Efficient solid acid catalysts for esterification of free fatty acids with methanol for the production of biodiesel
K.
Srilatha
a,
Ch.
Ramesh Kumar
a,
B. L. A.
Prabhavathi Devi
b,
R. B. N.
Prasad
b,
P. S.
Sai Prasad
a and
N.
Lingaiah
*a
aCatalysis Laboratory, I&PC Division, Indian Institute of Chemical Technology, Hyderabad 500607, India. E-mail: nakkalingaiah@iict.res.in; Fax: +91-40-27160921; Tel: +91-40-27193163
bCentre for Lipid Science and Technology Division, Indian Institute of Chemical Technology, Hyderabad 500607, India
First published on 9th May 2011
Abstract
12-Tungstophosphoric acid (TPA) with varying contents supported on SnO2 catalysts was prepared and their efficacy as solid acid catalysts for the esterification of palmitic acid with methanol was investigated. The catalysts were characterized by X-ray diffraction (XRD), Fourier transform infrared (FTIR), temperature programmed desorption (TPD) of NH3, Laser Raman, Brunner–Emmett–Teller (BET) surface area and scanning electron microscopy (SEM) techniques. The esterification activity depends on the amount and dispersion of TPA on SnO2 which in turn relates to the amount of acidity. Among the catalysts, 15 wt% TPA/SnO2 was the most promising one with the highest fatty acid conversion. Further, reaction parameters such as catalyst concentration, methanol to palmitic acid mole ratio and reaction temperature were also optimized. The catalyst was recycled and reused with consistent activity. A pseudo first order model was applied to correlate the experimental kinetic data and kinetic parameters were evaluated. The activation energy of the catalysts is comparable to that of mineral acids and lower than usual solid acid catalysts.
Introduction
Biodiesel, a renewable source of energy seems to be an ideal solution for global energy demands.1 However, biodiesel is currently not cost competitive with the conventional diesel fuel because of its high raw material and production costs. The economics can be improved with low cost oils that contain high free fatty acids (FFAs). The use of these types of feed results in the reduction of the overall biodiesel production cost. However, the presence of FFAs is not compatible with the homogeneous base catalysts used in the transesterification reaction for biodiesel production.2 One way to overcome this problem is to subject the FFAs to esterification before the transesterification of triglycerides. Thus, esterification forms an essential step in the production of biodiesel.3Even though homogeneous acid catalysts have been used in the industrial production of esters, a major drawback lies in separation and purification of the product. They are also not environmentally benign as a considerable amount of waste is generated to be disposed off into the atmosphere.4 The significance of solid acid catalysts over homogeneous acid catalysts is in their low corrosive effect and negligible environmental problems. The other advantage of the solid acid catalysts is that they can be used in the reactive distillation which is an advantageous method for the synthesis of biodiesel from the feed stock containing large amounts of FFAs.5,6 The pros and cons of using heterogeneous catalysts for the esterification of fatty acids are explained in detail from a chemistry and environmental point of view.7 The solid acids have recently received considerable attention. Esterification of FFAs via heterogeneous acid catalysis has already been explored by a number of researchers.8–19Catalysts such as Cs-doped heteropolyacid,8WO3/ZrO2,9,10SO4−2/TiO2–SiO2,11carbohydrate-derived solid acid,12cerium tridodecyl sulfate,13propylsulfonic acid-functionalized mesoporous silica,14polymers with sulfonic acid groups,15ammonium salt of TPA,16carbon material from D-glucose,17 ion exchange resins,18 and metal oxides19 were used for esterification of fatty acids and most of these exhibited considerable catalytic activity.
