A novel method to synthesize diphenyl carbonate from carbon dioxide and phenol in the presence of methanol†
Guozhi
Fan
*a,
Haitao
Zhao
b,
Zhenxiao
Duan
a,
Tao
Fang
a,
Minghai
Wan
a and
Liangnian
He
*c
aSchool of Chemical and Environmental Engineering, Wuhan Polytechnic University, Wuhan, 430023, China. E-mail: fgzcch@whpu.edu.cn; Fax: +86 27 83956762; Tel: +86 27 83943956
bDepartment of Chemistry, School of Science, Tianjin University, Tianjin, 300072, China
cKey Laboratory and Institute of Elemento-Organic Chemistry, Nankai University, Tianjin, 300071, China. E-mail: heln@nankai.edu.cn
First published on 25th July 2011
Abstract
A novel method for the direct synthesis of diphenyl carbonate (DPC) from carbon dioxide and phenol catalyzed by Lewis acid in the presence of methanol was developed. It was found that the simple Lewis acids are not effective for catalyzing the production of DPC, but (salen)Co(OAc) containing a quaternary phosphonium salt unit anchored on the ligand displayed excellent catalytic activity under mild conditions using dense carbon dioxide as reactant and solvent. A possible mechanism for the formation of DPC was proposed based on the GC-MS analysis and DFT calculation.
Diphenyl carbonate (DPC) is an important chemical intermediate traditionally synthesized from phosgene and phenol. However, the hazardous phosgene process has to be improved or replaced by more environmentally friendly processes due to increasing demand for safer and more environmentally benign processes.1 An alternative method is synthesis of DPC through the reaction of phenol with carbon monoxide, which is considered the most likely route to prepare DPC without employing toxic phosgene.2 However, oxidative carbonylation of carbon monoxide is usually catalyzed by a noble metal Pd catalyst in the presence of a co-catalyst, and the complexity of the system often leads to more by-products and poor selectivity.3
Meanwhile, carbon dioxide (CO2) is not only a greenhouse gas but is also the greatest carbon resource in the world. In recent years, much attention has been paid to the chemical fixation of CO2 to synthesize useful intermediates and chemicals.4 The replacement of traditional C1 chemicals, such as carbon monoxide, phosgene and syngas, by CO2 is considered an effective way to utilize CO2.5 A number of compounds have been reported to react with CO2 in the presence of metal catalysts, including hydrogen, alkenes, acetals, epoxides, amines, and carbon–carbon unsaturated compounds.6
The synthesis of cyclic carbonates from CO2 is an attractive methodology in performing the chemical fixation of CO2,7 especially the direct synthesis of dimethyl carbonate (DMC) from CO2, methanol and epoxide,8 but only a few examples of DPC.9 An alternative method for providing DPC from CO2, which involves the intermediacy of cyclic carbonates from epoxides and CO2 followed by transesterification processes, has been investigated,10 in which DMC is first obtained from cyclic carbonates and methanol, and DPC is then obtained from DMC and phenol. Too many reactants are involved although excellent yield of DPC has been provided. In fact, the direct synthesis of DPC can be achieved through the reaction of CO2 with phenol catalyzed by Lewis acid in the presence of carbon tetrachloride (CCl4).9 There are phenoxides and salicylic acid-like compounds formed from CO2 and phenol catalyzed by Lewis acid, and the salicylic acid-like compounds can be further transformed into DPC in the presence of CCl4.9,11 However, the reaction between CO2 and phenol catalyzed by zinc chloride in the presence of CCl4 is also accompanied by the formation of hydrogen chloride, which often leads to pollution and corrosion.12 Moreover, CCl4 as the ozone-depleting substance (ODS) is one of the controlled substances to protect public health and the environment.
Thus, the aim of this investigation is to explore a “greener” synthetic methodology for DPC from CO2. Methanol (CH3OH) has been employed as an alternative for CCl4. Based on the formation of salicylic acid from phenol and CO2,11methyl phenyl carbonate (MPC) is expected to be formed via the reaction of salicylic acid-like compounds with CH3OH.‡DPC is then formed by the transesterification between MPC and phenol.1c,13 Acceptable yield and selectivity were obtained catalyzed by the (salen)Co(OAc) complex under relatively mild conditions (90 °C, 8.0 MPa and 10 h) using compressed CO2 as reactant and solvent.
