Catalytic performance and mechanism of KF-loaded catalysts for biodiesel synthesis†
Chunli
Xu
*ab and
Qiang
Liu
ab
aKey Laboratory of Applied Surface and Colloid Chemistry (Shaanxi Normal University), Ministry of Education, Xi'an 710062, P. R. China
bSchool of Chemistry and Materials Science, Shaanxi Normal University, 199 Chang'an South Street, Xi'an 710062, P. R. China. E-mail: xuchunli@snnu.edu.cn; Fax: 86-29-85307774; Tel: 86-29-85300970
First published on 4th July 2011
Abstract
A number of KF-loaded heterogeneous base catalysts were prepared by doping KF on mixed oxide or single oxide supports containing Mg, Cu, Zn, Co, Al, Cr, Ni or Fe. The catalysts were characterized and tested for the transesterification of vegetable oil with methanol to produce biodiesel, in order to elucidate the active phase(s). The experimental results showed that KF doping increased activity irrespective of the nature of the support (i.e. whether it was mixed oxide or single oxide). For all catalysts KOH, formed during the treatment of each support with KF, was demonstrated to be the active phase. The activity of KF doped catalysts is promoted by the surface F-species. On this basis, it is possible to rationalize the general effect that KF has in promoting base catalyzed activity and select suitable supports in the design of highly active KF-loaded catalysts.
1. Introduction
Heterogeneous catalysts are economically and ecologically important because they are non-corrosive, environmentally benign and present fewer disposal problems. They are also much easier to separate from liquid products than their homogeneous counterparts and they can be designed to give high activity, selectivity and long catalyst lifetimes.1–3 As a consequence of these advantages, there is increasing interest in the application of heterogeneous base catalysts for processes of importance to the fine chemical industry.4–7 Many different types of bases, such as alkaline earth metal oxides, anion exchange resins and alkali metal compounds supported on alumina or zeolite, can be applied as catalysts to base catalyzed reactions such as isomerization, aldol condensation, Knoevenagel condensation, Michael condensation, oxidation and transesterification.8KF/Al2O3 catalysts were originally reported in 1979 by Yamawaki and Ando,9 and since then interest in KF-loaded systems has grown considerably, with hundreds of papers being published detailing their application as heterogeneous catalysts.10–12 Due to their importance as heterogeneous base catalysts, a significant degree of attention has been focused upon the elucidation of the active phase and mechanism of reaction and currently no consensus has been reached. KF/Al2O3 is an illustrative example in this respect. Ando et al.13 concluded that there were origins of the basicity of KF/Al2O3 of importance for catalytic activity: (1) the presence of active fluoride, (2) the presence of the [Al–O−] ion, which generates OH− when water is added, and (3) co-operation of F− and [Al–OH]. However, Kabashima et al.14 reported that the KF/Al2O3 surface species relevant to catalytic activity (particularly for the double bond isomerization and Tishchenko reactions), was a F− containing species, which gave a peak at −150 ppm in 19F MAS NMR. In contrast, Weinstock et al.15 have proposed that the basicity of KF/Al2O3 is induced by the formation of KOH by reaction of KF with the alumina support during preparation. Recently, Verziu et al.16 investigated the nature of the active site for mesoporous alumina supported alkaline fluorides applied to the transesterification of vegetable oils. They concluded that the active sites of alkaline fluorides were generated by co-operation between the fluorine and oxygen species. In addition to Al2O3, many other supports have been applied to KF based catalysts. These include ZnO,17 zeolites,18 fluorapatite,19 Zn(Al)O,20 and Mg–Al hydrotalcite.21 The application of different supports complicates the study of the catalytic mechanism and this results in significant difficulty in the identification of the most suitable support for a given application.9–21
The number and strength of base sites are highly pertinent parameters for the activity of heterogeneous base catalysts.8 In KF-loaded catalysts, it is known that the basicity is generated as a consequence of the KF loading process.9–21 The identification of the basicity is crucial for the elucidation of the catalytic mechanism and the identification of the active site. Two explanations can be advanced to explain why the source of basicity (i.e. the active sites and catalytic mechanism) is still not understood despite 30 years of extensive study on KF-loaded catalysts. One reason is the complexity of the catalyst surface. The other reason is that most of the studies performed to date have concentrated upon systems employing a single support.9–21 However, since each system is complex, it may be possible to gain a greater degree of understanding by taking an overview of the common features of supported KF catalysts employing a very wide range of supports. This general approach has been adopted in the present study as detailed below.
In recent years, hydrotalcite-like compounds (hereafter denoted HTlcs) have been applied to numerous processes, and have found use as catalyst precursors or supports, ion-exchangers, stabilizers and adsorbents.4,22 HTlcs are layered double hydroxides belonging to the class of anionic clays. Their general formula can be represented as: [M(II)1−xM(III)x(OH)2·(An−x/n)·mH2O], where M(II) (e.g. Mg, Zn, Cu, Mn, Co or Fe) and M(III) (e.g. Al, Cr, Fe, Ni or La) are divalent and trivalent metals, respectively; the value of x is equal to the molar ratio of M(II)/(M(II) + M(III)) and is generally in the range 0.2–0.33; An− is the anion that compensates for the positive charge of the hydroxide layers. Thermal decomposition of HTlcs leads to mixed oxides. The structure of HTlcs can accommodate a wide variation of different M(II) ions, M(III) ions, mixtures of M(III) and M(II) ions, M(II)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M(III) atomic ratios (represented by x = M(III)/M(II) + M(III) ranging from 0.1 to 0.33), and different types of inter-layered anion with charges ranging from −1 to −3.4,7,23,24 As a result of this, it is possible to tune the structure and properties of mixed oxide catalyst supports by employing HTlcs as precursors. Accordingly, the structure and properties of KF supported upon mixed oxides can be modified. In this way, it is possible to generate a wide range of supported KF systems, which can be investigated to yield information on the nature of the reaction system and/or the active sites.
M(III) atomic ratios (represented by x = M(III)/M(II) + M(III) ranging from 0.1 to 0.33), and different types of inter-layered anion with charges ranging from −1 to −3.4,7,23,24 As a result of this, it is possible to tune the structure and properties of mixed oxide catalyst supports by employing HTlcs as precursors. Accordingly, the structure and properties of KF supported upon mixed oxides can be modified. In this way, it is possible to generate a wide range of supported KF systems, which can be investigated to yield information on the nature of the reaction system and/or the active sites.
