An anionic D-valine–palladium(II) complex supported on a hydroxy double salt with a Brønsted basic phosphate anion: application for a heterogeneous catalyst toward aerobic alcohol oxidation†
Received
17th June 2011
, Accepted 25th July 2011
First published on 22nd August 2011
Abstract
An anionic D-valine–Pd(II) complex intercalated into an anion exchangeable Ni–Zn mixed basic salt (NiZn), which is classified as a layered hydroxy double salt, was synthesised in situ via a simple anion exchange procedure. A Brønsted basic PO43− anion was also intercalated into the NiZn interlayer along with the anionic D-valine–Pd(II) complex. The Brønsted basic clay–Pd(II) nanocomposite catalysed the aerobic oxidation of a wide variety of alcohols to the corresponding aldehydes and ketones. In the aerobic oxidation of 1-phenylethanol, for example, a turnover number (TON) of 2000 based on Pd was achieved. During the aerobic alcohol oxidation, the D-valine–Pd(II) complex in the NiZn interlayer maintained its original monomeric Pd(II) structure due to the strong electrostatic interaction between the NiZn host and the anionic Pd(II) complex. This catalyst could be reused without loss of either catalytic activity or selectivity for the aerobic oxidation of alcohols.
Introduction
Nanostructured catalysts with precisely designed structures have been extensively investigated to achieve cooperatively enhanced catalytic performance. For this purpose, significant effort has been extended toward developing catalyst materials, such as grafted transition metal complexes,1a metal-organic frameworks,1b metal nanoparticles,1c metal oxides,1d mesoporous materials,1e and nanosheets1f in order to fine-tune the catalyst characteristics. One of the key steps in designing the high-performance heterogeneous catalysts from solid materials is integrating the individual functions that belong to each component, which would be an influential approach for enhancing the catalytic performance.
Clays and clay minerals are widespread in nature and their high surface area, sorptive, and ion-exchange properties have been utilised in catalytic applications for decades.2 Layered hydroxy double salts, which consist of positively charged layers and exchangeable interlayer anions, have received considerable interest as anion-exchangeable layered compounds3 due to their potential applications as catalyst supports. We so far continue to maintain an awareness of the catalyst design using a Ni–Zn mixed basic salt (NiZn), Ni1−xZn2x(OAc)2x(OH)2·nH2O (0.15 < x < 0.25).4 Recently, the interlayer space-controlled NiZn with intercalated alkyl carboxylate anions was found to be an effective heterogeneous catalyst for the Knoevenagel condensation in water.4b Our motivational factors for the selection of NiZn as a catalyst support are the following characteristics: (i) simple preparation, (ii) high crystallinity, (iii) strong electrostatic interactions between guest anions and Zn2+ cations, (iv) high anion exchange capacity, and (v) the ability to fine-tune the interlayer space by modifying the size of the guest anion.
Selective alcohol oxidation using molecular oxygen as a sole oxidant is an atom-efficient molecular transformation, and this oxidative dehydrogenation is known to be catalysed by a range of transition metals under mild conditions.5 In our previous study, we developed a nanostructured NiZn-intercalated Pd(II) complex, [Pd(OH)4]2−/CH3COO−/NiZn, that acts as an efficient heterogeneous catalyst for the aerobic alcohol oxidation.4a The results of previous investigations have shown that the addition of a Brønsted base significantly improves the rate of alcohol oxidation. For example, Stahl and his group have demonstrated that “a base assisted mechanism”6 is predominant in the Pd(OAc)2/pyridine catalyst system. In particular, the results of a detailed kinetic analysis revealed that a Brønsted basic molecular sieve 3A has a stimulating effect on the formation of the Pd(II)-alcoholate species. A similar effect has also been observed in a heterogeneous catalyst system composed of gold nanoparticles and a strong basic hydrotalcite as a catalyst support.7 From an environmental and a synthetic perspective, the incorporation of Brønsted basic functions into solid supports is imperative for creating highly effective heterogeneous catalysts.
We describe here the synthesis of a new, environmentally friendly alcohol oxidation catalyst consisting of NiZn, an anionic amino acid–Pd(II) complex, and a Brønsted basic PO43− anion. Since the carcinostatic activities of organometallic complexes of group 10 metal and α-amino acids were discovered by Rosenberg and co-workers,8 interest in their synthesis, reactivity, and applications has rapidly increased.9 α-Amino acids are highly versatile ligands and can afford organometallic complexes by chelating to transition metal centres viaamino, carboxylato, or other basic functional groups, so that the research on the border between biochemistry and organometallic chemistry will be expanded rapidly. In this report, we synthesised a novel anionic D-valine–Pd(II) complex, and intercalated into a NiZn interlayer as an active species for aerobic alcohol oxidation. Furthermore, to create a more efficient heterogeneous catalyst, a Brønsted basic PO43− anion was intercalated into the NiZn interlayer along with the anionic D-valine–Pd(II) complex.