Lewis acids have been confirmed as efficient catalysts for the carboxylation of phenol with CO2 to form salicylic acid.11 In this study, several common Lewis acids were first selected as catalysts for the direct synthesis of DPC from compressed CO2 and phenol in the presence of CH3OH (Table 1, entries 1–8). The formation of DPC and MPC was confirmed by GC-MS (see ESI†). AlBr3 was found to be the most active catalyst under identical conditions (Table 1, entry 5). The Lewis acidity of AlBr3 is believed to be the strongest among the selected Lewis acids.11 Thus, the catalytic activity of the simple Lewis acids may be related to the acidity. Besides DPC and MPC, a small amount of 3-bromophenol and 4-bromophenol were also detected by GC-MS in the liquid product catalyzed by AlBr3 (see ESI†). The formation of these by-products leads to the relatively low selectivity to products, and the overall selectivity to products was only 65% (Table 1, entry 8).
| Entry | Catalyst | Conversion of phenolb (%) | Yield of DPCb (%) | Yield of MPCb (%) | Selectivityb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 10 mmol phenol, 30 mmol CH3OH and 4 mmol Lewis acid at 100 °C and 9 MPa for 2 h. b Determined by GC, based on the charged phenol. c 3 mmol AlBr3 was employed. d 5 mmol AlBr3 was employed. | |||||
| 1 | ZnCl2 | 2.5 | 0.8 | 0.5 | 52 |
| 2 | ZnBr2 | 4.4 | 1.9 | 0.6 | 57 |
| 3 | ZnI2 | 6.4 | 2.4 | 0.9 | 52 |
| 4 | AlCl3 | 2.7 | 1.2 | 0.5 | 63 |
| 5 | CoCl2 | 7.6 | 2.5 | 1.4 | 51 |
| 6 | Co(OAc)2 | 3.0 | 1.8 | 0.5 | 76 |
| 7 | Zn(OAc)2 | 2.7 | 1.5 | 0.5 | 74 |
| 8 | AlBr3 | 11 | 5.9 | 1.1 | 65 |
| 9 | AlBr3c | 10 | 6.0 | 1.3 | 70 |
| 10 | AlBr3d | 14 | 5.0 | 2.3 | 52 |
No salicylic acid, which has been reported to be the main product from phenol and CO2 without other reactants involved,11 but MPC in the liquid product was ascertained. The formation of MPC indicates that our assumption is probably correct. To confirm this point further, we have carried out an experiment on the mechanistic pathways for the direct synthesis of DPC using DFT calculations (see ESI†). Two possible reaction pathways were proposed, and MPC was observed as an intermediate in one of the paths. It is reasonable to conclude that the formation of DPC undergoes the transesterification of MPC with phenol based on the results of GC-MS and the DFT calculation. A possible reaction path is then suggested in Scheme 1. First, phenoxyaluminium 1 was generated from phenol catalyzed by AlBr3.11 Then, the insertion of CO2 into the O–Al bond of 1 would give the salicylic acid-like compound 2, the rearrangement of 2 resulted in an aluminium carbonate active species 3. The reaction between 3 and CH3OH would lead to MPC 4. DPC was finally formed via the transesterification of 1 with 4.
 | ||
| Scheme 1 Plausible mechanism for the synthesis of DPC from CO2 and phenol in the presence of CH3OH. | ||
The total synthetic pathway of DPC from CO2 and phenol in the presence of CH3OH can be concluded from the suggested mechanism above, just as shown in Scheme 2. It seems from the stoichiometric equation of chemical reaction that CH3OH isn't involved. It has been reported that salicylic acid is the main product from phenol and CO2 catalyzed by AlBr3 without other additive,11 but no salicylic acid was detected in the liquid product in the presence of CH3OH. We speculated that the formation of salicylic acid may be inhibited by the reaction between CH3OH and 3. It can be seen from Scheme 1 that MPC resulted from CH3OH and 3 is a crucial intermediate for the formation of DPCvia transesterification. Thus, CH3OH is essential and may be just a promoter for the transesterification.
