Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts
Chun-Hui
Zhou
*ab,
Jorge N.
Beltramini
*b,
Chun-Xiang
Lin
b,
Zhi-Ping
Xu
b,
G. Q. (Max)
Lu
b and
A.
Tanksale
b
aResearch Group for Advanced Materials & Sustainable Catalysis, College of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou, Zhejiang, 310032, P. R. China. E-mail: catalysis8@yahoo.com.cn
bARC Centre of Excellence for Functional Nanomaterials, The Australian Institute for Bioengineering and Nanotechnology and School of Engineering, The University of Queensland, St. Lucia, QLD 4072, Australia. E-mail: jorgeb@uq.edu.au
First published on 4th February 2011
Abstract
To convert glycerol, a by-product of biodiesel, into value-added fine chemicals is of great industrial and economic importance and represents a great challenge. This paper reports the influence of various metal elements (M = Ni, Zn, Cu, Co, etc.) incorporated into MgAl layered double hydroxide (LDH) materials used as catalysts for the liquid phase oxidation of glycerol to fine chemicals. The catalytic behaviour was also studied and discussed in terms of structure and texture of the synthesized materials that were determined using PXRD, FE-SEM, TEM, TG-DTA, FTIR, XPS and nitrogen adsorption–desorption isotherms. The results revealed that CuAlMg LDH materials are active in converting glycerol to glyceric acid (GLYAC) and formic acid, while yielding oxalic and hydroxyehthanoic acid. The selectivity to glyceric acid remarkably increased over Cu-containing catalysts, compared with those over similar materials with the addition of Ni, Zn, Co metal elements. The increase of Cu content not only affects the catalyst activity but also the selectivity. Moreover, an improvement of activity and selectivity was found on calcined CuAlMg samples. The calcined catalysts, with a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg molar ratio of 20
Mg molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, exhibited superior performances with a 97.3% conversion of glycerol and a 70% glyceric acid selectivity. It was concluded that the nature of the metal added to the LDH structure, such as Cu as active sites, in combination with the chemical state and microenvironment in calcined and uncalcined materials significantly affects the catalytic performance of the synthesized catalysts in the liquid phase oxidation of glycerol in terms of product activity and distribution.
200, exhibited superior performances with a 97.3% conversion of glycerol and a 70% glyceric acid selectivity. It was concluded that the nature of the metal added to the LDH structure, such as Cu as active sites, in combination with the chemical state and microenvironment in calcined and uncalcined materials significantly affects the catalytic performance of the synthesized catalysts in the liquid phase oxidation of glycerol in terms of product activity and distribution.
1. Introduction
The use of biorenewable feedstocks to produce commodity chemicals and clean fuels is an essential pathway to sustainable development. Particularly, it provides an alternative to global green-house gas emitting fuel, while mitigating the reliability of limited fossil fuel reserves.1,2 In this context, glycerol (1,2,3-propanetriol) is of interest as a biorenewable platform feedstock because of its wide availability.3,4Glycerol can be commercially produced by the fermentation of sugars such as glucose, either directly or as a by-product of the industrial conversion of lignocellulose into ethanol.5 More importantly, glycerol is essentially generated as a by-product during biodiesel production at the rate of 1 mol of glycerol for every 3 mol of biodiesel methyl esters generated.6,7 Notably, glycerol is an unavoidable by-product of the biodiesel process, so an increase in fatty acid ester consumption for fuels and related products will result in a significant increase in glycerol production. The projected production volume of both crude and purified glycerol will exceed the commercial demand in view of present techniques of glycerol utilization. Therefore, an effective way to convert glycerol into fine chemicals must be established. Any new routes to convert glycerol into value-added chemicals is of great industrial and scientific significance, not only because glycerol is a biosustainable feedstock, but because it is also a waste by-product of biodiesel production, either from fatty oils or algae, that must be used or eliminated.8–10Recently, much effort has been directed toward novel catalytic conversion processes that transform glycerol into useful chemicals. Most of the investigations reported in the scientific literature have been critically mentioned in our recent review.11 Among those, glycerol feedstocks provide a real alternative for the production of valuable oxygenated derivatives as glycerol is already a highly functionalized molecule. In the context of atom economy, the oxidation of glycerol could be more economical than hydrogenation, namely deoxygenation of glycerol. Nevertheless, when compared with glycerol hydrogenation, glycerol oxidation could lead to a more complex reaction pathway and as such selectivity would be more difficult to control. Theoretically, six potential C3 oxygenated products: dihydroxyacetone (DHA), hydroxypyruic acid, mesoxalic acid, glyceraldehydes (GLYALD), glyceric acid (GLYAC), tartronic acid, together with C2 (oxalic acid, hydroxyehthanoic acid, glyoxylic acid) and C1 products (formic acid, COx), can be obtained from oxidation of glycerol. Selective vs. non-selective oxidation is therefore a challenge associated with these possible catalytic oxidation reactions. Therefore, the first important step in the industrialization of these new processes requires the design of new effective heterogeneous catalysts to control the chemoselective orientation of the glycerol oxidation reaction towards either the oxidation of the primary alcohol –OH functions, to give glyceric acid, or the oxidation of the secondary alcohol function, giving dihydroxyacetone and hydroxypyruvic acid. Given its high boiling point, the selective oxidation of glycerol with air or oxygen is normally carried out in the liquid phase using water as the solvent.12–14 However, most previous studies dealing with the chemoselective catalytic oxidation of glycerol mainly use supported noble metals (palladium, platinum, gold) as catalysts.15–19 Apart from the cost of noble metals, the other disadvantage of supported Pt and Pd catalysts is the catalyst deactivation with increasing reaction time. In particular, catalytic sites that are based on the platinum group metal suffer oxygen poisoning that is proportional to the oxygen partial pressure.20,21 Therefore, there is still a need at laboratory and industry levels to search for new-generation catalysts for the selective oxidation of glycerol.