Results and discussion
In situ preparation of the D-valine–palladium(II) complex
The anionic D-aline–Pd(II) complex solution was prepared in situ following the cyclopalladation reaction between K2PdCl4 and D-valine (denoted by 1) (1/1 mol/mol) in aqueous solution at pH 4.9 adjusted by KOH. In contrast to the Hao method,9c a water-insoluble [Pd(D-valine)2]·H2O complex was not obtained. To confirm the coordinated structure of the D-valine–Pd(II) (1–Pd) complex, UV-vis, 1H NMR, and PdK-edge XAFS analyses were conducted. In the UV-vis spectra of the 1–Pd complex and an aqueous solution of K2PdCl4, the peak corresponding to the d–d transition at 419 nm in the aqueous K2PdCl4 solution shifted to 373 nm due to the addition of KOH and 1 (Fig. 1). The 1H NMR spectrum of the 1–Pd complex was taken in D2O solution, and the chemical shifts including the difference between free 1 and the 1–Pd complex are shown in Table 1. Negative values of Δδ correspond to downfield shifts of the coordinated 1 as compared to those of the free 1, while positive values correspond to upfield shifts. The α-CH proton of 1 showed a doublet peak at 3.43 and 3.42 ppm, both shifted downfield by −0.18 and −0.25 ppm, respectively, relative to the zwitterionic form due to the coordination of the amine nitrogen to Pd(II). The β-protons showed a multiple resonance centred at 2.19 ppm that was shifted downfield by −0.04 ppm. In contrast to the free 1, two peaks with opposing shifts corresponding to the protons at the γ-position were observed. The methyl protons at the γ-position were shifted upfield by + 0.09 and + 0.13 ppm, and the corresponding downfield shifts were −0.07 and −0.05 ppm, respectively. The γ-methyl protons likely displayed opposing shifts due to the rigid conformation of the isopropyl group, which was attributed to the formation of a complex between 1 and Pd(II).9a Consequently, the signals corresponding to the free 1 cannot be observed in the 1H NMR profile of the 1–Pd complex. In the PdK-edge XANES spectrum of a solution of the 1–Pd complex, the edge energy did not resemble that of Pd foil but was similar to that of a solution of K2PdCl4 (Fig. 2a, b and e), indicating that divalent Pd species were present in solution. In the Fourier transforms (FT) of k3-weighted PdK-edge EXAFS spectra of a solution of the 1–Pd complex, two peaks corresponding to the Pd–O and Pd–Cl bonds were observed around 0.15 and 0.19 nm, respectively (Fig. 3a). Moreover, the peaks based on Pd–O–Pd and Pd–Pd bonds around 0.25 and 0.30 nm in the Pd oxide or the Pd foil were not detected (Fig. 3b, d, and e). In the water-insoluble [Pd(D-valine)2]·H2O complex,9c the peak of the Pd–Cl bond around 0.19 nm was not observed (Fig. 3c). The two peaks around 0.15 and 0.19 nm in Fig. 3a were well fitted by using the Pd–O and Pd–Cl shell parameters, and the curve-fitting analysis suggested that two oxygen atoms at 0.198 nm and two chlorine atoms at 0.256 nm were present in the aqueous solution (Table 2).10 On the basis of 1H NMR and PdK-edge XAFS results, 1 coordinates to Pd(II)via the α-NH2 and the COO−groups, similar to the other previous reports.9 Thus, we can conclude that anionic (1–PdCl2)− species was formed in aqueous solution, as shown in Scheme 1a.11
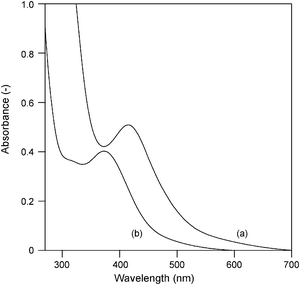 |
| | Fig. 1
UV-vis spectra of (a) aqueous K2PdCl4 solution (pH = 4.0) and (b) 1–Pd complex solution (pH = 4.9). Pd concentration of the solution was 2.0 mM. | |
Table 1
1H NHR chemical shifts of the 1–Pd complex in D2O
| Position |
δ/ppm |
Δδ/ppma |
mb, J/Hz |
|
Difference of chemical shift between 1 and the 1–Pd complex.
Multiplicity of signal: d: doublet; m: multiplet.
|
|
α-CH
|
3.43, 3.42 |
−0.18, −0.25 |
d, 4.3 |
|
β-CH
|
2.19 |
−0.04 |
m |
|
γ-CH3 (1) |
1.15, 1.08 |
−0.07, +0.09 |
d, 7.2 |
|
γ-CH3 (2) |
1.18, 0.94 |
−0.05, +0.13 |
d, 7.2 |
![Pd
K-edge XANES spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(d-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn.](/image/article/2011/CY/c1cy00223f/c1cy00223f-f2.gif) |
| | Fig. 2
Pd
K-edge XANES spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(D-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn. | |
![FT of k3-weighted PdK-edge EXAFS spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(d-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn.](/image/article/2011/CY/c1cy00223f/c1cy00223f-f3.gif) |
| | Fig. 3 FT of k3-weighted PdK-edge EXAFS spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(D-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn. | |
Table 2 Curve-fitting results of PdK-edgeEXAFS spectraa
| Sample |
Shell |
C.N.b |
r
c/nm |
σ
d/nm |
|
Inverse Fourier transformations were performed for the regions of 0.104–0.224 nm.
Coordination number.
Bond distance.
σ is Debye–Waller factors.
Data from X-ray crystallography.