The Keggin-type heteropolyacids (HPAs) are of interest as catalysts since they are strong Bronsted acids and are known for their efficient catalysis of reactions in both homogeneous and heterogeneous phases by offering cleaner processes.20–24 The disadvantages of HPAs as catalysts lie in their low thermal stability, low surface area (1–10 m2 g−1) and high solubility in polar media. HPAs can be made eco-friendly insoluble solid acid catalysts with high thermal stability and high surface area by supporting them on suitable supports. The support provides HPAs an opportunity to be dispersed over a large surface area, which increases catalytic activity. Various supports like titania,20 active carbon,21zirconia–alumina,22 clays,23 niobia,24ZrO2,25–27 mesoporous silica,28tantalum oxide,29SnO230,31 for dispersion of HPAs have already been published. Tin oxide is an interesting material due to its potential applications in solar cells, transistors etc. Recently, it has received attention as a catalyst and also as a carrier for supported catalysts. Furuta et al.32 compared the activities of sulfated zirconia and sulfated tin oxide for esterification reaction and reported that sulfated tin oxide is active due its high acidity. Thus, it is of interest to study the novelties of supported tin oxide catalysts for esterification of FFAs.
In the present study, a series of TPAs supported on SnO2 catalysts with varying TPA contents from 5–25 wt% were prepared and evaluated for the esterification of palmitic acid with methanol. The textural, structural and acidic properties of the catalyst were studied in detail and reported in our earlier publication.31 As these catalysts possess strong acidity and stability, it is of interest to know the activity of these catalysts for esterification of free fatty acids. Palmitic acid was selected as a representative of FFA. Further effect of various reaction parameters such as catalyst concentration, reaction temperature and molar ratio of the reactants on methyl palmitate formation was evaluated to optimize the reaction conditions. The experimental data correlated with a pseudo first order model. The model parameters were also obtained. The applicability of the catalysts was studied for the esterification of oils containing excess amounts of different fatty acids.
Results and discussion
Characterization of the catalysts
The TPA/SnO2 catalysts were characterized by XRD, FT-IR, NH3-TPD, Laser Raman, SEM, BET surface area. XRD, FTIR, TPD of NH3 and Raman characterization results of the TPA/SnO2 catalysts were already discussed in detail in our previous paper.31 In the context of the present work few salient features of these results are mentioned. XRD data of the catalysts reveal the well-dispersion of TPA Keggin ions up to 15 wt% beyond which they attain bulk nature. The FT-IR analysis established the preservation of the Keggin structure on a support. The NH3-TPD analysis suggests the presence of strong acid sites for the catalyst with 15 wt% of TPA. XRD, FT-IR and Raman analyses of the 15 wt% TPA/SnO2 catalyst calcined at different temperatures revealed the decomposition of TPA above a calcination temperature of 400 °C. The catalyst acidity decreased gradually with increase in calcination temperature.The BET results of 5 to 25 wt% TPA/SnO2 catalysts along with support SnO2 are shown in Table 1. It was observed that the surface area decreased with increasing TPA loading presumably due to the pore blockage of the surface with TPA.
SEM pictures of 5 to 25 wt% TPA/SnO2 catalysts calcined at 300 °C for 2 h along with that of support SnO2 are shown in Fig. 1 which clearly indicate an increase in the size of the catalyst particles with increase in TPA loading. SEM images are also supporting the phenomenon of agglomeration of the TPA particles. Thus SEM observations further support the attainment of the bulk nature of catalysts at higher TPA loadings.
 | ||
| Fig. 1 SEM pictures of TPA/SnO2 catalysts. (a) SnO2, (b) 5 wt% TPA/SnO2, (c) 10 wt% TPA/SnO2, (d) 15 wt% TPA/SnO2, (e) 20 wt% TPA/SnO2, (f) 25 wt% TPA/SnO2. | ||
Since the particle size of the catalyst is a paramount criterion in evaluating diffusional limitations of a catalyst, it was decided to study the particle size distribution of the best catalyst. The particle size distribution of the catalyst was done using the sieve analysis and the distribution data are summarized in Table 2. The sizes of particles were measured in the range of 5–200 μm.