In this work, we have applied different HTlcs and single hydroxides as support precursors, from which we have prepared a series of KF-loaded catalysts. These KF-loaded catalysts have been characterized and their catalytic activity for the transesterification of vegetable oil with methanol to produce biodiesel has been determined. The aim of this study was to determine the catalytic mechanism and the structural and catalytic influence exerted by the support. Our experimental results demonstrate that KOH is the source of the basicity and is the active phase for the supported KF systems. This work is of interest in relation to the on-going discussion of the active phase and catalytic mechanism of KF-loaded catalysts, and it is useful for selecting suitable supports with the aim of designing highly active KF-loaded catalysts.
2. Experimental
2.1. Preparation of catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M3+atomic ratios of 3
M3+atomic ratios of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were prepared using a standard aqueous co-precipitation method at constant pH and temperature.25 An aqueous solution (166 mL) of the metal nitrates in the desired M2+
1 were prepared using a standard aqueous co-precipitation method at constant pH and temperature.25 An aqueous solution (166 mL) of the metal nitrates in the desired M2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M3+ molar ratio with a total concentration of 1.5 M was mixed slowly with an alkaline solution of NaCO3/NaOH with continuous stirring. The molar quantity of NaCO3 employed was twice that of M3+. The pH value of the mixture was kept constant, typically at values between 9 and 10, by adjusting the rate of addition of the alkaline solution. The temperature was maintained at 60 °C. Following this, which resulted in the formation of heavy slurry, the mixture was aged at 60 °C for 18 h with stirring, to enhance the selective formation of the precipitated hydrotalcite phase. The slurry was then cooled to 25 °C, filtered, and washed with water until the pH value of the filtrate was near 7. The precipitate was dried at 90 °C for 16 h. The resulting materials were HTlcs, which were then calcined at 500 °C for 3 h in static air to generate mixed oxides (hereafter denoted as M2+(M3+)O). In this study, the M2+(M3+)O materials prepared were Mg(Al)O, Cu(Al)O, Co(Al)O, Ni(Al)O, Mg(Fe)O, Cu(Fe)O, Ni(Fe)O, Co(Cr)O and Zn(Cr)O. Table 1 summarizes the systems prepared.
M3+ molar ratio with a total concentration of 1.5 M was mixed slowly with an alkaline solution of NaCO3/NaOH with continuous stirring. The molar quantity of NaCO3 employed was twice that of M3+. The pH value of the mixture was kept constant, typically at values between 9 and 10, by adjusting the rate of addition of the alkaline solution. The temperature was maintained at 60 °C. Following this, which resulted in the formation of heavy slurry, the mixture was aged at 60 °C for 18 h with stirring, to enhance the selective formation of the precipitated hydrotalcite phase. The slurry was then cooled to 25 °C, filtered, and washed with water until the pH value of the filtrate was near 7. The precipitate was dried at 90 °C for 16 h. The resulting materials were HTlcs, which were then calcined at 500 °C for 3 h in static air to generate mixed oxides (hereafter denoted as M2+(M3+)O). In this study, the M2+(M3+)O materials prepared were Mg(Al)O, Cu(Al)O, Co(Al)O, Ni(Al)O, Mg(Fe)O, Cu(Fe)O, Ni(Fe)O, Co(Cr)O and Zn(Cr)O. Table 1 summarizes the systems prepared.
In order to compare the activities of the catalysts containing mixed oxides with their single oxide component counterparts, single oxides (hereafter denoted as MO) were prepared by calcining single hydroxides (hereafter indicated as M(OH)x) in air at 500 °C for 5 h. The M(OH)x precursors were obtained by precipitation using aqueous NaOH from the corresponding aqueous nitrate salt solutions. The MO supports prepared are listed in Table 2. The γ-Al2O3 used was a commercial sample (Alfa Aesar, SBET = 220 m2 g−1).
2.2. Catalyst characterization
X-Ray diffraction patterns were recorded using a D/Max-3C X-ray powder diffractometer (Rigaku Co., Japan), using a Cu-Kα source fitted with an Inel CPS 120 hemispherical detector.Infrared transmission spectra were recorded in the range 400–4000 cm−1 using an FTIR spectrometer (Bruker Tensor 27, Germany) and applying the KBr pellet technique. The IR spectra of the samples are shown in supplementary data (ESI, Fig. S1 and S2†).
The surface area and pore characteristics of the catalysts were determined using a Micromeritics ASAP 2020 instrument. The sample was degassed at 250 °C for 4 h in N2 prior to surface area measurement. Nitrogen adsorption and desorption isotherms were measured at −196 °C, and the specific surface areas of the catalysts were determined by applying the BET (Brunauer-Emmett-Teller) method to nitrogen adsorption data obtained in the relative pressure range from 0.06 to 0.30. Total pore volumes were estimated from the amount of nitrogen adsorbed at a relative pressure of 0.995. Pore volume and pore-size distribution curves were obtained from analysis of the desorption branches of the nitrogen isotherms using the BJH (Barrett-Joyner-Halenda) method (Table 1 and 2; ESI, Table S1 and Fig. S3†).
Hammett indicator experiments were conducted to determine the H− value range of the basic sites for white solids only, since the sensitivity of this method was affected for colored solids. The conditions of pre-treatment of the solid for the determination of basic strength using Hammett indicators are given in Table 3. The Hammett indicators used were methyl yellow (pKa = 3.3), neutral red (pKa = 6.8), bromthymol blue (pKa = 7.2), phenolphthalein (pKa = 9.3), alizarin yellow R (pKa = 11.0), indigo carmine (pKa = 12.2), 2,4-dinitroaniline (pKa = 15), 4-nitroaniline (pKa = 18.4), 4-chloroaniline (pKa = 26.5), and diphenylmethane (pKa = 35). Typically, 25 mg of the catalyst was mixed with 1 mL of a solution of Hammett indicator diluted with cyclohexane and allowed to equilibrate for at least 1 h, after which the color of the catalyst was noted. The basic strength of the catalyst was taken to be higher than the weakest indicator that underwent a color change and lower than the strongest indicator that underwent no color change.
| Sample | Pre-treatment T (°C) | Basic Strength |
|---|---|---|
| Mg–Al HTlcs | 100 | 7.2 < H− < 9.3 |
| Mg(Al)O | 500 | 12.2 < H− < 15.0 |
| KF/Mg(Al)O-cn | 500 | 18.4 < H− < 26.5 |
| MgO | 500 | 11.0 < H− < 12.2 |
| KF/MgO-cn | 500 | 18.4 < H− < 26.5 |
| γ-Al2O3 | 500 | 7.2 < H− < 9.3 |
| KF/γ-Al2O3-cn | 500 | 18.4 < H− < 26.5 |
CO2 temperature programmed desorption (TPD) experiments were performed using a microreator (AutoChem II 2920, Micromeritics Instrument Corporation, USA) loaded with 70 mg of sample. The sample was pre-treated under He (30 mL min−1) ramping at 15 °C min−1 up to 500 °C where it was held for 30 min, and then cooled to 40 °C prior to the adsorption of CO2. After the adsorption of CO2 (30 mL min−1) for 60 min, the catalyst was flushed with He (30 mL min−1) for 60 min at 40 °C to remove the physisorbed gas from the surface of the catalyst, and the desorption profile was recorded employing a heating ramp of 10 °C min−1 between 40 to 500 °C where it was held for 30 min. TPD profiles of CO2 adsorbed on M2+(M3+)O and KF/M2+(M3+)O-cn materials are shown in the ESI (ESI, Fig. S4†).