Inverse Fourier transformations were performed for the regions of 0.117–0.196 nm.
|
|
Pd foile |
Pd–Pd |
(12) |
(0.274) |
|
|
PdO
e |
Pd–O |
(4) |
(0.202) |
|
| Pd–(O)–Pd |
(4) |
(0.303) |
|
| Pd–(O)–Pd |
(8) |
(0.342) |
|
|
K2PdCl4 solution (pH 1)e |
Pd–Cl |
(4) |
(0.232) |
|
|
1–Pd complex solution |
Pd–O |
2.1 |
0.198 |
0.0038 |
| Pd–Cl |
2.6 |
0.230 |
0.0069 |
| Fresh [1–Pd(OH)2]−/PO43−/NiZnf |
Pd–O |
4.1 |
0.199 |
0.0078 |
| Recovered [1–Pd(OH)2]−/PO43−/NiZnf |
Pd–O |
3.9 |
0.200 |
0.0060 |
![Proposed structure of (a) anionic (1–PdCl2)− complex and (b) anionic [1–Pd(OH)2]− complex in an NiZn interlayer.](/image/article/2011/CY/c1cy00223f/c1cy00223f-s1.gif) |
| | Scheme 1 Proposed structure of (a) anionic (1–PdCl2)− complex and (b) anionic [1–Pd(OH)2]− complex in an NiZn interlayer. | |
Intercalation of the D-valine–palladium(II) complex into an NiZn interlayer
CH3COO
−/NiZn, Ni0.78Zn0.44(CH3COO)0.44(OH)2·0.86H2O (Ni/Zn = 1.77 and anion-exchange capacity = 2.65 mmol g−1), was synthesised according to a previously reported procedure.3f A simple anion-exchange process was conducted in water to exchange CH3COO− with PO43−. Specifically, treatment of CH3COO−/NiZn with an aqueous solution of K3PO4 at 60 °C for 72 h yielded the PO43−-exchanged NiZn catalysts (PO43−/NiZn) as a green powder. After intercalation of the PO43− anion, the d001 peak shifted to a large angle relative to that of the CH3COO−/NiZn catalyst, and the calculated C.S. (clearance space = basal spacing (d001) – thickness of the brucite layer (0.46 nm)) of PO43−/NiZn was 0.45 nm (Table 3).12 Immobilisation of the anionic 1–Pd complex in the NiZn interlayer was also achieved by a simple anion-exchange method in the following manner. Treating PO43−/NiZn with the as-prepared aqueous solution of the 1–Pd complex yielded the NiZn-intercalated 1–Pd complex catalyst (denoted by 1–Pd/PO43−/NiZn, Pd loading amount: 0.02 mmol g−1). According to XRD analysis, the d001 peak position did not change after intercalation of the anionic 1–Pd complex. The local structure of the 1–Pd/PO43−/NiZn catalyst was estimated by XAFS analyses. The features of the PdK-edge XANES spectrum and the edge energy of the 1–Pd/PO43−/NiZn were similar to those of Pd oxide (Fig. 2d and f). The peak corresponding to the Pd–O bond was observed in the second FT coordination sphere of the k3-weighted EXAFS data of the 1–Pd/PO43−/NiZn catalyst (Fig. 3f). Moreover, the curve-fitting results suggested that four oxygen atoms at 0.199 nm were coordinated to the monomeric Pd(II) centre (Table 2).13 Based on these results, we proposed that the local structure of Pd species in the NiZn interlayer is a monomeric [1–Pd(OH)2]−, accompanied with the ligand exchange from Cl− into HO−, as shown in Scheme 1b.
|
Catalyst
|
(110) p.p.a/° |
d
001/nm |
C.S.b/nm |
|
Peak position.
Clearance space. C.S. = d001 − thickness of brucite layer (0.46 nm).
|
|
CH3COO
−/NiZn |
6.78 |
1.30 |
0.84 |
|
PO43−/NiZn |
9.76 |
0.91 |
0.45 |
| [1–Pd(OH)2]−/CH3COO−/NiZn |
6.82 |
1.29 |
0.83 |
| [1–Pd(OH)2]−/PO43−/NiZn |
9.76 |
0.91 |
0.42 |
The catalytic activity of various NiZn catalysts was surveyed for the nonactivated 2-adamantanol oxidation in trifluorotoluene solvent under air flow conditions (10 mL min−1),14 and the results are shown in Table 4. The almost quantitative 2-adamantanone was obtained in the presence of [1–Pd(OH)2]−/PO43−/NiZn catalyst (entry 1).15 No oxidation proceeded in the absence of the catalyst or in the presence of CH3COO−/NiZn or PO43−/NiZn (entries 5–7). Our previously reported [Pd(OH)4]2−/CH3COO−/NiZn4a did not catalyse the reaction under these reaction conditions (entry 4), however, 57% of 2-adamantanone was formed after the intercalation of PO43− coexisted with [Pd(OH)4]2− (entry 3). Since the alcohol substrate was activated by the coordination to the Pd(II) center and the phosphate anion, the Pd(II)-alcoholate species was readily formed in the NiZn interlayer.16 The same activation effect on the PO43− anion was observed in the [1–Pd(OH)2]−/PO43−/NiZn catalyst (entries 3 and 4). Therefore, the integration of the anionic [1–Pd(OH)2]− complex and a Brønsted basic PO43− anion into a NiZn matrix is important for achieving the high catalytic activity.
| Entry |
Catalyst
|
C.S.b/nm |
Conv.c /% |
Yieldc /% |
|
2-Adamantanol (0.5 mmol), Pd catalyst (Pd: 1 mol%), trifluorotoluene (5 mL), 80 °C, 3 h, air flow (20 mL min−1).
C.S. was calculated by XRD pattern. C.S. = d001 − thickness of layer (0.46 nm).
Determined by GC using an internal standard technique.