As for the case of non-noble metal-based catalysts, McMorn et al.22 studied glycerol oxidation over a range of transition metal-containing silicates and aluminophosphate catalysts in the presence of hydrogen peroxide as an oxidant. The results revealed that variations in reaction conditions, such as temperature, glycerol/hydrogen peroxide ratio or type of catalysts, namely, silicalite containing Ti, V, Fe or AlPO-5 containing Cr, V, Mn, Co, did not lead to the formation of partial oxidation products of glycerol. Formic acid and a mono-formate ester of glycerol were observed to be the major products, together with a complex mixture of acetals. Generally, the possibility of glycerol oxidation over non-noble metal-based oxide catalysts is scarcely reported and poorly understood.23
Therefore, it was noted that synthetic hydrotalcite-like solids, conventionally also called layered double hydroxides (LDHs), have potential use as catalysts.24 Though naturally occurring hydrotalcite would clearly qualify as a clay,25 compared with cationic clay minerals, synthetic hydrotalcite-like solids have a wider range of chemical compositions of layers based on a different choice of metals in the framework and interlayer anions. Thus, synthetic hydrotalcite-like solids have shown potentials for their catalytic activities in organic synthesis, particularly catalytic oxidation.26 Firstly, the ratio of metal atoms within the layers can be finely tuned in a wide range. In addition, calcination of LDHs yielded metal oxide structures with well-dispersed active species, which in most cases can improve catalytic functions.27 Furthermore, the doping of additional transition metals into MgAl-based LDHs can further tailor the catalytic performance in the oxidation, for instance, the oxidation of phenol in aqueous solutions,28,29 aerial oxidation of benzoin,30 and the oxidation of volatile organic compounds.31 Nevertheless, the possible use of LDH materials as catalysts for glycerol oxidation has not been reported. Here, for the first time, we explore the possibility for activating molecular oxygen using a series of synthetic LDHs with hydrotalcite (HT)-like layered structures with the incorporation of a third metal as dopant as catalysts for the selective oxidation of glycerol. The effect of the amount of metal elements in LDHs on their oxidation activity and/or selectivity to glyceric acid is investigated. The catalytic activity of CuAlMg LDHs materials as catalysts is also compared with that of other transition metal-containing LDHs solids for glycerol oxidation under similar experimental conditions. In particular, the relationships between structure and texture of Cu-containing LDHs and their calcined solids with the resulted catalytic behaviour are probed using several characterization techniques.
2. Experimental
2.1 Catalysts preparation
A series of LDH materials were synthesized by simultaneous co-precipitation and hydrothermal treatments. Typically, a mixture of MgCl2·6H20 (20.39 g, 0.10 mol) and Al(Cl)3·6H20 (12.07 g, 0.05 mol), and the desired amount of Cu(NO3)2·3H2O was dissolved in 200 mL of deionized water. Then, 2 M of NaOH aqueous solution was added to the above Mg–Al solution while stirring at 25 °C until pH 10 was reached. The pH was then maintained by the continual addition of 0.2 M Na2CO3. Thereafter, the resulting suspension was kept in a shaking water bath at 70 °C for 24 h. The solid was isolated by centrifugation and then re-suspended in 250 mL of 0.1 M Na2CO3 aqueous solution. After stirring for 6 h, the solid was thoroughly washed with deionized water and dried overnight at 110 °C. These samples were kept for characterization and used as uncalcined catalysts. Following the same synthetic procedures, the other metallic elements (M) in the form of their corresponding soluble salts were used to replace Cu; the molar ratio of M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg was adjusted by changing the amount of metallic salts, thus obtaining a series of LDHs-based materials. The dried samples were further calcined at 650 °C for 6 h and denoted MAlMg–C, where M stands for metallic elements.
Mg was adjusted by changing the amount of metallic salts, thus obtaining a series of LDHs-based materials. The dried samples were further calcined at 650 °C for 6 h and denoted MAlMg–C, where M stands for metallic elements.