Thermogravimetric analysis (TGA) experiments were carried out using a Q600 SDT thermal analysis machine (TA Instruments, USA) under a flow of nitrogen. The sample weight used was about 20 milligrams, and the temperature ranged from 38 to 1000 °C with a ramping rate of 20 °C/min.
2.3. Reaction procedure
The catalytic transesterification reactions were carried out under vigorous stirring in a round-bottomed flask equipped with a reflux condenser. Typical reactions were performed with 19.6 mL of vegetable oil (100% soybean oil; Xi'an Jiali Grease Industrial Co., Ltd., Xi'an, China) and 4.6 mL of methanol (methanol to vegetable oil molar ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) using 3 wt% (catalyst to oil weight ratio) of catalyst at methanol reflux temperature (65 °C) for the specified reaction time. The reaction products were analyzed using the following procedure: the samples were separated from the catalyst and glycerol by centrifugation, with the separation of glycerol being achieved because it was insoluble in the esters and had a much higher density. The methanol was removed under vacuum and the product was added to chloroform-d for 1H-NMR spectroscopy. The methyl ester could be readily determined quantitatively from the NMR spectra using the method described by Gelbard et al.26
1) using 3 wt% (catalyst to oil weight ratio) of catalyst at methanol reflux temperature (65 °C) for the specified reaction time. The reaction products were analyzed using the following procedure: the samples were separated from the catalyst and glycerol by centrifugation, with the separation of glycerol being achieved because it was insoluble in the esters and had a much higher density. The methanol was removed under vacuum and the product was added to chloroform-d for 1H-NMR spectroscopy. The methyl ester could be readily determined quantitatively from the NMR spectra using the method described by Gelbard et al.26
3. Results and discussion
3.1. BET surface area and pore size
The surface area of the mixed oxides was a function of their composition. Mg(Al)O had the highest surface area (190 m2 g−1). Cu(Fe)O had the lowest surface area (9 m2 g−1). Most of the prepared mixed oxides had high surface area (>54 m2 g−1). The surface areas of single oxides were also determined and are shown in Table 2. Among them, MgO had the highest surface area (151 m2 g−1) and the surface areas of the remaining single oxides were lower than 30 m2 g−1. Hence, compared to the mixed oxides, most of the single oxides had lower surface area.3.2. XRD analysis
Fig. 1 presents the XRD patterns of the HTlcs, M2+(M3+)O, KF/M2+(M3+)O-dy and KF/M2+(M3+)O-cn materials. The X-ray diffractograms of the HTlcs display characteristic diffraction peaks at 11.4°, 23.0°, 34.9° (Fig. 1 (a)).4,27 The thermal pre-treatment of the HTlcs results in changed XRD patterns, caused by the structural changes associated with the loss of CO2 and H2O from the starting material (Fig. 1 (b)).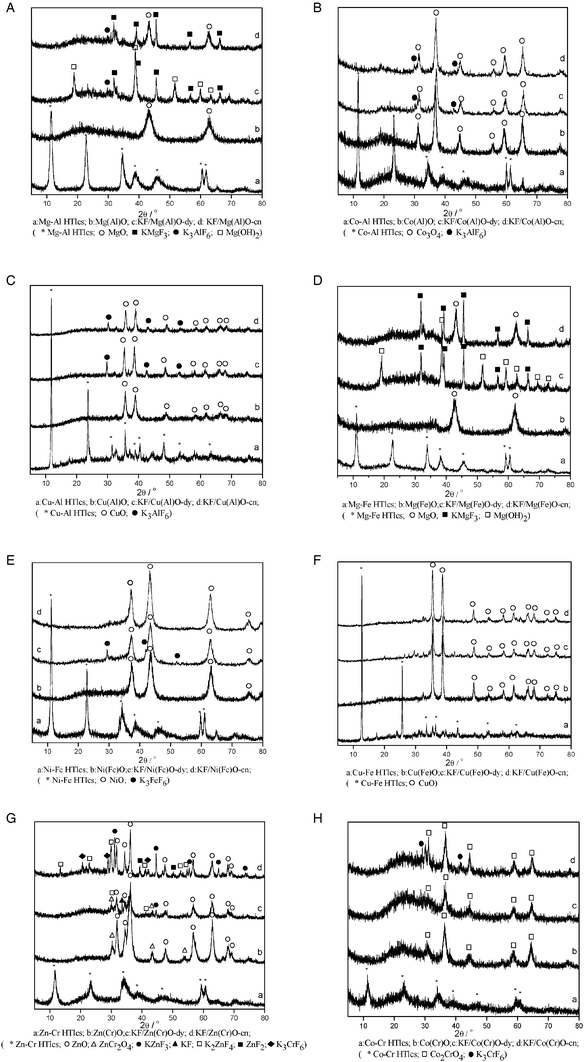 | ||
| Fig. 1 XRD patterns showing (a) HTlcs, (b) M2+(M3+)O, (c) KF/M2+(M3+)O-dy, and (d) KF/M2+(M3+)O-cn materials. | ||
Mg(Al)O displayed reflections characteristic of MgO (Fig. 1A (b)).27 MgO was rehydrated to form Mg(OH)2 in the process of preparing KF/Mg(Al)O-dy. The XRD pattern of KF/Mg(Al)O-dy showed, in addition to Mg(OH)2, a series of new diffraction peaks (Fig. 1A (c)). Among them, the reflections at 29.8° may correspond to K3AlF6 and the reflections at 31.8°, 39.2° and 45.6° could be associated with KMgF3. The XRD patterns of KF/Mg(Al)O-cn showed diffraction peaks of MgO, K3AlF6 and KMgF3.10,16,21,27 The MgO resulted from the removal of H2O from the Mg(OH)2 of KF/Mg(Al)O-dy. The formation of the new crystalline phases (KMgF3 and K3AlF6) can be ascribed to a chemical reaction between KF and the Mg(Al)O support (eqn (1) and (2)) during the preparation of KF/Mg(Al)O-dy.10,16,21,27 Stoichiometric considerations indicate that KOH will also be produced when KF is loaded on the Mg(Al)O support, despite the fact that no XRD reflections characteristic of KOH were found in the XRD pattern of the KF/Mg(Al)O catalysts, possibly as a consequence of high dispersion (Fig. 1A (d)).