| Diameter range/μm | Mass fraction |
|---|---|
| >150 | 0.0423 |
| 125–150 | 0.1064 |
| 112–125 | 0.1432 |
| 100–112 | 0.1327 |
| 80–100 | 0.1416 |
| 63–80 | 0.2045 |
| 50–63 | 0.0964 |
| 40–50 | 0.0941 |
| <40 | 0.0388 |
Catalytic activity
The acid catalyzed esterification is an equilibrium limited reaction. Palmitic acid reacts with methanol in the presence of TPA/SnO2 catalysts to produce the ester, methyl palmitate and water. Scheme 1 shows the mechanistic steps during the esterification reaction. The catalyst initiates the esterification reaction by donating a proton to the carboxylic acid molecule. The carboxylic acid is then subjected to nucleophilic attack by the hydroxyl group of alcohol (CH3OH), and the reaction continues with water elimination. | ||
| Scheme 1 Mechanism of acid catalyzed esterification of carboxylic acid. | ||
The esterification of palmitic acid with methanol was tested with 5–25 wt% TPA/SnO2 catalysts and the results are presented in Fig. 2. The conversion of palmitic acid increased with increase in the TPA content from 5 to 15 wt% and thereafter remained almost the same. Further increase in TPA loading resulted in a marginal increase only. The higher activity of 15 wt% TPA/SnO2 catalysts compared to others could be due to high acidic nature of catalysts. In solid acid catalyzed esterification reaction, the acidity of catalyst is a matter of great importance and it is reported that the catalyst with high accessible surface acid sites shows the optimum performance.3,4,12 From the acid strength distribution data of TPA/SnO2 catalysts (Table 1), it is evident that 15–25 wt% TPA/SnO2 catalysts possess more or less similar acidity. The constancy of the acidic nature beyond 15 wt% can be ascertained to the attainment of the bulk nature beyond this point which is clearly depicted in characterization results.
 | ||
| Fig. 2 Effect of TPA loading on SnO2 on esterification of palmitic acid with methanol. | ||
The catalyst 15 wt% TPA/SnO2 showed high activity when it was subjected to different calcination temperatures in the range of 300–750 °C and tested for the esterification reaction. The corresponding results are shown in Fig. 3. The conversion of palmitic acid increased with increase in calcination temperature from 300 to 400 °C and decreased thereafter. This is mainly related to the surface characterisation of the catalysts. High temperature calcination resulted in the decomposition of TPA on the support to its constituents like WO3. The degradation of Keggin ions results in the net decrease of the total acidity.
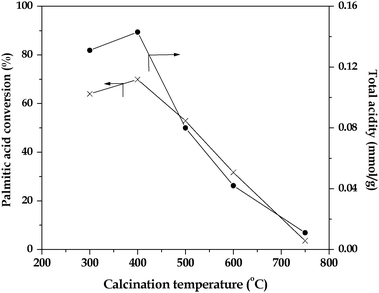 | ||
| Fig. 3 Effect of catalyst calcination temperature on the conversion of palmitic acid over the 15 wt%TPA/SnO2 catalyst. | ||
The influence of external resistances to mass transfer, the effect of the stirring speed on the esterification of palmitic acid with methanol was studied from 300 to 800 rpm. Results (figure not shown) revealed that there was no effect of stirring speed on the conversion of palmitic acid in the range of 500–800 rpm, which suggested that the reaction was free from external mass transfer resistance. All further experiments were carried out at 600 rpm.
The effect of catalyst concentration was studied in the range of 0.01–0.07 g cm−3. Fig. 4 shows the variation in palmitic acid conversion against catalyst concentration. The plot indicates the increased conversion of palmitic acid with increase in catalyst concentration from 0.014 to 0.055 g cm−3. However, beyond a catalyst loading of 0.055 g cm−3, there was no significant increase in the conversion. The increase in the catalyst amount leads to enhanced activity as the net available acidic sites are increased. This is a common feature of acid catalyzed reactions.