NiZn (0.1 g) was used as a catalyst.
|
| 1 |
[1–Pd(OH)2]−/PO43−/NiZn |
0.44 |
98 |
98 |
| 2 |
[1–Pd(OH)2]−/CH3COO−/NiZn |
0.83 |
20 |
20 |
| 3 |
[Pd(OH)4]2−/PO43−/NiZn |
0.44 |
57 |
57 |
| 4 |
[Pd(OH)4]2−/CH3COO−/NiZn |
0.83 |
Trace |
Trace |
| 5d |
CH3COO
−/NiZn |
0.83 |
Trace |
Trace |
| 6d |
PO43−/NiZn |
0.44 |
Trace |
Trace |
| 7 |
None |
— |
Trace |
Trace |
The [1–Pd(OH)2]−/PO43−/NiZn catalyst showed high performance for oxidising various alcohols using air as a sole oxidant, as summarized in Table 5. Primary benzylic alcohols with electron-donating substituents, such as p-methoxy and p-methylbenzyl alcohol, were converted to the corresponding aldehydes with an excellent yield, respectively (entries 3 and 4). Alternatively, the oxidation rate of p-chlorobenzyl alcohol was relatively low (entry 5). Similar to the results with other homogeneous Pd(II) catalysts,17p-chlorobenzyl alcohol was not oxidised well due to its electron deficiency. A small amount of benzaldehyde was formed under a N2 atmosphere, presumably due to the presence of residual O2 (entry 2). The consumption of O2 gas was measured, and a ca. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
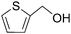
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of O2 uptake to benzaldehyde yield was observed, suggesting that molecular oxygen was used quantitatively as an oxidant in the oxidative dehydrogenation reaction. In all cases, the aldehydes were produced from primary alcohols without over-oxidation to the corresponding carboxylic acid. The oxidation of the secondary benzylic alcohols produced high yields of ketone (entries 6 and 10). Selective oxidation of the hydroxyl groups was observed for cyclopropyl phenyl carbinol with a radical clock, where any skeletal isomerisation or carbon–carbon bond cleavage did not occur (entry 10). After the oxidation, the spent [1–Pd(OH)2]−/PO43−/NiZn catalyst was easily separated by simple centrifugation or filtration. The recovered catalyst could be reused without additional treatment, and its high activity and selectivity were maintained (entries 7 and 8). In order to further demonstrate the requirement of the heterogeneous [1–Pd(OH)2]−/PO43−/NiZn catalyst, the catalyst was removed by hot filtration after the oxidation of 1-phenylethanol reached ca. 60% conversion. After removal of the catalyst, the reaction was monitored for an additional hour, and additional acetophenone formation was not observed.10 These results show that the reaction proceeds on the NiZn interlayer and that leaching of the dissolved Pd species does not occur under the reaction conditions. Sterically hindered 2-adamantanol (Table 4, entry 1) and 1-(2-naphthalenyl)ethanol (entry 11) were also oxidised to the corresponding ketones in the presence of [1–Pd(OH)2]−/PO43−/NiZn catalyst. Cinnamyl alcohol afforded cinnamaldehyde, accompanied by the formation of a small amount of the hydrogen transfer product of the double bonds (entry 12). Although the present catalytic system was effective for the oxidation of benzylic and allylic alcohols, several limitations for substrates were observed. Except for cyclopentanol, for example, the oxidation of the cyclic aliphatic alcohols with the [1–Pd(OH)2]−/PO43−/NiZn catalyst was relatively slow (entries 13–16). In the case of nonactivated linear aliphatic alcohols such as 1-octanol or 2-octanol, the selectivity for the corresponding carbonyl compounds was over 97% in all cases, however, the yields were low (entries 17 and 18). Unfortunately, heterocyclic alcohols, such as 2-pyridinemethanol and 2-thiophenemethanol, were not efficiently oxidised with the [1–Pd(OH)2]−/PO43−/NiZn catalyst, probably due to the strong coordination of nitrogen or sulfur atoms to the Pd(II) centre (entries 19 and 20).
2 ratio of O2 uptake to benzaldehyde yield was observed, suggesting that molecular oxygen was used quantitatively as an oxidant in the oxidative dehydrogenation reaction. In all cases, the aldehydes were produced from primary alcohols without over-oxidation to the corresponding carboxylic acid. The oxidation of the secondary benzylic alcohols produced high yields of ketone (entries 6 and 10). Selective oxidation of the hydroxyl groups was observed for cyclopropyl phenyl carbinol with a radical clock, where any skeletal isomerisation or carbon–carbon bond cleavage did not occur (entry 10). After the oxidation, the spent [1–Pd(OH)2]−/PO43−/NiZn catalyst was easily separated by simple centrifugation or filtration. The recovered catalyst could be reused without additional treatment, and its high activity and selectivity were maintained (entries 7 and 8). In order to further demonstrate the requirement of the heterogeneous [1–Pd(OH)2]−/PO43−/NiZn catalyst, the catalyst was removed by hot filtration after the oxidation of 1-phenylethanol reached ca. 60% conversion. After removal of the catalyst, the reaction was monitored for an additional hour, and additional acetophenone formation was not observed.10 These results show that the reaction proceeds on the NiZn interlayer and that leaching of the dissolved Pd species does not occur under the reaction conditions. Sterically hindered 2-adamantanol (Table 4, entry 1) and 1-(2-naphthalenyl)ethanol (entry 11) were also oxidised to the corresponding ketones in the presence of [1–Pd(OH)2]−/PO43−/NiZn catalyst. Cinnamyl alcohol afforded cinnamaldehyde, accompanied by the formation of a small amount of the hydrogen transfer product of the double bonds (entry 12). Although the present catalytic system was effective for the oxidation of benzylic and allylic alcohols, several limitations for substrates were observed. Except for cyclopentanol, for example, the oxidation of the cyclic aliphatic alcohols with the [1–Pd(OH)2]−/PO43−/NiZn catalyst was relatively slow (entries 13–16). In the case of nonactivated linear aliphatic alcohols such as 1-octanol or 2-octanol, the selectivity for the corresponding carbonyl compounds was over 97% in all cases, however, the yields were low (entries 17 and 18). Unfortunately, heterocyclic alcohols, such as 2-pyridinemethanol and 2-thiophenemethanol, were not efficiently oxidised with the [1–Pd(OH)2]−/PO43−/NiZn catalyst, probably due to the strong coordination of nitrogen or sulfur atoms to the Pd(II) centre (entries 19 and 20).