2.2 Catalysts characterization
The total surface area was investigated using an automated adsorption and pore size analyzer (Autosorb-1C, Quantachrome, USA). The samples were degassed overnight at 473 K prior to the adsorption measurements. The specific surface area was calculated by the multi point Brunauer, Emmett and Teller (BET) equation using the adsorption data at a relative pressure of 0.05–0.35. The total pore volume was obtained at a relative pressure of 0.99, and the pore size distribution was obtained based on BJH model using desorption curve.Powder X-ray diffraction was carried out on a Rigaku MiniFlex X-Ray diffractometer, equipped with an automatic slit and with Cu-Kα radiation at 30 kV and 15 mA. The data was collected at a scanning speed of 2° min−1.
Scanning electron microscopy (SEM) of the samples was obtained using a JEOL field emission JSM-6400F and 6300F SEM on the platinum coated samples. The TEM characterization was performed through a Philips Tecnai F20 operated at 200 kV equipped with an EDAX (Energy Dispersive Analysis of X-ray) microanalysis system, with a Si detector at 150 eV resolutions per channel. Samples were dispersed in ethanol and then deposited onto a copper microgrid. The distribution of elements in the samples was scanned and determined on JEOL SEM6460.
The infrared spectra were record on a Nicolet 6700 spectrometer directly using powder samples at room temperature and ambient atmosphere.
TG-DTA analyses were conducted on a Shimadzu TA-60 thermo-gravimetric/differential thermal analyzer. Data was processed using the Shimadzu TA60-WS collection software program. About 15 mg of powder sample was loaded into a platinum crucible. All samples were heated at a rate of 10 °C min−1 in an airflow of ca. 80 mL min−1.
The X-ray photoelectron spectroscopy (XPS) analyses were conducted using a Kratos Axis ULTRA X-ray Photoelectron Spectrometer incorporating a 165 mm hemispherical electron energy analyzer. The incident radiation came from monochromatic Al X-rays (1486.6 eV) at 225 W (15 kV, 15 mA). Survey (wide) scans were taken at an analyzer pass energy of 160 eV and multiplex (narrow) higher resolution scans at a pass energy of 20 eV. Survey scans were carried out over a 1200–0 eV binding energy range with 1.0 eV steps and a dwell time of 100 ms. Narrow higher resolution scans were run with 0.05 ev steps and a 250 ms dwell time. Base pressure in the analysis chamber was 1.0 × 10−9 Torr and during sample analysis 1.0 × 10−8 Torr. Atomic concentrations were calculated using CasaXPS software and a linear baseline.
2.3 Catalytic reaction oxidation of glycerol
The catalyticglycerol oxidation experiments were carried out under atmospheric pressure in a 50 mL batch glass reactor equipped with an automated controller over stirring and heating rate, and a constant reaction temperature (J-KEM personal reaction station PRS-T-230-CE). For each reactor, 200 mg of catalyst was suspended in 5 mL aqueous solution of glycerol (1 mol L−1) and NaOH (10 mL, 2 mol L−1), and the mixture was heated to 60 °C with a constant flow of oxygen bubbling through the suspension. After 3 h reaction, the products collected were diluted using distilled water to a concentration suitable for high performance liquid chromatography (HPLC) analysis. The quantitative analyses of the reaction mixtures were then performed by HPLC using a UV detector at 210 nm, injection volume 30 μL and flow rate of 0.6 mL min−1. The percentage product distribution was calculated from the ratio of mass of specific product divided by total mass of products. The conversion of glycerol is calculated from the carbon equilibrium before and after reaction. The molar selectivity is expressed as percentage of products whereas the amount of a specific product was divided by the total amount of all products.3. Results and discussion
3.1 Catalytic behaviour of metal doped LDHs
Over, the metal doped LDHs catalysts used, the product distribution for glycerol oxidation mainly comprises oxalic acid, glyceric acid, hydroxyehthanoic acid (i.e. glycolic acid), and formic acid (Table 1). With the exception of glyceric acid, all other product compounds are the result of an over-oxidation reaction. It was noted that when using synthetic AlMg, NiAlMg, ZnAlMg, CoAlMg, CuAlMg, NiAl, ZnAl, SnAlMg, MoAlMg, MnAl and AlCr LDH materials, formic acid was found to be the main dominant product. In contrast, when FeAl LDH was used as the catalyst, hydroxyehthanoic acid was obtained as the main product (68.03% in the products distribution). In addition, the results suggested that all the catalysts, with the exception of the MgAl LDH catalyst, exhibited a glycerol conversion as low as 10%. The most interesting results were obtained for the CuAlMg LDH catalyst with a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 of Cu
200 of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg, showing a 43.7% glycerol conversion with a 21% selectivity to glyceric acid when compared to Mo, Sn, Fe, Cr-containing LDH catalysts. These preliminary results suggested that Cu is the most active metal species for the glycerol selective oxidation reaction.