| 3KF + MgO + H2O → KMgF3 (K2MgF4) + 2KOH | (1) |
| 12KF + Al2O3 + 3H2O → 2 K3AlF6 + 6KOH | (2) |
| 12KF + Fe2O3 + 3H2O → 2 K3FeF6 + 6KOH | (3) |
| 3KF + ZnO + H2O → KZnF3 (K2ZnF4, ZnF2) + 6KOH | (4) |
| 12KF + Cr2O3 + 3H2O → 2 K3CrF6 + 6KOH | (5) |
Co(Al)O displayed the reflections characteristic of Co3O4 (Fig. 1B (b)).28 The XRD patterns of KF/Co(Al)O-dy and KF/Co(Al)O-cn showed, in addition to Co3O4, the presence of K3AlF6 (Fig. 1B (c) and (d)), the formation of which can be ascribed to the chemical reaction between KF and the Co(Al)O support (eqn (2)) during preparation.
The XRD patterns of Cu(Al)O and its supported materials were similar to that of Co(Al)O. Cu(Al)O displayed reflections characteristic of CuO (Fig. 1C (b)).29 The XRD patterns of KF/Cu(Al)O-dy and KF/Cu(Al)O-cn showed, in addition to CuO, K3AlF6 (Fig. 1C (c) and (d)). The formation of the new phase (K3AlF6) can be ascribed to the chemical reaction between KF and the Cu(Al)O support (eqn (2)) during preparation.
Mg(Fe)O exhibited diffraction reflections characteristic of MgO (Fig. 1D (b)) and KF/Mg(Fe)O-dy showed diffraction peaks characteristic of Mg(OH)2 and KMgF3 (Fig. 1D (c)).30 The XRD patterns of KF/Mg(Fe)O-cn indicated the presence of KMgF3 and MgO (Fig. 1D (d)).
Ni(Fe)O showed diffraction reflections characteristic of NiO (Fig. 1E (b)) and the XRD patterns of KF/Ni(Fe)O-dy showed, in addition to NiO, a series of new diffraction peaks characteristic of K3FeF6 (Fig. 1E (c)).31 The presence of K3FeF6 may be ascribed to chemical reaction between KF and the Ni(Fe)O support (eqn (3)) upon preparation of KF/Ni(Fe)O-dy. The diffraction peaks characteristic of K3FeF6 became weak after calcination (Fig. 1E (d)).
The Cu(Fe)O displayed reflections characteristic of CuO (Fig. 1F (b)).29 The XRD patterns of KF/Cu(Fe)O-dy (Fig. 1F (c)) and KF/Cu(Fe)O-cn (Fig. 1F (d)) were similar to that of the Cu(Fe)O support, suggesting that KF doping did not apparently change the crystalline structure of the support.
Zn(Cr)O exhibited diffraction reflections characteristic of ZnO and ZnCr2O4 (Fig. 1G (b)).32 The XRD patterns of KF/Zn(Cr)O-dy showed, in addition to ZnO and ZnCr2O4, a series of new diffraction peaks ascribed to KZnF3, KF and K2ZnF4 (Fig. 1G (c)).10,33 KF arises from residues from the preparation and the new crystalline phases (KZnF3 and K2ZnF4) may be ascribed to the chemical reaction between KF and the Zn(Cr)O support (eqn (4)). Calcination enhanced the crystallinity of KF/Zn(Cr)O-cn (Fig. 1G (d)). Compared with KF/Zn(Cr)O-dy catalysts, the diffraction peak intensity of ZnO, KZnF3 and K2ZnF4 was higher on KF/Zn(Cr)O-cn. Furthermore, the KF/Zn(Cr)O-cn displayed, in addition to ZnO, KZnF3 and K2ZnF4, reflections characteristic of ZnF2 and K3CrF6.34,35 The ZnF2 and K3CrF6 may have resulted from chemical reaction between KF and the Zn(Cr)O support (eqn (4) and (5)) during the calcination process.
Co(Cr)O displayed reflections characteristic of Co2CrO4 (Fig. 1H (b)). The XRD patterns of KF/Co(Cr)O-dy were similar to that of Co(Cr)O (Fig. 1H (c)). Calcination made KF/Co(Cr)O-cn more crystalline (Fig. 1H (d)). Furthermore, KF/Co(Cr)O-cn showed, in addition to Co2CrO4, a series of new diffraction peaks characteristic of K3CrF6 (Fig. 1H (d)).The new crystalline phase (K3CrF6) may be ascribed to the chemical reaction between KF and the Co(Cr)O support (eqn (5)) during calcination.
Fig. 2 presents the XRD patterns of the prepared M(OH)x, MO and KF/MO catalysts. The M(OH)x sample showed X-ray diffractograms typical of M(OH)x (Fig. 2A–F (a)). The thermal pre-treatment of the M(OH)x sample resulted in a change in the XRD pattern, caused by the removal of H2O from the starting material (Fig. 2A–F (b)).The XRD pattern was further changed by the KF doping on the MO support (Fig. 2A–F (c)). The XRD pattern of KF/MgO-cn showed, in addition to MgO, a series of new diffraction peaks ascribed to K2MgF4 (Fig. 2A (c)).