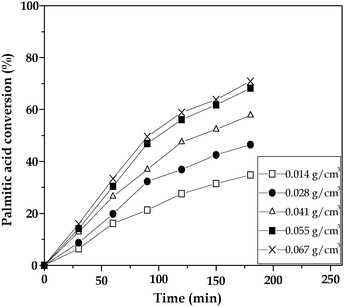 | ||
| Fig. 4 Influence of catalyst concentration on conversion of palmitic acid over the 15 wt% TPA/SnO2 catalyst. | ||
In order to assess the role of intra-particle diffusion resistance, the effect of the particle size of the catalyst on the reaction rate was studied by taking three different particle sizes in the range of 40–80 μm and the results are summarized in Fig. 5. No effect of the particle size on the yield of ester was observed for an average particle size of 55 μm, suggesting that there is no intra-particle diffusion and the entire process becomes surface reaction controlled. The average particle diameter of 55 μm was taken for a theoretical calculation based on the Wiesz–Prater criterion to assess the influence of intraparticle diffusion resistance. According to the Wiesz–Prater criterion, the dimensionless parameter Cwp which represents the ratio of the intrinsic reaction rate to intra-particle diffusion rate can be evaluated from the observed rate of reaction (robs), the particle radius (Rp), density of the catalyst particles (ρp), effective diffusivity of the limiting reactant (De) and concentration of the reactant at the external surface of the particle ([As]).33
 | ||
| Fig. 5 Effect of catalyst particle size on the conversion of palmitic acid over the 15 wt% TPA/SnO2 catalyst. | ||
then the reaction is limited by severe internal diffusion resistance.
then the reaction is intrinsically kinetically controlled.
The effective diffusivity of oil (De) inside the pores of the catalyst was obtained from the bulk diffusivity (DAB), porosity (ε) and tortuosity (τ) where De–A = DAB (ε/τ). In the present study, the value of Cwp was found to be 0.022 which is less than 1 thus it can be concluded that the reaction is intrinsically kinetically controlled.
The molar ratio of reactants is one of the most important parameters that needs to be optimised. Stoichiometrically, the methanolysis of fatty acid requires 1 mole of methanol for 1 mole of fatty acid. However, the use of excess methanol should benefit the conversion of fatty acid since its esterification is a reversible reaction. A high alcohol to fatty acid molar ratio (such as 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 63
1, 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has been reported to improve the rate of the heterogeneously catalyzed esterification reaction.12,19,34Fig. 6 reflects the effect of the methanol to palmitic acid molar ratio on the conversion of acid, which clearly indicates that with an increase in the mole ratio from 5
1) has been reported to improve the rate of the heterogeneously catalyzed esterification reaction.12,19,34Fig. 6 reflects the effect of the methanol to palmitic acid molar ratio on the conversion of acid, which clearly indicates that with an increase in the mole ratio from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 14
1 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion substantially increased. A further increase in the molar ratio shows no significant increase in the conversion of palmitic acid. The excess methanol used in the reaction can be collected by distillation and reused.
1, the conversion substantially increased. A further increase in the molar ratio shows no significant increase in the conversion of palmitic acid. The excess methanol used in the reaction can be collected by distillation and reused.
 | ||
| Fig. 6 Influence of the molar ratio on the conversion of palmitic acid over the 15 wt% TPA/SnO2 catalyst. | ||
The effect of reaction temperature on the palmitic acid conversion was studied over a range of 45–65 °C and the results are summarized in Fig. 7. It was observed that the conversion of palmitic acid increased with an increase in temperature from 45 to 65 °C. This would suggest that the reaction was not influenced by either external mass transfer or intraparticle diffusion resistance.
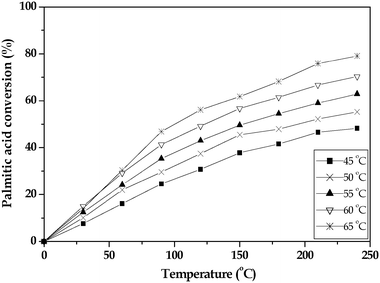 | ||
| Fig. 7 Effect of reaction temperature on the conversion of palmitic acid over the 15 wt% TPA/SnO2 catalyst. | ||
Stability and reusability studies of the catalyst
Since 15 wt% TPA/SnO2 calcined at 400 °C for 2 h was the best catalyst, its stability and reusability studies were performed. The stability of the catalyst and intactness of active species (TPA) on the tin oxide support, the reaction mixture was filtered after 1 h of reaction to take out the catalyst and the reaction with filtrate, in the absence of any externally added catalyst, was further continued. No significant increase in yield was observed; even after 10 h. This result confirmed the absence of leached active species in the filtrate of the reaction mixture under present reaction conditions.In order to prove the reusability of the catalyst, after reaction the catalyst was separated by filtration and washed thoroughly with methanol. The washed catalyst was dried in an oven for 1 h and used for the next experiment. The same procedure was adopted and continued five times. The activities upon reuse of the catalyst are presented in Fig. 8. It is noteworthy to mention that the catalyst is reusable without any appreciable loss in activity.
