrossRef CAS.
- H. van Bekkum and H. W. Kouwenhoven, in Introduction to Zeolite Science and Practice, ed. J. Čejka, H. van Bekkum, A. Corma and F. Schüth, Elsevier, Amsterdam, 3rd Revised edn, 2007, pp. 947–998 Search PubMed.
- T. F. Degnan, J. Catal., 2003, 216, 32 Search PubMed.
- J. Li, Y. Wei, G. Liu, Y. Qi, P. Tian, B. Li, Y. He and Z. Liu, Catal. Today, 2011, 171, 221 Search PubMed.
- S. Teketel, U. Olsbye, K.-P. Lillerud, P. Beato and S. Svelle, Microporous Mesoporous Mater., 2010, 136, 33 CrossRef CAS.
- M. S. Holm, E. Taarning, K. Egelblad and C. H. Christensen, Catal. Today, 2011, 168, 3 CAS.
- J. Pérez-Ramírez, C. H. Christensen, K. Egeblad, C. H. Christensen and J. C. Groen, Chem. Soc. Rev., 2008, 37, 2530 RSC.
- C. Fernandez, I. Stan, J.-P. Gilson, K. Thomas, A. Vicente, A. Bonilla and J. Pérez-Ramírez, Chem.–Eur. J., 2010, 16, 6224 CrossRef CAS.
- C. Kerby, T. F. Degnan Jr., D. O. Marler and J. S. Beck, Catal. Today, 2005, 104, 55 Search PubMed.
- J. A. Martens, W. Souverijns, W. Verrelst, R. Parton, G. F. Froment and P. A. Jacobs, Angew. Chem., Int. Ed. Engl., 1995, 34, 2528 CAS.
- M. Schenk, B. Smit, T. J. H. Vlugt and T. L. M. Maesen, Angew. Chem., Int. Ed., 2001, 40, 736 CrossRef CAS.
- D. Verboekend, S. Mitchell, M. Milina, J. C. Groen and J. Pérez-Ramírez, J. Phys. Chem. C, 2011, 115, 14193 Search PubMed.
- D. Verboekend, A. M. Chabaneix, K. Thomas, J.-P. Gilson and J. Pérez-Ramírez, CrystEngComm, 2011, 13, 3408 RSC.
- P. Matias, C. S. Couto, I. Graça, J. M. Lopes, A. P. Carvalho, F. R. Ribeiro and M. Guisnet, Appl. Catal., A, 2011, 399, 100 Search PubMed.
- C. S. Laxmi Narasimhan, J. W. Thybaut, G. B. Marin, P. A. Jacobs, J. A. Martens, J. F. Denayer and G. V. Baron, J. Catal., 2003, 220, 399 Search PubMed.
- T. F. Degnan and C. R. Kennedy, AIChE J., 1993, 39, 607 Search PubMed.
- O.-L. Pérez, D. Romeu and M. J. Yacaman, J. Catal., 1983, 79, 240 Search PubMed.
- C. Li-feng and L. V. C. van Rees, Zeolites, 1988, 8, 310.
- F. Thibault-Starzyk, I. Stan, S. Abelló, A. Bonilla, K. Thomas, C. Fernandez, J.-P. Gilson and J. Pérez-Ramírez, J. Catal., 2009, 264, 11 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