Table 5 Substrate scope for the aerobic alcohol oxidation with the [1–Pd(OH)2]−/PO43−/NiZn catalysta
| Entry |
Substrate |
Time/h |
Product |
Conv.b /% |
Yieldb /% |
|
Substrate (0.5 mmol), [1–Pd(OH)2]−/PO43−/NiZn catalyst (Pd: 1 mol%), TFT (5 mL), 80 °C, air flow (10 mL min−1).
Determined by GC analysis using an internal standard technique.
Under N2 atmosphere.
1st recycle.
2nd recycle.
Byproduct was benzenepropanal.
Reaction was carried out at 100 °C with catalyst (Pd: 2 mol%).
|
| |

|
|

|
|
|
| 1 |
R1 = H |
R2 = H |
1 |
R1 = H |
R2 = H |
>99 |
>99 |
| 2c |
R1 = H |
R2 = H |
1 |
R1 = H |
R2 = H |
Trace |
Trace |
| 3 |
R1 = OMe |
R2 = H |
0.5 |
R1 = OMe |
R2 = H |
>99 |
99 |
| 4 |
R1 = Me |
R2 = H |
1 |
R1 = Me |
R2 = H |
96 |
92 |
| 5 |
R1 = Cl |
R2 = H |
10 |
R1 = Cl |
R2 = H |
12 |
12 |
| 6 |
R1 = H |
R2 = Me |
1 |
R1 = H |
R2 = Me |
>99 |
>99 |
| 7d |
R1 = H |
R2 = Me |
1 |
R1 = H |
R2 = Me |
>99 |
>99 |
| 8e |
R1 = H |
R2 = Me |
1 |
R1 = H |
R2 = Me |
99 |
98 |
| 9 |
R1 = H |
R2 = Ph |
9 |
R1 = H |
R2 = Ph |
98 |
98 |
| 10 |
R1 = H |
R2 = cyclopropyl |
9 |
R1 = H |
R2 = cyclopropyl |
92 |
91 |
| 11 |

|
24 |
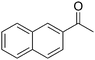
|
63 |
61 |
| 12 |

|
1 |

|
>99 |
86f |
| 13 |
n = 1 |
3 |
n = 1 |
>99 |
92 |
| 14 |
n = 2 |
24 |
n = 2 |
43 |
40 |
| 15g |
n = 4 |
24 |
n = 4 |
77 |
75 |
| 16 |

|
24 |

|
74 |
74 |
| 17g |

|
24 |

|
27 |
27 |
| 18g |
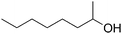
|
24 |
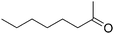
|
49 |
47 |
| 19 |
|
24 |

|
23 |
21 |
| 20 |

|
24 |

|
Trace |
Trace |
It should be noted that no spectral change was observed in the PdK-edge XANES, FT of k3-weighted PdK-edge EXAFS spectra (Fig. 2g and Fig. 3g), or the curve-fitting analysis (Table 2) between the fresh and the recovered [1–Pd(OH)2]−/PO43−/NiZn catalyst. These results suggest that the Pd species remain in the divalent monomeric state throughout the course of the reaction due to the strong electrostatic interactions between the anionic [1–Pd(OH)2]− species and the layered NiZn host.4a This strong electrostatic interaction successfully suppressed the formation of deactivated Pd black, and the [1–Pd(OH)2]−/PO43−/NiZn catalyst actualized the high turnover number (TON, the mole of the product per mole of the Pd content) of 2000 for the aerobic oxidation of 1-phenylethanol at 100 °C for 48 h.18 Furthermore, the addition of catalytic amounts of radical scavengers (1 equivalent relative to Pd), such as 2,2′,6,6′-tetramethylpiperidine-N-oxyl (TEMPO) or 2,6-di-tert-butyl-p-cresol, did not affect the oxidation rate. This result, in combination with the radical clock studies and selective formation of aldehydes, suggests that the catalytic system did not contain free radical intermediates. Furthermore, the oxidation of primary hydroxyl groups was selective in the presence of secondary hydroxyl groups. For instance, an intramolecular competition experiment was performed using 4-(1′-hydroxyethyl) benzylalcohol. At 10% conversion, 4-(1′-hydroxyethyl) benzaldehyde (91% selectivity) and 4-acetylbenzylalcohol (9% selectivity) were observed, and further oxidation into 4-acetylbenzaldehyde did not occur. These results suggest that the oxidation rate of primary alcohols is faster than that of secondary alcohols and that a Pd2+-alcoholate intermediate was formed during the reaction.19 Competitive oxidations of para-substituted benzyl alcohols gave a Hammett ρ value of −0.562 (R2 = 0.95),10 indicating a transition state with positive charge on the carbon is formed. Thus, β-hydride elimination via the above cationic transition state is involved in the oxidation. Based on the aforementioned results, the following reaction mechanism for the oxidation of alcohols by the [1–Pd(OH)2]−/PO43−/NiZn catalyst was proposed. The PO43− anion in the NiZn interlayer abstracts a proton from the alcohol substrate coordinated to the Pd(II) center giving rise to a Pd(II)-alcoholate species and H2O