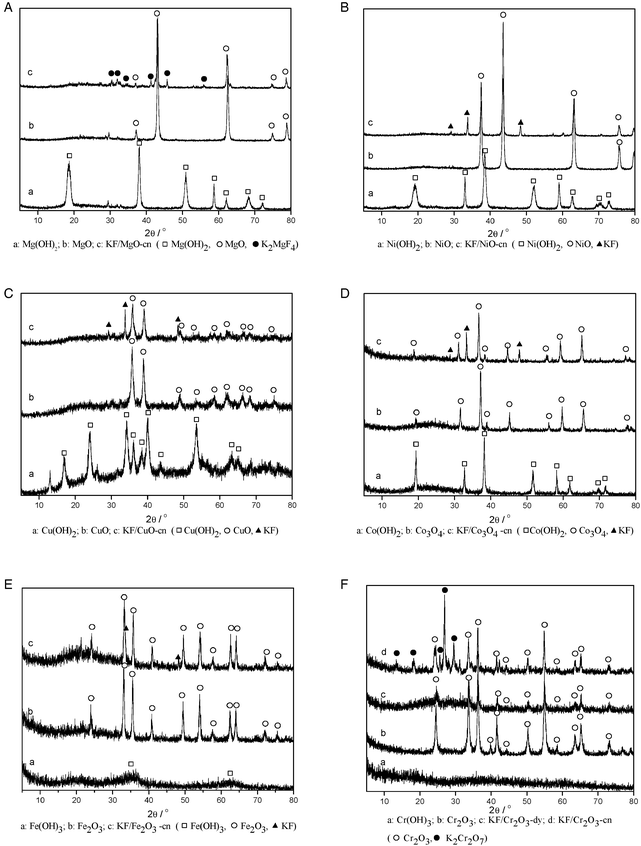 | ||
| Fig. 2 XRD patterns of (a) M(OH)x, (b) MO, and (c) KF/MO catalysts. | ||
KF/Cr2O3-dy displayed only diffraction peaks characteristic of Cr2O3 (Fig. 2F (c)). However, the XRD pattern of KF/Cr2O3-cn showed, in addition to Cr2O3, a series of new diffraction peaks ascribed to K2Cr2O7 (Fig. 2F (d)). The K2Cr2O7 compound was further validated by qualitative analysis. The newly formed K2Cr2O7 compound was first separated from the KF/Cr2O3-cn material, and then treated with a solution of AgNO3 or BaCl2. A red or yellow precipitate was observed for Ag2CrO4 or BaCrO4, respectively, demonstrating that the new compound was K2Cr2O7. The production of K2Cr2O7 during calcination is given by (eqn (6)):
| 4KOH + 2Cr2O3 + 3O2 → 2 K2Cr2O7 + 2H2O | (6) |
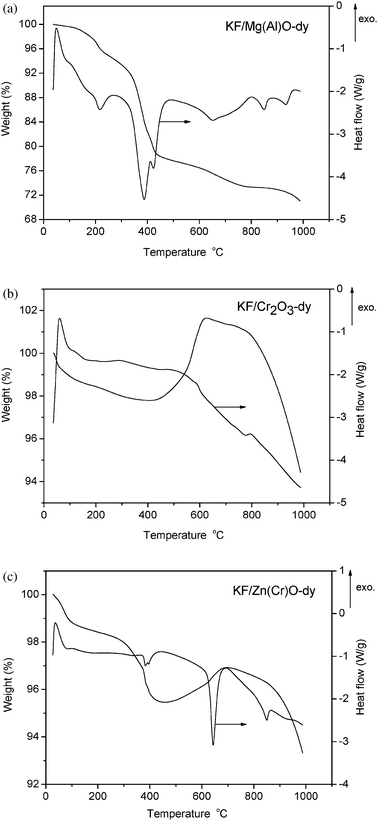 | ||
| Fig. 3 DSC/TG curves of (a) KF/Mg(Al)O-dy under a flow of nitrogen, (b) KF/Cr2O3-dy under a flow of oxygen, and (c) KF/Zn(Cr)O-dy under a flow of oxygen. | ||
The XRD patterns of the other four MO supported KF catalysts (KF/NiO, KF/CuO, KF/Co3O4, KF/Fe2O3) showed, in addition to their corresponding supports, diffraction peaks ascribed to residual KF (Fig. 2B–E (c)), suggesting that KF was not well dispersed and crystallized on the MO supports, which may be a consequence of their low surface area. The results also indicated that no other crystalline phases were formed in the process of preparing these MO supported catalysts.
Analysis of the XRD patterns demonstrates that the formation of KOH and KMxFy occurs for some supports when they are treated with KF. These supports are Mg(Al)O, Co(Al)O, Cu(Al)O, Mg(Fe)O, Ni(Fe)O, Zn(Cr)O, Co(Cr)O and MgO. However, these new compounds were not observed in the XRD patterns of KF supported on Cu(Fe)O, NiO, CuO, Co3O4 and Fe2O3, suggesting that any KOH and KMxFy formed may be in an amorphous state since KF can react with all the supports during preparation.
3.3. Thermogravimetric analysis
The structure characterization by XRD analysis showed that calcination affected the structure of KF-loaded catalysts. In order to explain the effect of calcination, we have investigated the calcination process of KF/Mg(Al)O-dy, KF/Cr2O3-dy and KF/Zn(Cr)O-dy by thermogravimetric analysis. The DSC/TG measurements of KF/Mg(Al)O-dy (Fig. 3a) showed the temperatures at which the precursors decomposed when heated in a nitrogen atmosphere. The weight of KF/Mg(Al)O-dy decreased with temperature until 1000 °C. The weight loss that occurred below 211 °C was ascribed to the removal of physisorbed water, and the weight loss above 211 °C was attributed to dehydroxylation and loss of CO2.The discussion of KF/Cr2O3-cn in the previous section showed that O2 participated in the process of calcining the KF/Cr2O3-dy samples. The proposed reaction pathway was illustrated by eqn (6), which indicated that sample mass increased after reaction. In order to verify the proposed reaction mechanism, thermal analysis of KF/Cr2O3-dy was undertaken in an O2 atmosphere. The DSC/TG result (Fig. 3b) showed that its weight decreased with temperature until 400 °C, after which the weight began to increase reaching a maximum at about 626 °C. Above 626 °C, the mass started to drop. The weight loss below 400 °C may be ascribed to the removal of physisorbed or chemisorbed water and CO2. The apparent increase of weight between 400 and 626 °C may be attributed to the chemical reaction between O2 and the sample, as indicated in eqn (6). Above 626 °C, the mass loss may possibly result from the decomposition of the sample and the removal of volatile gas. The DSC/TG result of KF/Zn(Cr)O-dy (Fig. 3c) was similar to that of KF/Cr2O3-dy. Its weight also increased between 400 and 626 °C, and decreased below 400 °C as well as above 626 °C, indicating that O2 also reacts with KF/Zn(Cr)O-dy as illustrated in eqn (6).