Mg, showing a 43.7% glycerol conversion with a 21% selectivity to glyceric acid when compared to Mo, Sn, Fe, Cr-containing LDH catalysts. These preliminary results suggested that Cu is the most active metal species for the glycerol selective oxidation reaction.
| Catalysts | M/Mg/Al molar ratio | Glycerol conversion (%) | Products distribution (m/m %) | ||||
|---|---|---|---|---|---|---|---|
| Oxalic acid (%) | Glyoxylic acid (%) | Glyceric acid (%) | Hydroxyethanoic acid (%) | Formic acid (%) | |||
| AlMg | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
26.4 | 0.00 | 0.57 | 2.06 | 9.57 | 87.80 |
| NiAlMg | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
6.4 | 0.42 | 0.00 | 2.07 | 9.25 | 88.27 |
| ZnAlMg | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4.8 | 1.78 | 0.00 | 10.20 | 38.06 | 49.96 |
| CuAlMg | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
43.7 | 0.09 | 0.00 | 20.01 | 10.58 | 69.32 |
| CoAlMg | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.1 | 0.95 | 0.00 | 0.00 | 17.94 | 81.11 |
| NiAl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (no Mg) 5 (no Mg) |
4.4 | 0.00 | 8.25 | 0.00 | 32.06 | 59.69 |
| ZnAl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (no Mg) 5 (no Mg) |
4.3 | 0.00 | 0.00 | 7.92 | 30.90 | 61.18 |
| SnAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.8 | 0.00 | 0.00 | 7.28 | 29.49 | 63.23 |
| MoAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.5 | 0.00 | 0.00 | 0.00 | 25.39 | 74.61 |
| MnAl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (no Mg) 5 (no Mg) |
3.5 | 1.44 | 0.00 | 6.19 | 21.98 | 70.39 |
| FeAl | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (no Mg) 5 (no Mg) |
7.6 | 0.00 | 0.00 | 14.21 | 68.03 | 17.76 |
| MgCr | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (no Mg) 5 (no Mg) |
3.5 | 12.22 | 0.00 | 5.90 | 11.02 | 70.86 |
Moreover, the distribution of products of glycerol oxidation was dependent upon both the elemental composition and the ratio of active metal in LDH catalysts (Table 2). With regards to NiAlMg, ZnAlMg and CuAlMg catalysts, it was found that lower amounts of transition metal atom doping led mainly to the formation of glyceric acid, hydroxyehthanoic acid and formic acid. As can also be seen from Table 2, the yield variation for glyceric acid, hydroxyehthanoic acid and formic acid was related to the presence of different kinds of metal atoms attached to the LDH surface. Particularly, the presence of Cu on the LDH surface at 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 molar ratio results in the formation of glyceric acid as a main product. NiAlMg and ZnAlMg catalysts produced formic acid as the main product. This also reflected that different metal species have a distinct tendency to oxidise primary alcohol –OH function or the secondary alcohol function, thereby clearly allowing different selectivity. This unique property of the Cu metal was further confirmed by increasing the Cu metal concentration during the LDH synthesis, which resulted in the selective formation of glyceric acid and formic acid in the products yield. Similarly, the conversion of glycerol proportionally increased with an increase in the amount of metal Cu incorporated to the LDH catalyst. A decrease in the formation of glyceric acid, with a sharp increase in the hydroxyehthanoic acid and formic acid yields was also observed. Further, among all the metals investigated (Ni, Zn, Cu, Co, etc.), Co was the least active during the glycerol oxidation reaction. In contrast, CuAlMg proved to be an effective solid catalyst to produce glyceric acid. As Cu content on LDH support increases, the yield of formic acid increases as the expenses of hydroxyehthanoic acid decomposition into formic acid. However, further increasing the amount of Cu added to the LDH catalyst produces no change in the product distribution, while the total glycerol conversion still sharply increases from 55 to 86%. Such findings further confirmed Cu metal as the most active species that promotes the glycerol oxidation reaction among all the tested materials.
200 molar ratio results in the formation of glyceric acid as a main product. NiAlMg and ZnAlMg catalysts produced formic acid as the main product. This also reflected that different metal species have a distinct tendency to oxidise primary alcohol –OH function or the secondary alcohol function, thereby clearly allowing different selectivity. This unique property of the Cu metal was further confirmed by increasing the Cu metal concentration during the LDH synthesis, which resulted in the selective formation of glyceric acid and formic acid in the products yield. Similarly, the conversion of glycerol proportionally increased with an increase in the amount of metal Cu incorporated to the LDH catalyst. A decrease in the formation of glyceric acid, with a sharp increase in the hydroxyehthanoic acid and formic acid yields was also observed. Further, among all the metals investigated (Ni, Zn, Cu, Co, etc.), Co was the least active during the glycerol oxidation reaction. In contrast, CuAlMg proved to be an effective solid catalyst to produce glyceric acid. As Cu content on LDH support increases, the yield of formic acid increases as the expenses of hydroxyehthanoic acid decomposition into formic acid. However, further increasing the amount of Cu added to the LDH catalyst produces no change in the product distribution, while the total glycerol conversion still sharply increases from 55 to 86%. Such findings further confirmed Cu metal as the most active species that promotes the glycerol oxidation reaction among all the tested materials.