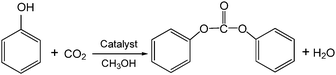 | ||
| Scheme 2 Synthesis of DPC from CO2 and phenol in the presence of CH3OH. | ||
The results shown in Table 1 clearly illustrate that the simple Lewis acids including AlBr3 are not effective catalysts for the synthesis of DPC since an AlBr3 loading of 30 mol% (based on phenol) was required (Table 1, entry 9). The salen complexes have been reported to possess excellent performance in the reactions involving CO2 in our previous work,14 it appears reasonable to assume that they may have activity in the synthesis of DPC from CO2. The structure of the salen complexes used in this work is shown in Scheme 3.
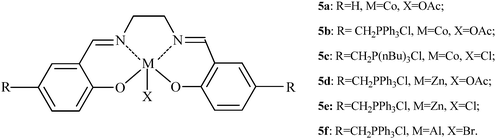 | ||
| Scheme 3 The structure of the salen complexes. | ||
The complexes derived from salicylaldehyde exhibited very poor activity (Table 2, entry 1) possibly due to their low solubility.15 This observation is in agreement with our previous findings.16 The catalytic performance of the salen complexes from (triphenylphosphonium)methyl chloride substituted salicylaldehyde was greater than that in the presence of AlBr3 (Table 2, entries 2–6), both the yield and the selectivity were enhanced although the amount of catalyst was decreased. Almost no by-products were detected by GC-MS (see ESI†) in the liquid product catalyzed by salen complexes, which is contributed to the noticeable improvement of the selectivity. The complex 5b was found to be superior to the others (Table 2, entry 2). 7.5 mol% loading of 5b resulting in 15% yield of DPC, 2.9% yield of MPC, and 87% overall selectivity to products, respectively. Although a significant influence of the amount of complex on the yield of DPC was observed, the yield almost keeps constant when the catalyst loading reached 7.5 mol% (based on phenol).
| Entry | Catalyst | Conversion of phenolb (%) | Yield of DPCb (%) | Yield of MPCb (%) | Selectivityb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 10 mmol phenol, 30 mmol CH3OH and 0.75 mmol catalyst at 100 °C and 9 MPa for 10 h. b Determined by GC, based on the charged phenol. c 0.5 mmol 5b was employed. d 1 mmol 5b was employed. | |||||
| 1 | 5a | 8.9 | 6.1 | 1.7 | 88 |
| 2 | 5b | 21 | 15 | 2.9 | 87 |
| 3 | 5c | 13 | 8.1 | 2.2 | 81 |
| 4 | 5d | 18 | 13 | 2.8 | 89 |
| 5 | 5e | 14 | 8.7 | 2.4 | 82 |
| 6 | 5f | 15 | 9.0 | 2.7 | 80 |
| 7 | 5b c | 15 | 11 | 2.3 | 87 |
| 8 | 5b d | 22 | 16 | 3.0 | 86 |
Fig. 1 shows the effect of CO2 pressure on the conversion, selectivity, and yield obtained at different pressures from 5 to 12 MPa. Results showed that the conversion was dependent on pressure below 8 MPa. The variation of density around the critical point generally causes a change in chemical or physical equilibrium and affects the interaction of CO2 with the reactant and/or the intermediates in the liquid phase.17 The unique properties appearing near the critical point are probably responsible for the maximal conversion obtained around 8 MPa. Above 8 MPa, almost no significant change in the conversion of phenol occurred with further increase in CO2 pressure. This can be ascribed to the mutual solubility of CO2 in the organic phase. Both the solubility of CO2 in the organic phase and the amount of organic compounds dissolved in the gas phase increased along with increase in CO2 pressure.9d,18 The former is beneficial to the reaction, but the latter is not. Thus, it can be said that the two opposite factors could compensate each other, resulting in the marginal variation of the conversion with CO2 pressure.