3.4. The basic strengths and basicity of the catalysts
The strength of the basic sites in the white samples has been analyzed qualitatively using Hammett indicators. As shown in Table 3, the Mg–Al HTlcs, Mg(Al)O, MgO and γ-Al2O3 samples were found to be weakly basic, possessing H− values in the range 7.2–15.0. Loading KF onto the support material promoted the basic strengths of the catalysts. KF/Mg(Al)O, KF/γ-Al2O3 and KF/MgO were found to possess H− values in the range of 18.4–26.5.3.5. Catalytic transesterification
| Catalyst | Biodiesel yield (%) | |
|---|---|---|
| -dy | -cn | |
| KF/Mg(Al)O | 100 | 100 |
| KF/Mg(Fe)O | 93 | 96 |
| KF/Cu(Al)O | 92 | 90 |
| KF/Ni(Fe)O | 83 | 86 |
| KF/Ni(Al)O | 94 | 100 |
| KF/Co(Al)O | 81 | 95 |
| KF/Cu(Fe)O | 78 | 87 |
| KF/Co(Cr)O | 82 | 70 |
| KF/Zn(Cr)O | 87 | 4 |
| KF/γ-Al2O3 | 82 | 86 |
The activity of KF/Cr2O3 was also affected by calcination (Table 5, entry 12). The biodiesel yield was 70% with KF/Cr2O3-dy as the catalyst; it decreased to 0 with KF/Cr2O3-cn as the catalyst. It seems that calcination leads to a decrease of activity for KF-loaded catalysts with supports containing Cr. The deactivation of KF/Zn(Cr)O-cn, KF/Co(Cr)O-cn and KF/Cr2O3-cn may be due to the decrease of KOH, resulting from reaction between Cr2O3 and KOH (eqn (6)) during calcination. The results suggest that the major active component of the prepared KF-loaded catalysts may be KOH.
The single oxide supports did not show any activity for biodiesel production under the reaction conditions studied (Table 5). However KF loading was observed to increase activity. The KF/MgO catalyst showed the highest activity, with a biodiesel yield of 98%; followed by the KF/CuO catalyst (biodiesel yield 92%). Other KF/MO systems had lower activity, with biodiesel yields in the range of 70–80%. Comparing the activity between the mixed oxide and single oxide systems, it was found that the activity of the mixed oxides was similar to, or a little higher, than their corresponding single oxide counterparts.
The results also showed that KF doping increases catalyst activity irrespective of whether the support was mixed oxide or singe oxide. It is apparent that the yield of the methyl esters does not directly correlate with the surface area of the catalyst support. The highest surface area was observed for the Mg(Al)O (190 m2 g−1) and the lowest surface area was for CuO (8 m2 g−1), yet the difference in their biodiesel yield was only 6%. The increased activity of the KF-loaded catalyst could be mainly ascribed to the active sites on the surface. The KF/M2+(M3+)O catalysts contained KF, KOH, KMxFy and M2+(M3+)O on their surfaces, while KF/MO catalysts contained KF, KOH, KMxFy and MO compounds on their surfaces (Table 6, entry 6 and 7). Of the surface compounds, KF was the dopant and M2+(M3+)O or MO was the support, which all showed low activity (Table 6, entry 1, 3 and 4). Since KOH and KMxFy were formed during the reatment of supports with KF, the high activity of the KF-loaded catalysts may result from the formation of either or both of the two new compounds. As the true active component should be common to all the prepared KF-loaded catalysts, KMxFy can be ruled out. In addition, since KMxFy are salts with no apparent basic properties it is not possible for them to act as basic catalysts for biodiesel synthesis. Furthermore, the commercially available K3AlF6, which has been tested in the transesterification of vegetable oil with methanol to biodiesel (Table 6, entry 5) showed no activity, as was expected. KMgF3 has also been proved to be ineffective for biodiesel synthesis as described in section 3.6. On this basis it can be deduced that KOH should be the most active component because KMxFy, KF and the supports were inactive for biodiesel synthesis (Table 6, entry 6 and 7). This conclusion was also supported by the structure analysis and activity of the KF-loaded catalysts whose supports contained Cr. It suggests that any oxide could be the active support of KF-loaded catalysts, if the support reacts with KF to produce KOH. On this basis a further question can be posed as to whether materials other than oxides can be active supports if they react with KF to produce KOH.
| Entry | Catalysts | Comprised compounds on the surface | Active componentsb | Biodiesel yield (%)c |
|---|---|---|---|---|
| a Characterized by structure analysis. b Deduced from the correlation between structure and activity of catalyst. c Experimental results. | ||||
| 1 | KF | KF | None | <1 |
| 2 | HTlcs | HTlcs | None | <3 |
| 3 | M2+(M3+)O | M2+(M3+)O | None | <8 |
| 4 | MO | MO | None | <8 |
| 5 | K3AlF6 | K3AlF6 | None | 0 |
| 6 | KF/M2+(M3+)O | KF | KOH | 80–98 |
| M2+(M3+)O | ||||
| KMxFya | ||||
| KOHa | ||||
| 7 | KF/MO | KF | KOH | 70–98 |
| MO | ||||
| KMxFy (K2Cr2O7)a | ||||
| KOHa | ||||
| 8 | KF/Mg–Al HTlcs | KF | KOH | 95 |
| Mg–Al HTlcs | ||||
| KMxFya | ||||
| KOHa |
In order to answer this question, a KF/Mg–Al HTlcs catalyst was prepared by doping KF on the Mg–Al HTlcs support. Powder X-ray diffraction showed that the KF/Mg–Al HTlcs catalyst contained KMgF3 (ESI, Fig. S5†). It is evident that Mg–Al HTlcs can react with KF to produce KOH. The activity of the KF/Mg–Al HTlcs catalyst was tested under the same conditions as the KF/M2+(M3+)O and KF/MO catalysts. A high yield (>95%) was obtained (Table 6, entry 8), indicating that Mg–Al HTlcs could be an active support of KF-loaded catalysts. Therefore it can be concluded that materials other than oxides are effective supports if they can also react with KF to produce KOH.