| Catalysts | M/Mg/Al molar ratio | Glycerol conversion (%) | Products distribution (m/m %) | ||||
|---|---|---|---|---|---|---|---|
| Oxalic acid (%) | Glyoxylic acid (%) | Glyceric acid (%) | Hydroxyethanoic acid (%) | Formic acid (%) | |||
| NiAlMg | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4.4 | 0.60 | 0.00 | 8.09 | 34.47 | 56.84 |
| ZnAlMg | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
6.5 | 0.56 | 0.00 | 10.32 | 44.95 | 44.17 |
| CuAlMg | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
10.5 | 0.30 | 0.00 | 47.38 | 28.32 | 24.00 |
| NiAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
27.6 | 0.00 | 4.14 | 10.75 | 39.88 | 45.22 |
| ZnAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
34.8 | 0.13 | 0.53 | 2.45 | 8.47 | 88.43 |
| CuAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
55.0 | 0.10 | 0.00 | 28.08 | 13.03 | 58.79 |
| CoAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.1 | 1.00 | 0.00 | 10.02 | 0.00 | 88.98 |
| NiAlMg | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
28.1 | 0.12 | 0.00 | 1.41 | 7.11 | 91.36 |
| ZnAlMg | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
27.7 | 0.07 | 0.00 | 1.53 | 7.13 | 91.26 |
| CuAlMg | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
86.0 | 0.28 | 0.00 | 35.69 | 12.76 | 51.27 |
| CoAlMg | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.7 | 0.75 | 0.00 | 10.67 | 12.11 | 76.47 |
In addition, the catalytic behaviour of Cu LDH materials was evaluated after catalyst calcination at 650 °C over 6 h. Catalytic results (Table 3) showed that when CuAlMg catalysts were calcined, the glycerol oxidation product distribution differed greatly from those results obtained over uncalcined materials. As an example, no traces of oxalic acid were detected. Particularly, independent of Cu content on calcined CuAlMg materials, glyceric acid was found to be the main product. At Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg catalysts molar ratio greater than 5
Mg catalysts molar ratio greater than 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, the formic acid yield is markedly reduced when compared with uncalcined catalysts. Over calcined Cu
200, the formic acid yield is markedly reduced when compared with uncalcined catalysts. Over calcined Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg catalysts with a molar ratio 20
Mg catalysts with a molar ratio 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, a higher glycerol conversion of 97.3% and 70% selectivity to glyceric acid can be produced (Table 3). In comparison to uncalcined Cu LDHs catalyst, over such a calcined catalyst, not only the selectivity to glyceric acid approximately doubled, but also there is a sharp increase in the hydroxyehthanoic acid selectivity. Interestingly, calcined Zn, Ni and Co-containing LDHs-based catalysts did not show improvement either in conversion or in products distribution. On the contrary, on calcined Zn and Ni-containing LDHs-based catalysts, the catalytic activity towards glycerol conversion decreases.
200, a higher glycerol conversion of 97.3% and 70% selectivity to glyceric acid can be produced (Table 3). In comparison to uncalcined Cu LDHs catalyst, over such a calcined catalyst, not only the selectivity to glyceric acid approximately doubled, but also there is a sharp increase in the hydroxyehthanoic acid selectivity. Interestingly, calcined Zn, Ni and Co-containing LDHs-based catalysts did not show improvement either in conversion or in products distribution. On the contrary, on calcined Zn and Ni-containing LDHs-based catalysts, the catalytic activity towards glycerol conversion decreases.
| Catalyst a | M/Mg/Al molar ratio | Glycerol conversion (%) | Products distribution (m/m %) | |||
|---|---|---|---|---|---|---|
| Glyoxylic acid (%) | Glyceric acid (%) | Hydroxyethanoic acid (%) | Formic acid (%) | |||
| a MalMg–C: calcined at 650 °C for 6 h using synthetic LDHs. | ||||||
| NiAlMg–C | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.8 | 0.00 | 12.66 | 27.09 | 60.25 |
| ZnAlMg–C | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4.3 | 0.00 | 13.46 | 33.10 | 53.43 |
| CuAlMg–C | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
20.8 | 0.00 | 55.38 | 29.24 | 15.38 |
| MgAlMg–C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.8 | 0.00 | 6.29 | 34.79 | 58.92 |
| NiAlMg–C | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.5 | 0.00 | 6.09 | 28.70 | 65.21 |
| ZnAlMg–C | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.4 | 0.00 | 7.01 | 26.74 | 66.25 |
| CuAlMg–C | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
41.2 | 0.09 | 70.16 | 24.43 | 5.32 |
| NiAlMg–C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.9 | 0.00 | 6.62 | 18.58 | 74.81 |
| ZnAlMg–C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4.3 | 0.00 | 0.00 | 41.24 | 58.76 |
| CuAlMg–C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
74.5 | 0.66 | 72.39 | 21.95 | 4.99 |
| CoAlMg–C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.5 | 0.00 | 0.00 | 17.86 | 82.14 |
| NiAlMg–C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.1 | 0.00 | 0.00 | 31.37 | 68.63 |
| ZnAlMg–C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
4.3 | 0.00 | 4.93 | 38.00 | 57.07 |
| CuAlMg–C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
97.3 | 2.90 | 70.73 | 21.09 | 5.28 |
| CoAlMg–C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.2 | 0.00 | 0.00 | 8.84 | 91.16 |
| MgAl–C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.6 | 0.00 | 13.71 | 55.25 | 31.04 |
| NiAl–C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
3.0 | 0.00 | 9.43 | 18.80 | 71.77 |
| ZnAl–C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.0 | 0.00 | 0.00 | 40.60 | 59.40 |
| CuAl–C | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
99.2 | 5.00 | 67.34 | 22.59 | 5.07 |
Table 3 also shows that calcined CuAL materials, using similar LDH precursors during synthesis, produced similar catalytic behaviour as the CuAlMg catalysts. These results showed that the existence of elemental Mg on LDH catalysts had little effect in changing the catalyst selectivity. In contrast, as previously observed, the enhancement of Cu content on LDHs-based uncalcined catalyst increases both the activity and the yield of glyceric acid, with an optimal Cu content of around 10–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Cu
200 Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio. Moreover, results suggested that Cu in LDHs or their calcined oxides acted as active species, which are more favourable for facilitating the glycerol oxidation reaction on the primary alcohol –OH functions, instead of secondary alcohol function. This unique function led to higher selectivity to glyceric acid. Furthermore, Considering that during calcinations the surface basicity of LDH-based materials could change as a result of the transformation of the hydroxides groups to oxides, and if also take into account the nature of transition metal atoms present on the catalyst surface, it could be preliminarily concluded that Cu has the highest catalytic activity, and that both the catalytic conversion and selectivity can be adjusted by changing the basicity which at the end can be tuned by calcination.
Mg ratio. Moreover, results suggested that Cu in LDHs or their calcined oxides acted as active species, which are more favourable for facilitating the glycerol oxidation reaction on the primary alcohol –OH functions, instead of secondary alcohol function. This unique function led to higher selectivity to glyceric acid. Furthermore, Considering that during calcinations the surface basicity of LDH-based materials could change as a result of the transformation of the hydroxides groups to oxides, and if also take into account the nature of transition metal atoms present on the catalyst surface, it could be preliminarily concluded that Cu has the highest catalytic activity, and that both the catalytic conversion and selectivity can be adjusted by changing the basicity which at the end can be tuned by calcination.
3.2 Structure analysis
As detected by the powder X-ray diffraction (PXRD, Fig. 1–3), those catalysts proved typical of layered materials containing sharp, symmetric and intense lines at low 2 theta values and less intense, asymmetric lines, at higher 2 theta angular values.32 The PXRD diffraction pattern of the carbonate exchanged samples that were dried at 110 °C showed sharp and symmetrical reflections for (003), (006), (110), (113) planes and broad asymmetrical peaks for (012), (015), (018) planes. In particular, a sharp peak at (003) plane indicates the formation of a highly crystalline material. The reflections were indexed to a hexagonal lattice with an R3m rhombohedral symmetry. All diffraction peaks have characteristics of a well crystalline single hydrotalcite-like phase as previously reported (LDH: JCPDS file no. 38-487).33 Moreover, when comparing any of these patterns to the hydrotalcite [Mg6A12(OH)16](CO3)·4H2O pattern, it was clear that M–AlMg materials have the same layered structure as hydrotalcite, whatever the metal Co, Cu, Zn, or Ni was used. No other crystalline phases such as malachite, brucite and gibbsite were detected, leading to the conclusion that non-LDH-like phases were not formed during the catalyst synthesis. | ||
Fig. 1 Typical PXRD patterns of uncalcined LDHs consisting of (a) AlMg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (b) NiAlMg, (c) ZnAlMg, (d) CuAlMg, (e) CoAlMg, (M 2) and (b) NiAlMg, (c) ZnAlMg, (d) CuAlMg, (e) CoAlMg, (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 5 Mg = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, molar ratio, M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). 200, molar ratio, M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). | ||