with PO43−regeneration. A Pd(II)-hydride species and the carbonyl product are produced after β-hydride elimination. The Pd(II)-hydride can then reductively eliminate an equivalent of H2O to form Pd(0), which is readily reoxidised by molecular oxygen to form a Pd(II)-peroxide species. The reaction of Pd(II)-peroxide species with H2O completes the catalytic cycle. The kinetic isotope effect for the intramolecular competitive oxidation of α-deuterio-p-methylbenzyl alcohol gave a kH/kD value of 1.98 at 80 °C in toluene-d8, which is consistent with the kinetic isotope effects observed in similar systems. These data strongly suggest that the elimination of β-hydride from the Pd(II)-alcoholate species is the rate-determining step.20
Conclusions
We demonstrated that a function-integrated [1–Pd(OH)2]−/PO43−/NiZn catalyst system effectively catalyses the aerobic alcohol oxidation into the corresponding carbonyl compounds. We are currently investigating the application of our clay–Pd(II)-amino acid catalyst toward asymmetric syntheses, for example the kinetic resolution of racemic secondary alcohols.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (21760623). Some of the experiments were carried out at a facility in the Photon Factory (KEK-PF, Proposal No. 2009G069).
References
- For excellent reviews, see
(a)
S. L. Scott and E. W. Deguns, in Nanostructured Catalysts, ed. S. L. Scott, C. M. Crudden and C. W. Jones, Kluwer Academic/Plenum Publishers, New York, 2003, p. 1 Search PubMed;
(b) J. Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. B. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC;
(c) J. Guerra and M. A. Herrero, Nanoscale, 2010, 2, 1390 RSC;
(d) I. Lee, M. A. Albiter, Q. Zhang, J. Ge, Y. Yin and F. Zaera, Phys. Chem. Chem. Phys., 2011, 13, 2449 RSC;
(e) N. R. Shiju and V. V. Guliants, Appl. Catal., A, 2009, 356, 1 CrossRef CAS;
(f) R. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082 CrossRef CAS.
- For reviews, see
(a) J. M. Fraile, J. I. García, C. I. Herrerías, J. A. Mayoral and E. Pires, Chem. Soc. Rev., 2009, 38, 695 RSC;
(b) K. Zhu, D. Wang and J. Liu, Nano Res., 2009, 2, 1 Search PubMed;
(c) A. De Stefanis and A. A. G. Tomlinson, Catal. Today, 2006, 114, 126 Search PubMed;
(d) R. S. Varma, Tetrahedron, 2002, 58, 1235 CrossRef CAS;
(e) S. Shimazu, Clay Sci., 2001, 40, 166 Search PubMed;
(f) H. H. Murray, Appl. Clay Sci., 2000, 17, 207 CrossRef CAS;
(g) A. Vaccari, Catal. Today, 1998, 41, 53 CrossRef CAS;
(h) A. Vaccari, Appl. Clay Sci., 1999, 14, 161 CrossRef CAS;
(i) S. Shimazu and T. Uematsu, J. Synth. Org. Chem. Jpn., 1993, 51, 664 Search PubMed.
-
(a) R. M. R. Bull, C. Markland, G. R. Williams and D. O'Hare, J. Mater. Chem., 2011, 21, 1822 RSC;
(b) E. Kandare and J. M. Hossenlopp, Inorg. Chem., 2006, 45, 3766 CrossRef CAS;
(c) R. Rojas, C. Barriga, M. A. Ulibarri, P. Malet and V. Rives, J. Mater. Chem., 2002, 12, 1071 RSC;
(d) H. Morioka, H. Tagaya, M. Karasu, J. Kadokawa and K. Chiba, Inorg. Chem., 1999, 38, 4211 CrossRef CAS;
(e) J.-H. Choy, Y.-M. Kwon, K.-S. Han, S.-W. Song and S. H. Chang, Mater. Lett., 1998, 34, 356 CrossRef CAS;
(f) S. Yamanaka, K. Ando and M. Ohashi, MRS Online Proc. LIbr., 1995, 371, 131 Search PubMed;
(g) M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1993, 32, 1209 CrossRef CAS.
-
(a) T. Hara, M. Ishikawa, J. Sawada, N. Ichikuni and S. Shimazu, Green Chem., 2009, 11, 2034 RSC;
(b) T. Hara, J. Kurihara, N. Ichikuni and S. Shimazu, Chem. Lett., 2010, 39, 304 CrossRef CAS.
- For reviews, see
(a) T. Tsukuda, H. Tsunoyama and H. Sakurai, Chem.–Asian J., 2011, 6, 736 CrossRef CAS;
(b) C. P. Vinod, K. Wilson and A. F. Lee, J. Chem. Technol. Biotechnol., 2011, 86, 161 Search PubMed;
(c) K. Kaneda and T. Mizugaki, Energy Environ. Sci., 2009, 2, 655 RSC;
(d) T. Matsumoto, M. Ueno, N. Wang and S. Kobayashi, Chem.–Asian J., 2008, 3, 196 CrossRef CAS;
(e) M. J. Schultz and M. S. Sigman, Tetrahedron, 2006, 62, 8227 CrossRef CAS.