3.6. Comparison of KF doped catalyst and an equivalent KOH doped catalyst
The role of KF was investigated by comparing the activity of KF doped catalysts with that of an equivalent KOH doped catalyst (Table 7). The amount of KOH liberated from the KF-support reaction was determined by titrimetric analysis according to literature procedures.13 Aqueous washing of the KF doped reagents gives an alkaline solution, titration of which gives a guide to the amount of soluble base formed during the reagent preparation. Table 7 gives the measured basicities for the similarly prepared KF doped reagents (KF/Mg(Al)O, KF/Al2O3, KF/MgO, and KF/Fe2O3). The amount of soluble base for KF/Mg(Al)O was the highest (0.0493 g). Next was that of KF/Al2O3 (0.0474 g). The amount of soluble base for KF/Fe2O3 was far less than that of others (0.0007 g), being about 1.4% of the amount for the KF/Mg(Al)O and KF/Al2O3 catalysts. Table 7 also gives the activity for pure KOH and KOH doped catalysts. The activity of 0.0493 g of pure KOH (equivalent to the amount of base liberated by KF/Mg(Al)O) was similar to that of KF/Mg(Al)O (Table 4, biodiesel yield 100%), but higher than that of KOH/Mg(Al)O (Table 7, entry 2; biodiesel yield 81%). A similar phenomenon was observed for the KF/Al2O3 catalyst (Table 7, entry 3 and 4; Table 4). The activity of KF/MgO (Table 5, entry 2; biodiesel yield 98%) was higher than that of both pure KOH (Table 7, entry 5; biodiesel yield 87%) and KOH/MgO (Table 7, entry 6; biodiesel yield 60%). The activity of the pure KOH (Table 7, entry 7; biodiesel yield 1%) and KOH/Fe2O3 (Table 7, entry 8; biodiesel yield 1%) was far less than that of KF/Fe2O3 (Table 5, entry 8; biodiesel yield 83%). The results demonstrate that the activity of the KF doped catalyst is higher than that of an equivalent KOH doped support. Furthermore, except the case about KF/Fe2O3, even the activity of pure KOH is higher than that of an equivalent KOH doped support. This indicates that the KF doped catalysts can not be prepared directly by adding KOH to the supports. The different activity of the KF doped catalyst and KOH doped catalyst may be related to the difference of their structure. The main surface species of the KOH doped catalysts is KOH, while KOH, KF and KMxFy are the main surface species of the KF doped catalysts. Therefore, the higher activity of the KF doped catalysts may be ascribed to the synergistic relationship between the KOH (active sites) and the other surface species (KF or KMxFy). The KOH pure is an active homogeneous catalyst for biodiesel synthesis, while both KF pure and KMxFy pure show no activity. Hereby, the surface F-species (KF or KMxFy) may work as cocatalysts for KF doped catalysts.| Entry | Catalyst | Amount (g) | Biodiesel yield (%) | |
|---|---|---|---|---|
| -dy | -cn | |||
| a The amount of KOH liberated from 0.5 g of KF/Mg(Al)O-cn. b The amount of KOH liberated from 0.5 g of KF/γ-Al2O3-cn. c The amount of KOH liberated from 0.5 g of KF/MgO-cn. d The amount of KOH liberated from 0.5 g of KF/Fe2O3-cn. | ||||
| 1 | KOH | 0.0493a | 97 | |
| 2 | KOH/Mg(Al)O | KOH (0.0493)/Mg(Al)O (0.278) | 81 | 81 |
| 3 | KOH | 0.0474b | 87 | |
| 4 | KOH/γ-Al2O3 | KOH (0.0474)/γ-Al2O3 (0.278) | 69 | 50 |
| 5 | KOH | 0.0295c | 87 | |
| 6 | KOH/MgO | KOH (0.0295)/MgO (0.278) | 63 | 60 |
| 7 | KOH | 0.0007d | 6 | |
| 8 | KOH/Fe2O3 | KOH (0.0007)/Fe2O3 (0.278) | 10 | 10 |
3.7. Recycling experiments for catalysts
Three catalysts KF/Mg(Al)O, KF/Cu(Fe)O and KF/Fe2O3 were reused (Table 8). The biodiesel yield of the three fresh catalysts was in the range of 84–99% (Table 8, entry 1). Upon second use (Table 8, entry 2) a decrease in the activity of KF/Mg(Al)O and KF/Fe2O3 of about 30% was evident, with a greater decrease for KF/Cu(Fe)O being apparent, for which the biodiesel yield sharply dropped to 16%. Upon third use, activity was further reduced, and the catalysts were completely deactivated after being used more than 4 times. Although there were differences in the stability, the activity of the three tested catalysts declined with each reuse, which is a similar observation to other reported KF-loaded catalysts.16,20 The XRD results of KF/Mg(Al)O-cn before use and after being used 5 times are shown in Fig. 4, in which similar patterns are evident. Reflections characteristic of KMgF3 were observed in the XRD patterns of KF/Mg(Al)O-cn before use and after being used 5 times, further indicating that KMgF3 was not an active phase for the biodiesel synthesis since the catalyst was almost totally deactivated after being used 5 times. As discussed previously, analysis of the structure–activity relationship revealed that KOH was the active phase. Since KOH is soluble in the reactant methanol, the deactivation of reused catalysts may be due to the leaching of the active component KOH. Based on the experimental results, it is suggested that future work should be concentrated on the development of methods to prevent the leaching of the active component, KOH, and to improve the stability of KF-loaded catalysts.| Entry | Used times | Biodiesel yield (%) | ||
|---|---|---|---|---|
| KF/Mg(Al)O-cnb | KF/Cu(Fe)O-cnb | KF/Fe2O3-cnc | ||
| a The used catalyst was separated from the reaction solution by centrifugation. It was taken as the catalyst for the repeated reactions. The repeated reactions were run under the same reaction conditions to the last reaction. b Reaction conditions were supplied in experimental section. c Catalyst amount was 5 wt%, other reaction conditions were supplied in experimental section. | ||||
| 1 | First use | 99 | 84 | 97 |
| 2 | Second use | 59 | 16 | 72 |
| 3 | Third use | 46 | <1 | 10 |
| 4 | Fourth use | 16 | — | <1 |
| 5 | Fifth use | 8 | — | — |
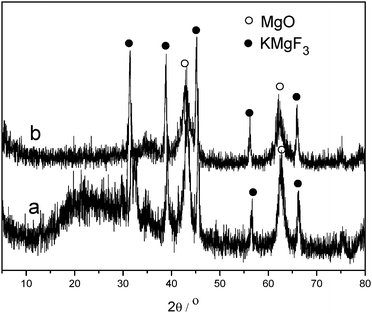 | ||
| Fig. 4 XRD patterns of KF/Mg(Al)O-cn (a) before use and (b) after being used 5 times. | ||
4. Conclusions
In this work, a series of KF-loaded heterogeneous base catalysts have been prepared, characterized and tested for the transesterification of vegetable oil with methanol to yield biodiesel. The deposition of KF on supports generates KMxFy and KOH species. The results showed that KF loading could increase the activity of each prepared catalyst irrespective of whether the support was a mixed oxide or singe oxide. The supports showed no activity (biodiesel yield <8%), but when loaded with KF high activities were apparent with biodiesel yields in the range of 73–98% being observed. The active phase of the KF-loaded catalysts was studied by systematically investigating the relationship between structure and activity. It was demonstrated that the active site of the KF-loaded catalysts is KOH, which is formed during the treatment of supports with KF. The activity of the KF doped catalysts is promoted by the other surface species (KF or KMxFy).Based on the results above, it is proposed that materials other than oxides can be active supports if they react with the KF dopant to produce KOH. This supposition is further supported by the experimental data concerning the direct application of hydrotalcite as a support for KF. On this basis, it is possible to identify potential novel highly active supports and these will not solely be limited to γ-Al2O3 and other reported supports. Furthermore, this study also shows that future work on the KF-loaded catalysts should be directed towards the development of methods to prevent leaching of the active KOH phase, hence improving the stability of the KF doped catalysts. Therefore, this work will be useful for selecting suitable supports in the design of highly active KF-loaded catalysts.