 | ||
| Fig. 1 Effect of CO2 pressure on the synthesis of DPC. Reaction conditions: 10 mmol phenol, 30 mmol CH3OH and 0.75 mmol 5b at 100 °C for 10 h. | ||
The influence of reaction temperature was also examined in the range of 70–130 °C. Results shown in Fig. 2 revealed that the conversion, selectivity and yield were also dependent on temperature. Excellent selectivity of 98%, 17% yield of DPC and 2.5% yield of MPC were obtained at 90 °C, respectively. Further increase in temperature led to a decrease in yield, although the conversion monotonously increased. The different changes in the conversion, selectivity and yield can be attributed to the intermediates formed during the reaction of phenol with CO2. Several intermediates, such as salicylic acid-like compounds, p-hydroxybenzoic acid-like compounds and metal phenoxide–CO2 complex, are formed during the activation of CO2.9d,19 Among them, salicylic acid-like compounds and metal phenoxide–CO2 complex further change into the desired product at moderate temperature, resulting in an increase in selectivity with increasing temperature below 90 °C. However, further increase in temperature is favorable for the formation of p-hydroxybenzoic acid-like compounds that cannot be transferred into the desired product.9d,19 Thus, a decrease in selectivity and yield at a temperature above 90 °C was observed in this work.
 | ||
| Fig. 2 Effect of reaction temperature on the synthesis of DPC. Reaction conditions: 10 mmol phenol, 30 mmol CH3OH and 0.75 mmol 5b at 8 MPa for 10 h. | ||
In summary, we have developed a new approach for the direct synthesis of DPC from CO2 and phenol catalyzed by Lewis acids in the presence of CH3OH. Dense CO2 was used as reactant and solvent, and no additional organic solvent was required. The simple Lewis acid reveals poor catalytic activity but (salen)Co(OAc) complexes containing a quaternary phosphonium salt unit anchored on the ligand complex revealed excellent catalytic performance. The conversion, selectivity and yield have been found to be dependent on the CO2 pressure and reaction temperature. 19% yield of DPC, 3.1% yield of MPC and 98% overall selectivity were obtained at 8 MPa and 90 °C for 10 h. A possible mechanism for the formation of DPC was proposed based on the GC-MS analysis and DFT calculation.
Acknowledgements
This work was supported by the Research Project of Hubei Provincial Department of Education of China (Q20091810).Notes and references
- (a) I. Hatanaka, N. Mitsuyasu, G. Yin, Y. Fujiwara, T. Kitamura, K. Kusakabe and T. Yamaji, J. Organomet. Chem., 2003, 674, 96–100 CrossRef CAS; (b) X. Zhao, N. Sun, S. Wang, F. Li and Y. Wang, Ind. Eng. Chem. Res., 2008, 47, 5298–5303 CrossRef; (c) J. Gong, X. Ma and S. Wang, Appl. Catal., A, 2007, 316, 1–21 CrossRef CAS.
- (a) K. Okuyama, J. Sugiyama, R. Nagahata, M. Asai, M. Ueda and K. Takeuchi, J. Mol. Catal. A: Chem., 2003, 203, 21–27 CrossRef CAS; (b) H. Yasuda, N. Maki, J. C. Choi and T. Sakakura, J. Organomet. Chem., 2003, 682, 66–72 CrossRef CAS; (c) H. Ishii, M. Goyal, M. Ueda, K. Takeuchi and M. Asai, Appl. Catal., A, 2000, 201, 101–105 CrossRef CAS.
- (a) G. Z. Fan, T. Li and G. X. Li, Appl. Organomet. Chem., 2006, 20, 656–662 CrossRef CAS; (b) G. Z. Fan, J. Huang, Z. Q. Li, T. Li and G. X. Li, J. Mol. Catal. A: Chem., 2007, 267, 34–40 CrossRef CAS.
- (a) I. Omae, Catal. Today, 2006, 115, 33–52 CrossRef CAS; (b) T. Sakakura and K. Kohno, Chem. Commun., 2009, 1312–1330 RSC.
- (a) M. V. Schilt, M. Kemmere and A. Keurentjes, Catal. Today, 2006, 115, 162–169 CrossRef; (b) S. Dinda, A. V. Patwardhan, S. R. Panda and N. C. Pradhan, Chem. Eng. J., 2008, 136, 349–357 CrossRef CAS.