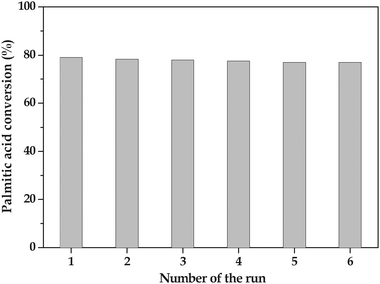 | ||
| Fig. 8 Reusability studies of the 15 wt% TPA/SnO2 catalyst. | ||
Kinetic studies
As the mole ratio of methanol to palmitic acid was greater than 5, it could be safely assumed to be a pseudo-first-order reaction for a fixed catalyst concentration. From the esterification conversion data obtained at different reaction temperatures over the 15 wt% TPA/SnO2 catalyst, plots of −ln (1-conversion) versus reaction time are shown in Fig. 9(a). The slopes of these lines are equal to rate constants from which Arrhenius plots were made to determine the apparent energy of activation (Fig. 9(b)). The activation energy was obtained as 8.68 kcal mol−1. This value is less than that of other reported solid acid catalysts.24,34 Thus, the present catalyst appears to be a promising candidate for esterification as its activity is comparable with that of mineral acids and it is more active than other solid acid catalysts. | ||
Fig. 9 (a) Plots of −ln(1-conversion) vs. time at different temperatures, (b) Arrhenius plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T for reaction of palmitic acid with methanol over the 15 wt% TPA/SnO2 catalyst. k vs. 1/T for reaction of palmitic acid with methanol over the 15 wt% TPA/SnO2 catalyst. | ||
Esterification of free fatty acids in the presence of triglycerides
As the studies were carried out on single fatty acids, it is thought to know the applicability of the 15 wt% TPA/SnO2 catalyst for real biodiesel feedstock. For this investigation, model high FFA containing oils were prepared by mixing 5 to 35 wt% of sunflower fatty acids with refined sunflower oil. The reaction was performed using the optimized reaction conditions and results are presented in Table 3. It was observed that conversion is more or less similar for all the samples indicating the negligible effect of triglycerides on esterification activity of the catalyst. There are few reports on the effect of FFAs on esterification in the presence of triglycerides.26,34 The influence of FFAs present in triglycerides of canola oil using TPA supported on hydrous zirconia is reported.26 It is observed that the yield of ester increased with increase in FFAs content, which supports the simultaneous occurrence of esterification and transesterification reactions. Srilatha et al.35 reported the decrease in the conversion of a mixture of fatty acids when compared to a single fatty acid over the Nb2O5 catalyst. However, with the present catalyst there was no decrease in activity for the mixture of fatty acids. This might be due to simultaneous occurrence of esterification and transesterification reactions. The present results are in support of the observation made by Kulkarni et al.26 This finding has commercial importance as high FFA containing oils are potential feedstock for biodiesel production. Further work is under progress at authors' laboratory to determine the activity of the present catalytic systems for simultaneous esterification and transesterification reactions along with a detailed kinetic modeling.| Fatty acid content (wt%) | Fatty acid conversion (%) |
|---|---|
| 5 | 78.5 |
| 15 | 78.0 |
| 25 | 79.0 |
| 35 | 81.2 |
Experimental
Catalyst preparation
The chemicals TPA and tin oxide were obtained from Aldrich Chemicals. The TPA supported tin oxide catalysts with different TPA loadings (5–25 wt%) were prepared by a wet impregnation method according to the procedure reported by us previously.31 The samples were dried at 120 °C and subsequently calcined at desired temperature for 2 h.Catalyst characterization