Acknowledgements
This work was supported by the Scientific Research Foundation for the Returned Overseas Chinese Scholars and the Project funded by Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2010JM2003). We thank Dr J. S. J. Hargreaves (Department of Chemistry, University of Glasgow, UK) for language help.References
- X. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. Shen, Nature, 2009, 458, 746 CrossRef CAS.
- R. Juárez, P. Concepción, A. Corma, V. Fornés and H. García, Angew. Chem., Int. Ed., 2010, 49, 1286 CrossRef.
- A. Thomas and M. Driess, Angew. Chem., Int. Ed., 2009, 48, 1890 CrossRef CAS.
- D. P. Debecker, E. M. Gaigneaux and G. Busca, Chem.–Eur. J., 2009, 15, 3920 CrossRef CAS.
- S. Shylesh, A. Wagener, A. Seifert, S. Ernst and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 184 CrossRef CAS.
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS.
- C. M. Jinesh, C. A. Antonyraj and S. Kannan, Catal. Today, 2009, 141, 176 CrossRef CAS.
- G. Busca, Chem. Rev., 2010, 110, 2217 CrossRef CAS.
- J. Yamawaki and T. Ando, Chem. Lett., 1979, 8, 755 CrossRef.
- J. M. Clacens, D. Genuit, L. Delmotte, A. Garcia-Ruiz, G. Bergeret, R. Montiel, J. Lopez and F. Figueras, J. Catal., 2004, 221, 483 CrossRef CAS.
- B. E. Blass, Tetrahedron, 2002, 58, 9301 CrossRef CAS.
- N. Boz, N. Degirmenbasi and D. M. Kalyon, Appl. Catal., B, 2009, 89, 590 CrossRef CAS.
- T. Ando, J. H. Clark, D. G. Cork, T. Hanafusa, J. Ichihara and T. Kinura, Tetrahedron Lett., 1987, 28, 1421 CrossRef CAS.
- H. Kabashima, H. Tsuji, S. Nakatab, Y. Tanaka and H. Hattori, Appl. Catal. A, 2000, 194–195, 227 CrossRef CAS.
- L. M. Weinstock, J. M. Stevenson, S. A. Tomellin, S. H. Pan, T. Utne, R. B. Jobson and D. F. Reinhold, Tetrahedron Lett., 1986, 27, 3845 CrossRef CAS.
- M. Verziu, M. Florea, S. Simon, V. Simon, P. Filip, V. I. Parvulescu and C. Hardacre, J. Catal., 2009, 263, 56 CrossRef CAS.
- W. Xie and X. Huang, Catal. Lett., 2006, 107, 53 CrossRef CAS.
- J. Zhu, Y. Chun, Y. Qin and Q. Xu, Microporous Mesoporous Mater., 1998, 24, 19 CrossRef CAS.
- A. Smahi, A. Solhy, H. E. Badaoui, A. Amoukal, A. Tikad, M. Maizi and S. Sebti, Appl. Catal., A, 2003, 250, 151 CrossRef CAS.
- C. Xu, J. Sun, B. Zhao and Q. Liu, Appl. Catal., B, 2010, 99, 111 CrossRef CAS.
- L. Gao, B. Xu, G. Xiao and J. Lv, Energy Fuels, 2008, 22, 3531 CrossRef CAS.
- S. Abelló, F. Medina, D. Tichit, J. Pérez-Ramírez, J. C. Groen, J. E. Sueiras, P. Salagre and Y. Cesteros, Chem.–Eur. J., 2005, 11, 728 CrossRef.
- G. Centi and S. Perathoner, Microporous Mesoporous Mater., 2008, 107, 3 CrossRef CAS.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173 CrossRef CAS.
- W. T. Reichle, J. Catal., 1985, 94, 547 CrossRef CAS.
- G. Gelbard, O. Bres, R. Vargas, F. Vielfaure and U. Schuchardt, J. Am. Oil Chem. Soc., 1995, 72, 1239 CrossRef CAS.
- A. H. Padmasri, A. Venugopal, V. Durga Kumari, K. S. Rama Rao and P. Kanta Rao, J. Mol. Catal. A: Chem., 2002, 188, 255 CrossRef CAS.
- J. Pérez-Ramíraz, G. Mul and J. A. Moulijn, Vib. Spectrosc., 2001, 27, 75 CrossRef.
- A. Alejandre, F. Medina, P. Salagre, X. Correig and J. E. Sueiras, Chem. Mater., 1999, 11, 939 CrossRef CAS.
- P. S. Kumbhar, J. Sanchez-Valente and F. Figueras, Tetrahedron Lett., 1998, 39, 2573 CrossRef CAS.
- M. Del Arco, P. Malet, R. Trujillano and V. Rives, Chem. Mater., 1999, 11, 624 CrossRef CAS.
- L. Zou, X. Xiang, J. Fan, F. Li and D. G. Evans, J. Phys. Chem. Solids, 2008, 69, 1075 CrossRef CAS.
- R. Hua, Z. Jia, D. Xie and C. Shi, Mater. Res. Bull., 2002, 37, 1189–1195 CrossRef CAS.
- D. S. Jacob, L. Bitton, J. Grinblat, I. Felner, Y. Koltypin and A. Gedanken, Chem. Mater., 2006, 18, 3162 CrossRef CAS.
- F. Heras, J. Dufour, A. López-Delgado, C. Negro and F. López-Mateos, Environ. Eng. Sci., 2004, 21, 583 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00022e |
| This journal is © The Royal Society of Chemistry 2011 |