- B. A. Steinhoff, A. E. King and S. S. Stahl, J. Org. Chem., 2006, 71, 1861 CrossRef CAS.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Green Chem., 2009, 11, 793 RSC.
-
(a) B. Rosenberg, L. Van Camp and T. Krigas, Nature, 1965, 205, 698 CAS;
(b) B. Rosenberg, L. Van Camp, J. E. Trosko and V. H. Mansour, Nature, 1969, 222, 385 CrossRef CAS.
- For α-amino acid–palladium complexes, see
(a) J. Zhang, L. Li, L. Wang, F. Zhang and X. Li, Eur. J. Med. Chem., 2010, 45, 5337 Search PubMed;
(b) J. Albert, M. Crespo, J. Granell, J. Rodríguez, J. Zafrilla, T. Calvet, M. Font-Bardia and X. Solans, Organometallics, 2010, 29, 214 CrossRef CAS;
(c) Y.-Z. Hao, Z.-X. Li and J.-L. Yian, J. Mol. Catal. A: Chem., 2007, 265, 258 CrossRef CAS;
(d) V. B. Valodkar, G. L. Tembe, M. Ravindranathan, R. N. Ram and H. S. Rama, J. Mol. Catal. A: Chem., 2003, 202, 47 Search PubMed;
(e) R. Navarro, J. García, E. P. Urriolabeitia, C. Cativiela and M. D. Doaz-de-Villegas, J. Organomet. Chem., 1995, 490, 35 CrossRef CAS.
- See ESI†.
- As a single crystal of the (1–PdCl2)− complex was not successfully obtained, we cannot explain the detailed crystal structural data of this anionic [1–PdCl2]− complex.
- The FT-IR spectrum of the PO43−/NiZn catalyst showed two peaks at 1009 cm−1 and 1078 cm−1 based on νP–O of PO43− and νP–O of HPO42−, respectively. See ESI†.
- In the FT-IR spectrum of this catalyst, no significant change of the spectrum was observed, due to the low loading amount of the guest anionic [1–Pd(OH)2]− complex.
- Following the screening of solvent for aerobic benzyl alcohol oxidation, trifluorotoluene showed the highest catalytic activity.
- The heterogeneous Pd catalyst, which is prepared by using L-valine instead of D-valine, can also promote aerobic alcohol oxidation effectively. For example, the quantitative amount of benzaldehyde was obtained after 1 h by using the L-valine–Pd(II)/PO43−/NiZn catalyst under the same reaction conditions.
- The proton abstraction from an activated substrate by a phosphate anion was also reported, see K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2003, 125, 11460 Search PubMed.
-
(a) N. Kakiuchi, Y. Maeda, Y. Nishimura and S. Uemura, J. Org. Chem., 2001, 66, 6620 CrossRef CAS;
(b) M. J. Schultz, S. S. Hamilton, D. R. Jensen and M. S. Sigman, J. Org. Chem., 2005, 70, 3343 CrossRef CAS;
(c) S. Shimazu, T. Uehara, A. Asami, T. Hara and N. Ichikuni, J. Mol. Catal. A: Chem., 2008, 282, 28 CrossRef CAS.
- Aerobic 1-phenylethanol oxidation using our [1–Pd(OH)2]−/PO43−/NiZn catalyst was carried out under the following reaction conditions: 1-phenylethanol (2 mmol), [1–Pd(OH)2]−/PO43−/NiZn catalyst (0.05 g, Pd: 0.05 mol%), PhCF3 (5 mL), 373 K, and air flow (20 mL min−1), and products were determined by GC analysis using an internal standard technique. After 48 h, the acetophenone was yielded quantitatively.
-
(a) P. A. Shapley, N. Zhang, J. L. Allen, D. H. Pool and H.-C. Liang, J. Am. Chem. Soc., 2000, 122, 1079 CrossRef CAS;
(b) A. Dijksman, A. Marino-González, A. M. I. Payeras, I. W. C. E. Arends and R. A. Sheldon, J. Am. Chem. Soc., 2001, 123, 6826 CrossRef CAS;
(c) K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2000, 122, 7144 CrossRef CAS;
(d) K. Yamaguchi and N. Mizuno, Chem.–Eur. J., 2003, 9, 4353 CrossRef CAS.