- (a) H. Kawanami, A. Sasaki, K. Matsui and Y. Ikushima, Chem. Commun., 2003, 896–897 RSC; (b) J. Sun, S. Fujita, F. Zhao, M. Hasegawa and M. Arai, J. Catal., 2005, 230, 398–405 CrossRef CAS; (c) J. Sun, S. Fujita, F. Zhao and M. Arai, Appl. Catal., A, 2005, 287, 221–226 CrossRef CAS; (d) B. M. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Green Chem., 2003, 5, 340–342 RSC; (e) Z. Zhang, S. Hu, J. Song, W. Li, G. Yang and B. Han, ChemSusChem, 2009, 2, 234–238 CrossRef CAS; (f) Z. Zhang, Y. Xie, W. Li, S. Hu, J. Song, T. Jiang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 1127–1129 CrossRef CAS.
- S. Udayakumar, S. W. Park, D. W. Park and B. S. Choi, Catal. Commun., 2008, 9, 1563–1570 CrossRef CAS.
- H. Yasuda, L. N. He, T. Sakakura and C. W. Hu, J. Catal., 2005, 233, 119–122 CrossRef CAS.
- (a) Z. Li and Z. Qin, J. Mol. Catal. A: Chem., 2007, 264, 255–259 CrossRef CAS; (b) K. Su, Z. Li, B. Cheng and Y. Ren, Catal. Commun., 2008, 9, 1666–1670 CrossRef CAS; (c) Z. Li, Z. Qin, H. Zhu and J. Wang, Chem. Lett., 2006, 35, 784–785 CrossRef CAS; (d) G. Z. Fan, S. I. Fujita, B. Zou, N. Masahiro, X. C Meng and M. Arai, Catal. Lett., 2009, 133, 280–287 CrossRef CAS.
- S. Fukuoka, M. Tojo, H. Hachiya, M. Aminnaka and K. Hasegawa, Polym. J. (Tokyo), 2007, 39, 91–114 CAS.
- T. Iijima and T. Yamaguchi, J. Mol. Catal. A: Chem., 2008, 295, 52–56 CrossRef CAS.
- T. Iijima and T. Yamaguchi, Appl. Catal., A, 2008, 345, 12–17 CrossRef CAS.
- D. S. Tong, J. Yao, Y. Wang, H. Y. Niu and G. Y. Wang, J. Mol. Catal. A: Chem., 2007, 268, 120–126 CrossRef CAS.
- X. Miao, J. Q. Wang, W. Ying, Y. Du and L. N. He, ChemSusChem, 2008, 1, 236–241 CrossRef.
- W. Clegg, R. W. Harrington, M. North and R. Pasquale, Chem.–Eur. J., 2010, 16, 6828–6843 CrossRef CAS.
- G. Z. Fan, Z. G. Wang, B. Zou and M. Wang, Fuel Process. Technol., 2011, 92, 1052–1055 CrossRef CAS.
- F. Zhao, R. Zhang, M. Chatterjee, M. Ikushima and M. Arai, Adv. Synth. Catal., 2004, 346, 661–668 CrossRef CAS.
- J. Song, Z. Zhang, S. Hu, T. Wu, T. Jiang and B. Han, Green Chem., 2009, 11, 1031–1036 RSC.
- Y. Kosugi, Org. Biomol. Chem., 2003, 1, 817–818 CAS.
Footnotes |
| † Electronic supplementary information (ESI): The preparation of salen complex, the effect of dehydrating agent, the results of GC-MS and DFT calculation. See DOI: 10.1039/c1cy00208b |
| ‡ General procedure for the catalytic test, 10 mmol phenol, 30 mmol CH3OH and catalyst were charged into a 50 ml stainless steel autoclave. The autoclave was sealed and flushed with 2 MPa CO2 for 3 times to wash out the air in it. The mixture was heated to the desired reaction temperature while stirring. CO2 was then introduced into the autoclave at the desired pressure using a high-pressure pump. After a certain reaction time, the autoclave was cooled, and the pressure was gradually released. The products in the reaction mixture were confirmed by GC-MS, and the yield based on the charged phenol was determined by GC. |
| This journal is © The Royal Society of Chemistry 2011 |