BET surface areas of the catalyst samples were calculated from N2 adsorption data acquired on an Autosorb-1 instrument (Quantachrome, USA) at liquid N2 temperature. Scanning electron micrographs (SEMs) of the catalysts were obtained in a Hitachi S-520 electron microscope at an accelerated voltage of 10 kV. Samples were mounted on aluminium stubs using double adhesive tape and were gold coated in a Hitachi HUS-5 GB vacuum evaporator. The size distribution of the catalyst was determined using standard-sized sieves (150 μm down to 20 μm). A known amount of catalyst was accurately weighed and placed in the top sieve. This was then sieved down through the tower of sieves with decreasing sieve diameters. The amount of catalyst in each sieve was weighed. This procedure was repeated several times to ensure reproducibility of results. The analysis of the products was done by estimating the acid value before and after the reaction. The acid value was determined by following the American Oil Chemists' Society (AOCS) method Te 1a-64.Catalytic reaction
Esterification of palmitic acid was carried out in a 100 cc Parr reactor (Parr Instrument Co.). In a typical reaction, 5 g (0.02 moles) of palmitic acid and 9.4 g (0.3 moles) of methanol were taken and about 1 g of catalyst was added. The initial sample was collected when the reaction mixture attained desired temperature after which agitation commenced. Samples were taken at periodic intervals of 30 min up to 4 h. Reproducibility of results was ensured by experimental triplicates under optimal conditions. The conversion of fatty acid was calculated by estimating the acid value before and after the reaction. The acid value was determined by following the American Oil Chemists' Society (AOCS) method Te 1a-64.Conclusions
From the above study the following conclusions can be drawn:1. The esterification activity depends on the TPA content and its dispersion on SnO2 and also on calcination temperature. 15 wt% TPA/SnO2 was the best composition of the catalyst and a calcination temperature of 400 °C was found to be optimum.
2. The effect of various parameters on the reaction was studied and the optimum conditions found were: stirring speed 600 rpm, catalyst concentration of 0.055 g cm−3, methanol to acid mole ratio of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction temperature of 65 °C.
1, reaction temperature of 65 °C.
3. The stability of the catalyst was ascertained by ensuring no leaching of TPA from the support. Studies to assess the catalyst reusability implied that its activity was consistent for five reaction cycles.
4. A pseudo first order model was applied to correlate the experimental kinetic data. Apparent activation was calculated to be 8.68 kcal mol−1.
5. A 15 wt% TPA/SnO2 catalyst is found to catalyze simultaneous esterification and transesterification reactions.
Acknowledgements
Authors KS and RK thank Council for Scientific and Industrial Research, India, for awarding Senior and Junior Research Fellowships respectively.References
- E. Lotero, Y. Liu, D. E. Lopez, K. Suwannakarn, D. A. Bruce and J. G. Goodwin, Jr., Ind. Eng. Chem. Res., 2005, 44, 5353 CrossRef CAS.
- M. Canakci, Bioresour. Technol., 2007, 98, 183 CrossRef CAS.
- M. Canacki and J. V. Gerpen, Trans. ASAE, 2003, 46, 945 Search PubMed.
- K. N. Rao, A. Sridhar, A. F. Lee, S. T. Tavener, N. A. Young and K. Wilson, Green Chem., 2006, 8, 790 RSC.
- A. A. Kiss, A. C. Dimian and G. Rothenberg, Energy Fuels, 2008, 22, 598 CrossRef CAS.
- A. A. Kiss, F. Omota, A. C. Dimian and G. Rothenberg, Top. Catal., 2006, 40, 141 CrossRef CAS.
- A. C. Dimian, Z. W. Srokol, M. C. M. Hazeleger and G. Rothenberg, Top. Catal., 2010, 53, 1197 CrossRef CAS.
- K. Narasimharao, D. R. Brown, A. F. Lee, A. D. Newman, P. F. Siril, S. J. Tavener and K. Wilson, J. Catal., 2007, 248, 226 CrossRef CAS.
- D. E. Lopez, K. Suwannakarn, D. A. Bruce and J. G. Goodwin Jr., J. Catal., 2007, 247, 43 CrossRef CAS.
- S. Ramu, N. Lingaiah, B. L. A. Prabhavathi Devi, R. B. N. Prasad, I. Suryanarayana and P. S. Sai Prasad, Appl. Catal., A, 2004, 276, 163 CrossRef CAS.