-
(a) K. Zaw, M. Lautens and P. M. Henry, Organometallics, 1985, 4, 1286 CrossRef CAS;
(b) B. A. Steinhoff, S. R. Fix and S. S. Stahl, Org. Lett., 2002, 4, 4179 CrossRef CAS;
(c) G.-J. ten Brink, I. W. C. E. Arends and R. A. Sheldon, Adv. Synth. Catal., 2002, 344, 355 CrossRef CAS;
(d) J. A. Mueller, D. R. Jensen and M. S. Sigman, J. Am. Chem. Soc., 2002, 124, 8202 CrossRef CAS;
(e) J. A. Mueller and M. S. Sigman, J. Am. Chem. Soc., 2003, 125, 7005 CrossRef CAS;
(f) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00223f |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![Pd
K-edge XANES spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(d-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn.](/image/article/2011/CY/c1cy00223f/c1cy00223f-f2.gif)
![FT of k3-weighted PdK-edge EXAFS spectra for (a) 1–Pd complex solution, (b) aqueous K2PdCl4 solution (pH = 1), (c) [Pd(d-valine)2]·H2O, (d) Pd oxide, (e) Pd foil, (f) fresh [1–Pd(OH)2]−/PO43−/NiZn, and (g) recovered [1–Pd(OH)2]−/PO43−/NiZn.](/image/article/2011/CY/c1cy00223f/c1cy00223f-f3.gif)
![Proposed structure of (a) anionic (1–PdCl2)− complex and (b) anionic [1–Pd(OH)2]− complex in an NiZn interlayer.](/image/article/2011/CY/c1cy00223f/c1cy00223f-s1.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of O2 uptake to benzaldehyde yield was observed, suggesting that molecular oxygen was used quantitatively as an oxidant in the oxidative dehydrogenation reaction. In all cases, the aldehydes were produced from primary alcohols without over-oxidation to the corresponding carboxylic acid. The oxidation of the secondary benzylic alcohols produced high yields of ketone (entries 6 and 10). Selective oxidation of the hydroxyl groups was observed for cyclopropyl phenyl carbinol with a radical clock, where any skeletal isomerisation or carbon–carbon bond cleavage did not occur (entry 10). After the oxidation, the spent [1–Pd(OH)2]−/PO43−/NiZn catalyst was easily separated by simple centrifugation or filtration. The recovered catalyst could be reused without additional treatment, and its high activity and selectivity were maintained (entries 7 and 8). In order to further demonstrate the requirement of the heterogeneous [1–Pd(OH)2]−/PO43−/NiZn catalyst, the catalyst was removed by hot filtration after the oxidation of 1-phenylethanol reached ca. 60% conversion. After removal of the catalyst, the reaction was monitored for an additional hour, and additional acetophenone formation was not observed.10 These results show that the reaction proceeds on the NiZn interlayer and that leaching of the dissolved Pd species does not occur under the reaction conditions. Sterically hindered 2-adamantanol (Table 4, entry 1) and 1-(2-naphthalenyl)ethanol (entry 11) were also oxidised to the corresponding ketones in the presence of [1–Pd(OH)2]−/PO43−/NiZn catalyst. Cinnamyl alcohol afforded cinnamaldehyde, accompanied by the formation of a small amount of the hydrogen transfer product of the double bonds (entry 12). Although the present catalytic system was effective for the oxidation of benzylic and allylic alcohols, several limitations for substrates were observed. Except for cyclopentanol, for example, the oxidation of the cyclic aliphatic alcohols with the [1–Pd(OH)2]−/PO43−/NiZn catalyst was relatively slow (entries 13–16). In the case of nonactivated linear aliphatic alcohols such as 1-octanol or 2-octanol, the selectivity for the corresponding carbonyl compounds was over 97% in all cases, however, the yields were low (entries 17 and 18). Unfortunately, heterocyclic alcohols, such as 2-pyridinemethanol and 2-thiophenemethanol, were not efficiently oxidised with the [1–Pd(OH)2]−/PO43−/NiZn catalyst, probably due to the strong coordination of nitrogen or sulfur atoms to the Pd(II) centre (entries 19 and 20).
2 ratio of O2 uptake to benzaldehyde yield was observed, suggesting that molecular oxygen was used quantitatively as an oxidant in the oxidative dehydrogenation reaction. In all cases, the aldehydes were produced from primary alcohols without over-oxidation to the corresponding carboxylic acid. The oxidation of the secondary benzylic alcohols produced high yields of ketone (entries 6 and 10). Selective oxidation of the hydroxyl groups was observed for cyclopropyl phenyl carbinol with a radical clock, where any skeletal isomerisation or carbon–carbon bond cleavage did not occur (entry 10). After the oxidation, the spent [1–Pd(OH)2]−/PO43−/NiZn catalyst was easily separated by simple centrifugation or filtration. The recovered catalyst could be reused without additional treatment, and its high activity and selectivity were maintained (entries 7 and 8). In order to further demonstrate the requirement of the heterogeneous [1–Pd(OH)2]−/PO43−/NiZn catalyst, the catalyst was removed by hot filtration after the oxidation of 1-phenylethanol reached ca. 60% conversion. After removal of the catalyst, the reaction was monitored for an additional hour, and additional acetophenone formation was not observed.10 These results show that the reaction proceeds on the NiZn interlayer and that leaching of the dissolved Pd species does not occur under the reaction conditions. Sterically hindered 2-adamantanol (Table 4, entry 1) and 1-(2-naphthalenyl)ethanol (entry 11) were also oxidised to the corresponding ketones in the presence of [1–Pd(OH)2]−/PO43−/NiZn catalyst. Cinnamyl alcohol afforded cinnamaldehyde, accompanied by the formation of a small amount of the hydrogen transfer product of the double bonds (entry 12). Although the present catalytic system was effective for the oxidation of benzylic and allylic alcohols, several limitations for substrates were observed. Except for cyclopentanol, for example, the oxidation of the cyclic aliphatic alcohols with the [1–Pd(OH)2]−/PO43−/NiZn catalyst was relatively slow (entries 13–16). In the case of nonactivated linear aliphatic alcohols such as 1-octanol or 2-octanol, the selectivity for the corresponding carbonyl compounds was over 97% in all cases, however, the yields were low (entries 17 and 18). Unfortunately, heterocyclic alcohols, such as 2-pyridinemethanol and 2-thiophenemethanol, were not efficiently oxidised with the [1–Pd(OH)2]−/PO43−/NiZn catalyst, probably due to the strong coordination of nitrogen or sulfur atoms to the Pd(II) centre (entries 19 and 20).
