 | ||
Fig. 2 Typical PXRD patterns of uncalcined material (a) NiAlMg, (b) ZnAlMg, (c) CuAlMg, (d) CoAlMg at M/AlMg = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). 200 (M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). | ||
 | ||
Fig. 3 Typical PXRD patterns of uncalcined material (a) NiAlMg, (b) ZnAlMg, (c) CuAlMg, (d) CoAlMg at M/AlMg = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). 200 (M = Ni, Zn, Co, Co). (X-ray: Cu-Kα 30 kV/15 mA). | ||
It is also clear that the presence of the metal did not affect the LDH structure. Assuming a hexagonal structure for the LDH, then it is possible to calculate the lattice parameters ‘a’ and ‘c’ of the LDH phase from (110) and (003) reflections as listed in Table 4. Nevertheless, a slight shift of reflection was observed for planes (015) and (018) caused by a minor change in the crystal unit cell, and ascribed to the differences in the metal atom sizes. It was also observed that the formation of the LDH phase during synthesis remains the same despite the increase of the metal doping in their structure (Fig. 2 and 3). Hence, it is reasonable concluded that the just well-crystallized layered structure does not play an essential role in the differences in catalytic performances, but the doping of a third element has the obvious effect on enhancing activity and selectivity during the glycerol oxidation reaction. XRD patterns of calcined LDH samples (Fig. 4) show peaks that are characteristic of a poorly crystalline cubic MgO phase that resulted from the collapse of the layered structure (JCPDS: 04-0829).34 This clearly confirms that the crystalline-layered material has been transformed into poorly ordered mixed oxides composed of MgO, amorphous Al2O3 and copper oxide following calcination. As a result, the crystal phase changes have a significant influence on the catalytic activity and selectivity in the glycerol oxidation reaction, as shown from the data in Table 3. This confirms that the state of Cu metal species present in the catalyst also plays a pivotal role in catalytic performances. These results support the findings that over calcined CuAlMg catalyst, glyceric acid is the main product, while on uncalcined catalysts the formation of formic acid is dominant, with a more prominent product distribution than when using calcined materials. Moreover, Cu in oxide form or in LDHs form lead to different catalytic activity, along with interaction with the primary alcohol –OH functions and secondary alcohol function, as exhibited by difference of selectivity.
 | ||
Fig. 4 Typical PXRD patterns of uncalcined material (a) AlMg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and (b) NiAlMg, (c) ZnAlMg, (d) CuAlMg, (e) CoAlMg at M/AlMg = 20 2), and (b) NiAlMg, (c) ZnAlMg, (d) CuAlMg, (e) CoAlMg at M/AlMg = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 after calcined for 6 h at 650 °C. (X-ray: Cu-Kα 30 kV/15 mA). 200 after calcined for 6 h at 650 °C. (X-ray: Cu-Kα 30 kV/15 mA). | ||
| Catalysts | Precursor Composition | x | a/Å | c/Å | Treatment | Surface area/m2 g−1 | Pore volume/mL g−1 |
|---|---|---|---|---|---|---|---|
| x is the molar ratio of Cu/[Cu + Al + Mg], c parameter is related to the thickness of the brucite-like layer and the interlayer distance, a parameter is corresponding to the cation–cation distance within the brucite-like layer. (Cu-Kα wavelength: 1.54056 Å). | |||||||
| CuAlMg | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0083 | 3.047 | 22.946 | Dried at 110 °C | 80.6 | 0.647 |
| CuAlMg | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0164 | 3.047 | 22.946 | Dried at 110 °C | 71.0 | 0.587 |
| CuAlMg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0323 | 3.048 | 22.946 | Dried at 110 °C | 78.8 | 0.628 |
| CuAlMg | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0625 | 3.053 | 22.946 | Dried at 110 °C | 75.6 | 0.649 |
| CuAlMg–C | 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0083 | — | — | Calcined at 650 °C | 75.3 | 0.349 |
| CuAlMg–C | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0164 | — | — | Calcined at 650 °C | 62.7 | 0.327 |
| CuAlMg–C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0323 | — | — | Calcined at 650 °C | 67.5 | 0.353 |
| CuAlMg–C | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.0625 | — | — | Calcined at 650 °C | 71.3 | 0.362 |
3.3 Thermal analysis
Effect of calcination conditions and phase transformation were studied for Cu containing LDH materials using thermal analysis techniques. The weight loss (TG) and differential thermal analysis (DTA) results of CuAlMg LDH material samples with a molar of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 20
200 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, respectively, are depicted in Fig. 5. The TG profile shows that there were three stages of weight loss, namely, before 222 °C, 222–440 °C, and 440–900 °C, respectively. The first two stages corresponded to dehydration and dehydroxylation, and the third stage was attributed to the decomposition of LDH and partial loss of the CO32− portion in the composite.35 Previous thermal studies conducted on LDH materials suggested that three stages of endothermic weight loss were due to loss of interlayer H2O and some loosely bound CO32− at temperature <250 °C, then loss of structural H2O and CO32− at 250–400 °C and finally above 400–500 °C due to the loss of some strongly held CO32− anions.36 As seen from the weight loss results in Fig. 5, Cu content on the samples increases, there is no substantial change in the structure and stability of the material. Only a slight variation in the three stage of weight loss as shown in Fig. 5(A) and (B) was observed. These findings were further confirmed by the DTA data.