- B. X. Peng, Q. Shu, J. F. Wang, G. R. Wang, D. Z. Wang and M. H. Han, Process Saf. Environ. Prot., 2008, 86, 441 Search PubMed.
- W. Y. Lou, M. H. Zong and Z. Q. Duan, Bioresour. Technol., 2008, 99, 8752 CrossRef CAS.
- G. F. Ghesti, J. L. de Macedo, V. C .I. Parente, J. A. Dias and S. C. L. Dias, Appl. Catal., A, 2009, 355, 139 CrossRef CAS.
- I. K. Mbaraka, K. J. McGuire and B. H. Shanks, Ind. Eng. Chem. Res., 2006, 45, 3022 CrossRef CAS.
- C. S. Caetano, L. Guerreiro, I. M. Fonseca, A. M. Ramos, J. Vital and J. E. Castanheiro, Appl. Catal., A, 2009, 359, 41 CrossRef CAS.
- B. Y. Giri, K. N. Rao, B. L. A. P. Devi, N. Lingaiah, I. Suryanarayana, R. B. N. Prasad and P. S. Sai Prasad, Catal. Commun., 2005, 6, 788 CrossRef CAS.
- A. Takagaki, M. Toda, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Catal. Today, 2006, 116, 157 CrossRef CAS.
- N. Ozbay, N. Oktar and N. A. Tapan, Fuel, 2008, 87(10–11), 1789 CrossRef.
- A. A. Kiss, A. C. Dimian and G. Rothenberg, Adv. Synth. Catal., 2006, 348, 75 CrossRef.
- P. G. Vazquez, M. N. Blanco and C. V. Cacares, Catal. Lett., 1999, 60, 205 CrossRef CAS.
- L. R. Pizzio, C. V. Cacares and M. N. Blanco, Appl. Catal., A, 1998, 167(2), 283 CrossRef CAS.
- K. M. Parida and S. Mallick, Catal. Commun., 2009, 11, 51 CrossRef CAS.
- G. D. Yadav and R. D. Bhagat, Clean Technol. Environ. Policy, 2005, 7, 245 Search PubMed.
- K. Srilatha, N. Lingaiah, B. L. A. P. Devi, R. B. N. Prasad, S. Venkateswar and P. S. Sai Prasad, Appl. Catal., A, 2009, 365, 28 CrossRef CAS.
- B. M. Devassy and S. B. Halligudi, J. Catal., 2005, 236, 313 CrossRef.
- M. G. Kulkarni, R. Gopinath, L. C. Meher and A. K. Dalai, Green Chem., 2006, 8, 1056 RSC.
- K. M. Parida and S. Mallick, J. Mol. Catal. A: Chem., 2007, 275, 77 CrossRef CAS.
- A. I. Tropecelo, M. H. Casimiro, I. M. Fonseca, A. M. Ramos, J. Vital and J. E. Castanheiro, Appl. Catal., A, 2010, 390, 183 CrossRef CAS.
- L. Xu, Y. Wang, X. Yang, X. Yu, Y. Guo and J. H. Clark, Green Chem., 2008, 10, 746 RSC.
- A. S. Khader and A. I. Ahmed, Appl. Catal., A, 2009, 354, 153 CrossRef CAS.
- Ch. Ramesh Kumar, P. S. Sai Prasad and N. Lingaiah, Appl. Catal., A, 2010, 384, 101 CrossRef.
- S. Furuta, H. Matsuhashi and K. Arata, Appl. Catal., A, 2004, 269, 187 CrossRef CAS.
- H. S. Fogler, Elements of Chemical Reaction Engineering, Prentice-Hall, New Delhi, 1995 Search PubMed.
- M. Berrios, J. Siles, M. A. Martin and A. Martin, Fuel, 2007, 86, 2383 CrossRef CAS.
- K. Srilatha, N. Lingaiah, P. S. Sai Prasad, B. L. A. Prabhavathi Devi, R. B. N. Prasad and S. Venkateswar, Ind. Eng. Chem. Res., 2009, 48, 10816 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |


