Homogeneously-catalyzed etherification of glycerol with 1-dodecanol†
Pierrick
Gaudin
a,
Roland
Jacquot
b,
Philippe
Marion
b,
Yannick
Pouilloux
*a and
François
Jérôme
*a
aLaboratoire de Catalyse en Chimie Organique, Université de Poitiers, ENSIP, 40 avenue du recteur Pineau, 86022 Poitiers, France. E-mail: francois.jerome@univ-poitiers.fr; yannick.pouilloux@univ-poitiers.fr; Fax: +33 (0)5 49 45 30 49; Tel: +33 (0)5 49 45 40 52
bRHODIA, Centre de Recherches et Technologies de Lyon, 85, rue des Frères Perret, 69192 Saint Fons Cédex, France
First published on 5th May 2011
Abstract
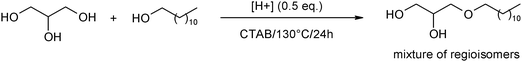
Here we describe the direct catalytic etherification of glycerol with 1-dodecanol. In particular, we show that addition of 10 mol% of 1-bromododecane in the reaction medium led to the nearly complete conversion of 1-dodecanol and to the formation of the targeted monododecyl glyceryl ethers with 60% yield, thus opening a direct route for the synthesis of bio-based surfactants.
Catalytic etherification of glycerol with long alkyl chain alcohols is a challenging reaction not only from a scientific point of view but also because of the important applications of the resulting monoalkylglyceryl ethers. Indeed, the etherification of glycerol with long alkyl chain alcohols provides a new generation of amphiphilic bio-based derivatives which are used (1) as water-stable surfactants and (2) as sustainable solvents for catalysis.1
Although amphiphilic monoalkylglyceryl ethers are now emerging as a promising new class of bio-based products, their complex synthesis hampers their industrial emergence and new innovative catalytic routes are needed. In the existing literature reports, all attempts to directly etherify glycerol with long alkyl chain alcohols failed and alternative routes are generally employed. For instance, Lemaire and co-workers reported that amphiphilic monoalkylglyceryl ethers can be produced by reductive etherification of glycerol with long alkyl chain aldehydes using Pd/C and camphorsulfonic acid under pressure of hydrogen.2 As another route, Weckhuysen and co-workers investigated the catalytic addition of various polyols, including glycerol, to alkenes.3 If the corresponding ethers have been obtained in good yields from ethylene and propylene glycol, this catalytic process was found to be inefficient starting from glycerol and 1-dodecene or 1-octene. Up to now, the direct and successful etherification of glycerol with alcohols has been only performed starting from activated alcohols such as tertiobutanol4 or benzyl alcohols.5 In 2009, Fajula and co-workers reported the acid catalyzed etherification of glycerol with ethanol which represents the first successful etherification of glycerol with alkyl alcohols.6 Although 40% yield of monoethoxy glyceryl ethers has been obtained, the production of diethylether in a very large extent represents the main drawback of this process. From a catalytic point of view, etherification of glycerol with long chain alkyl alcohols such as 1-dodecanol is even more complex not only because of their low reactivity but also due to the important difference of polarity between 1-dodecanol and glycerol which requires overcoming important mass transfer problems (biphasic system).
Here, in this paper, we wish to describe a new catalytic route allowing the direct etherification of glycerol with 1-dodecanol and affording the corresponding amphiphilic glyceryl ethers with 60% yield. For the sake of clarity, glyceryl ethers were noted here as DxGy where x represents the number of dodecyl chains and y the number of glyceryl units. Therefore, monododecyl glyceryl ethers, didodecylglyceryl ethers, monododecyl diglyceryl ethers, didodecylether and diglycerol will be named D11G11, D22G11, D11G22, D22 and G22, respectively (Scheme 1).
 | ||
| Scheme 1 Abbreviation of main products synthesized in this work. Note that only one regioisomer is drawn but glyceryl ethers and oligoglycerol described in this work are produced as a mixture of regioisomers. | ||
In a first set of experiments, 4 eq. of glycerol and 1 eq. of 1-dodecanol were heated at 130 °C for 24 h in the presence of a wet Amberlyst 70 (water content 53–59 wt%)‡. With the aim of rapidly optimizing the reaction, all tested reactions were first performed using 50 mol% of H+ (influence of the catalyst amount is discussed at the end of the manuscript). Under these conditions, only glycerol was converted (36%) while the conversion of 1-dodecanol remained lower than 5% (Table 1, entry 1). As a consequence, the monododecyl glyceryl ethers were produced with less than 5% yield. This low yield of the desired monododecyl glyceryl ethers can be ascribed to the poor solubility of 1-dodecanol in the glycerol phase. Indeed, in a biphasic glycerol/1-dodecanol system, the wet A70 catalyst has a stronger affinity for the glycerol phase resulting in the main oligomerization of glycerol to diglycerol (22%) and triglycerol (3%). From this first result, it is clear that the contact between the glycerol and 1-dodecanol phases is a crucial key for better controlling the selectivity of the reaction. In biphasic water/oil systems, mass transfer problems are frequently encountered and different strategies have been reported for facilitating a better contact between two phases. In particular, the assistance of a phase transfer agent has proved to be a suitable solution. Recently, we have shown that it was possible to transfer this concept to the biphasic glycerol/oil system.7 In this context, we decided to add cetyltrimetylammonium bromide (CTAB) in the reaction medium in order to favor a better contact between glycerol and 1-dodecanol. To this end, CTAB was progressively added to a solution of glycerol and 1-dodecanol (molar ratio 4/1) and heated at 130 °C. We found that it was necessary to add up to one equivalent of CTAB to observe the formation of a transparent and monophasic solution. This high amount of CTAB is consistent with previous results which have clearly shown that critical micellar concentrations of surfactants are much higher in glycerol than in water.8 As expected for a monophasic medium, when A70 (50 mol% H+) was added to the glycerol/dodecanol/CTAB solution and heated at 130 °C for 24 h, a significant improvement of the 1-dodecanol conversion was observed. Indeed, whereas 1-dodecanol was converted with less than 5% without CTAB, this conversion reached 43% in the presence of CTAB (Table 1, entries 1 and 2). This better contact between the glycerol and 1-dodecanol phases results in an enhancement of the monododecyl glyceryl ethers yield which reached 23% yield thus opening the first route for the direct etherification of glycerol with a long chain alkyl alcohol (Table 1, entry 2).
| Entry | Catalyst | Phase transfer agent | Conv glycerol (%) | Conv dodecanol (%) | Monoethers yield (%) | Diglycerol yield (%)b | Triglycerol yield (%)b | Didodecylether yield (%) | Carbon mass balance (%)c |
|---|---|---|---|---|---|---|---|---|---|
| a Glycerol (4 eq.), 1-dodecanol (1 eq.), catalyst (10 mol% H+), 130 °C, CTAB (1 eq.), 24 h. b Based on the total conversion of glycerol. c Based on 1-dodecanol. d 1-Bromododecane was used instead of 1-dodecanol. e Other products such as D22G11, D11G22, among others were detected. | |||||||||
| 1 | Wet A70 | — | 36 | 5 | 5 | 22 | 3 | 0 | 100 |
| 2 | Wet A70 | CTAB | 12 | 43 | 23 | 6 | 0 | 19 | 98 |
| 3 | Wet A31 | CTAB | 19 | 45 | 24 | 4 | 0 | 21 | 100 |
| 4 | Wet A35 | CTAB | 36 | 42 | 22 | 15 | 0 | 17 | 93 |
| 5 | Dry A70 | CTAB | 48 | 69 | 35 | 18 | 2 | 27 | 90 |
| 6 | CF3SO3H | CTAB | 48 | 59 | 37 | 11 | 0 | 19 | 95 |
| 7 | pTSA | CTAB | 54 | 61 | 37 | 8 | 0 | 22 | 97 |
| 8 | Pyrene–SO3H | CTAB | 18 | 56 | 38 | 9 | 0 | 18 | 100 |
| 9 | HBr | CTAB | 17 | 56 | 36 | 6 | 0 | 20 | 100 |
| 10d | — | CTAB | nd | — | 33 | 24 | 0 | 1 | 77e |
| 11 | Dry A70 | CTAH | 32 | 7 | 4 | 4 | 0 | 2 | 86 |
With the aim of increasing the monododecyl glyceryl ethers yield, we then screened different cation exchange resins and homogeneous Brønsted acid catalysts using the above described conditions. Over wet A31 and wet A35 similar monododecyl glyceryl ethers yields were obtained (22–24%) (Table 1, entries 3 and 4). It is noteworthy that the pre-drying of the A70 resin led to an increase of the monododecyl glyceryl ethers yield from 23 to 35% (Table 1, entry 5). This phenomenon can be ascribed to the initial stronger acidity of the grafted acid sites caused by the removal of water from the wet A70. Indeed, water is known to strongly solvate acid sites resulting in a drop of the acid strength.9 In the presence of homogeneous acid catalysts such as triflic, p-toluene sulfonic, pyrene sulfonic and bromhydric acids, yields to the desired monododecyl glyceryl ethers are similar to that obtained over dry A70 (37%) (Table 1, entries 6–9). To the best of our knowledge, this is the highest yield ever reported for the etherification of glycerol with 1-dodecanol.
Curiously, during the reaction, we clearly observed by gas chromatography (GC) the formation of an intermediate which led us to more deeply investigate the reaction mechanism. To this end, after 20% conversion of 1-dodecanol, the reaction medium was purified over silica gel and the intermediate was isolated and characterized by means of NMR and mass spectrometry. All these analytical techniques clearly evidenced that this intermediate corresponds to the formation of 1-bromododecane. Interestingly, as shown in Fig. 1, 1-bromododecane is a key intermediate in the reaction mechanism. Indeed, 1-bromododecane is initially produced as a major product and then is progressively consumed to the benefit of the formation of monododecyl glyceryl ethers and didodecylethers. In order to clarify the formation of 1-bromododecane, we performed various counter experiments. We10 and others11 have recently reported that in the presence of chloride-based ionic liquids, a rapid exchange between an acidic proton and the imidazolium moiety occurred leading to the liberation of HCl in the reaction medium. Having this reaction in mind, we rapidly suspected here an exchange of the proton with the ammonium part of the CTAB leading to the liberation of HBr and thus to the bromination of 1-dodecanol. In order to check this hypothesis, 10 wt% of wet A70 resin was placed in water in the presence of CTAB and we controlled the pH of the solution. Without CTAB, the pH of the solution is close to 7. Upon addition of CTAB, the pH rapidly dropped from 7 to 1.5, thus highlighting the liberation of HBr in the reaction medium. Therefore, whatever the Brønsted acid used, we can reasonably conclude here that, in the presence of CTAB, the etherification of glycerol with 1-dodecanol is mainly catalyzed by the released HBr. This result also explains the similar catalytic results collected with all tested Brønsted acids.
 | ||
| Fig. 1 Molar composition of the reaction medium during the catalytic reaction. | ||
In Scheme 2, we propose a plausible reaction mechanism for the formation of the monododecyl glyceryl ethers. First, 1-dodecanol is brominated owing to the liberation of HBr in the reaction medium leading to the formation of 1-bromododecane. Then, 1-bromododecane reacts with glycerol (known as the Williamson reaction) leading to (i) the formation of the desired monododecyl glyceryl ethers and (ii) the regeneration of HBr.
 | ||
| Scheme 2 Plausible catalytic cycle. | ||
In order to further support this reaction mechanism, two counter experiments were performed. First, 1-bromododecane (1 eq.), CTAB (1 eq.) and glycerol (4 eq.) were heated together at 130 °C. After 24 h of reaction, the glycerol and 1-bromododecane conversion reached nearly 80% and the corresponding monododecyl glyceryl ethers were obtained in 33% yield showing that the Williamson reaction can take place at 130 °C without the assistance of any catalyst (Table 1, entry 10). Note that the assistance of CTAB was here still necessary to ensure a better contact between the glycerol and 1-bromododecane phases. On the other hand, when the catalytic reaction was performed in the presence of cetyltrimethylammonium hydrogenosulfate (CTAH) instead of CTAB, only 4% yield of monododecyl glyceryl ether was obtained confirming the key role played by the bromide in the reaction medium (Table 1, entry 11). It should be noted that the bromination of glycerol is also a possible way (not represented in Fig. 1). However, we were unfortunately unable to isolate this intermediate presumably due to its very high reactivity and thus, to its rapid conversion to monododecyl glyceryl ethers and oligoglycerol. Nevertheless, the formation of bromoglycerol certainly occurs.
Considering that the reaction mechanism implies the formation of 1-bromododecane as an intermediate, it occurred to us that 1-bromododecane could be used as a generator of HBr for the reaction. Indeed, as mentioned above, etherification of glycerol with 1-bromododecane does not need the assistance of any catalyst and takes place at 130 °C with the liberation of HBr. To this end, a mixture of glycerol (4 eq.), 1-dodecanol (0.5 eq.) and 1-bromododecane (0.5 eq.) was heated at 130 °C in the presence of one equivalent of CTAB and in the absence of any acid catalyst. As expected, in these conditions, 1-dodecanol and 1-bromododecane were concomitantly converted with 75% and 82%, respectively (Fig. 2a). Without 1-bromododecane, the reaction did not proceed showing that the presence of 1-bromododecane in the reaction medium is essential for the conversion of 1-dodecanol (bromination of 1-dodecanol by released HBr). It should be noted that, under these conditions, the carbon mass balance (based on glycerol) dropped from 100% to 75%, indicating the formation of unidentified glycerol-based products presumably caused by the side bromination of glycerol (Fig. 2a). Under these conditions, monododecyl glyceryl ethers were produced with an unprecedented yield of 50% (Fig. 2b).
 | ||
| Fig. 2 Evolution of (a) the conversion; (b) the yield of glyceryl ethers and (c) the selectivity to glyceryl ethers during the reaction progress. | ||
At the same time, we clearly observed the formation of D22G11 and D11G22 with 7% and 3% yield, respectively, giving a total yield of glyceryl ethers of 60%. Note that the fatty chain (1-bromododecane and 1-dodecanol) was converted to the desired monododecyl glyceryl ethers with a selectivity of 70% up to 70% conversion (Fig. 2c). Over 70% conversion, this selectivity dropped to 60% due to the formation of D22G11 and D11G22. Additionally, didodecylether (D22) was also produced with 19% yield. It should be noted that when the glycerol/fatty chain molar ratio was increased from 4 to 8, the selectivity of the reaction to the desired monododecyl glyceryl ethers was even higher and these latter were obtained in 65% yield (Fig. S1, ESI†).
Next, we decided to find the optimal temperature in order to reduce as much as possible the energetic cost of the process. As shown in Fig. 3, a decrease of the reaction temperature from 130 °C to 115 °C did not change the selectivity to the monododecyl glyceryl ethers which were still produced with 50% yield. However, as expected, a decrease of 15 °C of the reaction temperature results in a decrease of the formation rate of monododecyl glyceryl ethers by a factor of 2.7. Similarly, the yield of the monododecyl glyceryl ethers (50%) was not affected by an increase of 15 °C of the reaction temperature. However, at 145 °C, the monododecyl glyceryl ethers were obviously produced 2.6 times faster than at 130 °C (Fig. 3). Note that at 145 °C, the glycerol mass balance is lower than at 130 °C due to the side conversion of glycerol to unidentified products. From these results, it seems that 130 °C is a good compromise between the required power, the reaction time and the glycerol mass balance.
 | ||
| Fig. 3 Influence of the reaction temperature on the reaction yield. | ||
HBr being generated in situ as a catalyst from 1-bromododecane and glycerol, we then decided to decrease the amount of 1-bromododecane in order to be closer from catalytic conditions. In this context, the 1-dodecanol/1-bromododecane molar ratio was increased from 1 to 9. As mentioned above, the reaction was stirred at 130 °C in the presence of one equivalent of CTAB. As shown in Fig. 4a, when the 1-dodecanol/1-bromododecane molar ratio was increased from 1 to 9, the amount of converted 1-dodecanol remained unchanged (80%). However, the conversion rate of 1-dodecanol was decreased by a factor of 5.4. These observations are consistent with a catalyst behavior of the 1-bromododecane. Indeed, a decrease of the amount of 1-bromododecane in the reaction medium is equivalent to a decrease in the amount of released HBr. Additionally, the selectivity of the reaction to monododecyl glyceryl ethers was similar whatever the 1-dodecanol/1-bromododecane molar ratio. Indeed, in all cases, the monododecyl glyceryl ethers were obtained in 50% yield at nearly 80% conversion of 1-dodecanol (Fig. 4b). Note that 10% yield of D22G11 was also obtained giving, in all cases, a global yield of 60% of glyceryl ethers.
 | ||
| Fig. 4 Influence of the 1-dodecanol/1-bromododecane molar ratio on (a) the conversion of 1-dodecanol and (b) the glyceryl ether yields. | ||
Conclusions
We report here that the direct etherification of glycerol with 1-dodecanol can be conveniently catalyzed by 1-bromododecane (10 mol%). In this process, the key step is the catalyst-free coupling of glycerol with a catalytic amount of 1-bromododecane (the so-called Williamson reaction) leading to the formation of the targeted monododecyl glyceryl ethers and the liberation of HBr. Then, 1-dodecanol is in situ brominated by the released HBr regenerating 1-bromododecane. Using this approach we found that introduction of 10 mol% of 1-bromododecane in the reaction medium led to the nearly complete conversion of 1-dodecanol and the formation of monododecyl glyceryl ethers with 60% yield. In such a catalytic process, the assistance of CTAB is needed in order to ensure a better contact between the polar glycerol phase and the hydrophobic 1-dodecanol phase.To the best of our knowledge, this is the first catalytic route describing the direct etherification of glycerol with 1-dodecanol, thus opening a direct route for the synthesis of bio-based surfactants.
Notes and references
- As recent selected articles see: (a) J. I. Garcia, H. Garcia-Marin, J. A. Mayoral and P. Perez, Green Chem., 2010, 12(3), 426–434 RSC; (b) H. García-Marín, J. C. Van Der Toorn, J. A. Mayoral, J. I. García and I. W. C. E. Arends, J. Mol. Catal. A: Chem., 2011, 334(1–2), 83–88 CrossRef CAS.
- (a) Y. Shi, W. Dayoub, A. Favre-Réguillon, G.-R. Chen and M. Lemaire, Tetrahedron Lett., 2009, 50, 6891–6893 CrossRef CAS; (b) Y. Shi, W. Dayoub, G.-R. Chen and M. Lemaire, Green Chem., 2010, 12, 2189–2195 RSC.
- A. M. Ruppert, A. N. Parvulescu, M. Arias, P. J. C. Hausoul, P. C. A. Bruijnincx, R. J. M. Klein Gebbink and B. M. Weckhuysen, J. Catal., 2009, 268, 251–259 CrossRef CAS.
- (a) K. Klepacova, D. Mravec and M. Bajus, Chem. Pap., 2006, 60, 224 Search PubMed; (b) K. Klepacova, D. Mravec and M. Bajus, Appl. Catal., A, 2005, 294, 141–147 CrossRef CAS.
- (a) Y. Gu, A. Azzouzi, Y. Pouilloux, F. Jérôme and J. Barrault, Green Chem., 2008, 10, 164–167 RSC; (b) R. Luque, V. Budarin, J. H. Clark and D. J. Macquarrie, Appl. Catal., B, 2008, 82, 157–162 CrossRef CAS.
- S. Pariente, N. Tanchoux and F. Fajula, Green Chem., 2009, 11, 1256–1261 RSC.
- (a) A. Karam, N. Villandier, M. Delample, C. Klein Koerkamp, J.-P. Douliez, R. Granet, P. Krausz, J. Barrault and F. Jérôme, Chem.–Eur. J., 2008, 14(33), 10196–10200 CrossRef CAS; (b) M. Delample, N. Villandier, J.-P. Douliez, S. Camy, J.-S. Condoret, Y. Pouilloux, J. Barrault and F. Jérôme, Green Chem., 2010, 12, 804–808 RSC.
- M. Delample, F. Jérôme, J. Barrault and J.-P. Douliez, Green Chem., 2011, 13, 64–68 RSC.
- As selected articles please see: (a) S. Talwalkar and S. Mahajani, Appl. Catal., A, 2006, 302(1), 140–148 CrossRef CAS; (b) E. D. Toit, R. Schwarzer and W. Nicol, Chem. Eng. Sci., 2004, 59(22–23), 5545–5550; (c) M. F. Gomez, L. A. Arrua and M. C. Abello, J. Chem. Technol. Biotechnol., 2004, 79(4), 391–396 CrossRef CAS.
- M. Benoit, Y. Brissonnet, E. Guélou, K. De Oliveira Vigier, J. Barrault and F. Jérôme, ChemSusChem, 2010, 11, 1298–1303.
- (a) R. Rinaldi, N. Meine, J. vom Stein, R. Palkovits and F. Schüth, ChemSusChem, 2010, 3, 266–276 CrossRef CAS; (b) N. Villandier and A. Corma, Chem. Commun., 2010, 46, 4408–4410 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Copies of NMR spectra and influence of the glycerol/1-dodecanol molar ratio. See DOI: 10.1039/c1cy00082a |
| ‡ Chemicals: glycerol was kindly provided by Stéarinerie Dubois and 1-dodecanol and 1-bromododecane were purchased from Sigma-Aldrich. The A70, A31 and A35 resins were kindly provided by Rohm et Hass. General procedure for the acid-catalyzed etherification of glycerol with 1-dodecanol: glycerol (20 mmol), 1-dodecanol (5 mmol) and CTAB (1 eq.) were mixed together and in the presence of an acid catalyst (0.5 eq. of H+) heated at 135 °C in an autoclave. The reaction progress was monitored on a Varian 3800 GPC equipped with a HT5 column (25 m × 0.32 mm; phase thickness: 0.25 μm) supplied by SGE, a Flame Detector Ionization and a PTV on-column injector. Prior to analysis, the sample was silylated as follows: to the sample (30 mg) was added 410 μL of pyridine, 210 μL of hexamethyldisilazane and 110 μL of trimethychlorosilane and the resulting mixture was stirred at room temperature for 10 min. Then, the solution was filtered in order to remove the pyridinium salts before injection. General procedure for the etherification of glycerol with 1-dodecanol in the presence of 1-bromododecane: glycerol (20 mmol), 1-dodecanol (5 mmol), 1-bromododecane (0.5 mmol) and CTAB (1 eq.) were mixed together and heated at 135 °C in an autoclave. As previously mentioned, the reaction progress was monitored by gas chromatography. Characterization of monododecylglycerylethers: monododecylglyceryl ethers are produced as a mixture of two regioisomers with the main etherification on the primary hydroxyl group. Exact determination of the regioselectivity is difficult to achieve in this case due to the overlapping of the two regioisomer retention times in gas chromatography. Purification of monododecylglyceryl ethers was carried out over silica gel using a gradient of heptane/ethyl acetate (7/3) as an eluent. Analytical data for the 1-monododecylglycerylethers: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.87 (t, 3H, 3JH−H = 7.9 Hz, –CH3), 1.28 (m, 18H, –CH2–), 1.57 (m, 2H, –CH2–), 2.38 (br t, 1H, –OH), 2.78 (d, 1H, 3JH−H = 4.1 Hz, –OH); 3.40–3.45 (m, 4H, –CH2–O–), 3.56–3.66 (m, 2H, –CH2–O–), 3.85 (m, 1H, –CH2–O–); 13C NMR (100 MHz, CDCl3) δ (ppm) 14.1 (–CH3), 22.7 (–CH2–), 26.1 (–CH2–), 29.3–29.7 (–CH2–), 31.9 (–CH2–), 64.3 (–CH2–O–), 70.5 (–CH2–O–), 71.9 (–CH2–O–), 72.5 (–CH2–O–); LC/MS (ESI) [M, Na]+ = 283.46. Characterization ofD22G11: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.88 (t, 6H, 3JH−H = 7.9 Hz, –CH3), 1.26 (m, 36H, –CH2–), 1.57 (m, 4H, –CH2–), 2.50 (d, 1H, 3JH−H = 3.9 Hz, –OH), 3.41–3.53 (m, 8H, –CH2–), 3.94 (m, 1H, –CH–); 13C NMR (100 MHz, CDCl3) δ (ppm) 14.2 (–CH3), 22.8 (–CH2–), 26.2 (–CH2–), 29.5–29.8 (–CH2–), 32.1 (–CH2–), 69.6 (–CH2–O–), 71.8 (–CH2–O–), 72.0 (–CH2–O–); LC/MS (ESI) [M, Na]+ = 451.26. Characterization ofD11G22: 1H NMR (400 MHz, CDCl3) δ (ppm) 0.87 (t, 3H, 3JH−H = 7.9 Hz, –CH3), 1.27 (m, 18H, –CH2–), 1.54 (m, 2H, –CH2–), 2.01 (br s, 1H, –OH), 2.95 (br s, 1H, –OH), 3.40–4.00 (m, 14H, –CH2–O–, –CH2–O–, –OH); 13C NMR (100 MHz, CDCl3) δ (ppm) 14.1 (–CH3), 22.7 (–CH2–), 26.1 (–CH2–), 29.3–29.7 (–CH2–), 31.9 (–CH2–), 63.8 (–CH2–O–), 69.5 (–CH2–O–, regioisomer 1), 70.8 (–CH2–O–, regioisomer 2), 71.7 (–CH2–O–, regioisomer 1), 73.0 (–CH2–O–, regioisomer 2), LC/MS (ESI) [M, Na]+ = 357.55. |
| This journal is © The Royal Society of Chemistry 2011 |