200, respectively, are depicted in Fig. 5. The TG profile shows that there were three stages of weight loss, namely, before 222 °C, 222–440 °C, and 440–900 °C, respectively. The first two stages corresponded to dehydration and dehydroxylation, and the third stage was attributed to the decomposition of LDH and partial loss of the CO32− portion in the composite.35 Previous thermal studies conducted on LDH materials suggested that three stages of endothermic weight loss were due to loss of interlayer H2O and some loosely bound CO32− at temperature <250 °C, then loss of structural H2O and CO32− at 250–400 °C and finally above 400–500 °C due to the loss of some strongly held CO32− anions.36 As seen from the weight loss results in Fig. 5, Cu content on the samples increases, there is no substantial change in the structure and stability of the material. Only a slight variation in the three stage of weight loss as shown in Fig. 5(A) and (B) was observed. These findings were further confirmed by the DTA data.
 | ||
Fig. 5
TG-DTA curves of (A) CuAlMg LDH sample at a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and (B) CuAlMg LDH sample at a molar ratio of 20 200 and (B) CuAlMg LDH sample at a molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20014. 20014. | ||
In addition, the DTA curves clearly show two major endothermic peaks at around 133 °C and 250 °C, respectively, which can be attributed to the dehydration of the LDH from water adsorbed on the outside of the anionic clay or, more likely, between the anionic clay layers. Variation in the amount of Cu loaded on the samples could be a function of the degree of substitution of Mg by Cu. In general, as the Cu doping increases, the temperature of the dehydroxylation, and/or decomposition steps shifts slightly towards low temperatures, while dehydration temperature remains similar. These findings suggest no difference in the surface physical adsorption of water. Nevertheless, the surface hydroxyl –OH and decomposition temperature could be slightly different, justifying the similar LDH phase formation as shown in the XRD results. As found from the experimental results, Cu present in LDH materials greatly improves the catalytic oxidation of glycerol. Moreover, as proved by TG-DTA analysis, new crystal phases can be formed after calcination at 650 °C, as a result of the collapse of the layered structure. The third major enthalpy change that took place around 433–443 °C led to the formation of a stable mixed oxide phase. As an example, CuCO3 formation will produce an additional rise in heat change at the higher temperature range, but this is not observed in DTA curves. These can explain why calcined CuAlMg LDH-based materials beyond 433–443 °C are dominantly in the fashion of mixed oxides, show different catalytic performances. Interestingly, this calcination treatment provided a notable increase in the glycerol to glyceric acid transformation in terms of activity and selectivity.
3.4 Texture and morphology of synthesized LDH catalysts
Evidently in Fig. 6, Cu-loaded LDH material’s nitrogen adsorption–desorption isotherms show type IV isotherms with an H3 hysteresis loop, confirming the presence of mesoporous structures with slit-shaped micropores or plate-like particles.37 The macro-mesopores formation is the result of aggregation of platelet-like particles of LDH materials, while micropores are composed of slit-like pores from the LDH lamellar structures. As indicated by the shape of the hysteresis loop, a change of Cu content in LDH catalysts result in a small change of the isotherm shape at low P/P0 range; while it is shown that the isotherm change is much greater at high-pressure range, indicating that the aggregation state and particle size have already been affected with the increase in Cu loading on the catalyst sample. However, the change of Cu contents in the samples does not produce any significant difference in crystal structure as observed from PXRD patterns and TG-DTA curves. The specific surface area as calculated by BET equation is in the range of 71.06 to 80.6 m2 g−1 as the Cu content varied (Table 4). | ||
Fig. 6 N2 adsorption–desorption isotherms of (A) CuAlMg LDH samples at ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = (a) 2.5, (b) 5, (c) 10 and (d) 20 Mg = (a) 2.5, (b) 5, (c) 10 and (d) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, respectively. (B) Samples calcined for 6 h at 650 °C at ratio of Cu 200, respectively. (B) Samples calcined for 6 h at 650 °C at ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlMg = (e) 2.5, (f) 5, (g) 10 and (h) 20 AlMg = (e) 2.5, (f) 5, (g) 10 and (h) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, respectively. 200, respectively. | ||
As shown in Fig. 6(B), the hysteresis loop of the materials after calcination is nearly horizontal and parallel over a wide P/P0 range, similar to a H4 loop, suggesting that its narrow slit-like pores are the result of the collapse of the lamellar structures.38 Moreover, total N2 adsorption values are lower for their uncalcined counterparts, resulting in a decrease in the specific surface area and total pore volume. However, such variations are too small to draw any finite conclusions on the influence in catalytic performances, but, as described previously, the nature of the metal atom in the structure plays a dominated role, instead of the texture.
As previously stated from the PXRD and TG-DTA results, the incorporation of Cu into the crystalline structure of the LDH materials does not affect the formation of the layered structure. In addition, Table 4 displays the calculated cation–cation distance (calculated by a = 2 × d110) and the interlayer thickness distance (calculated from c = 3 × d003) of the brucite-like layer.39 No major differences were found for those parameters as the amount of Cu incorporated into the LDH structure increases. These results suggest that as Cu is incorporated into the LDH layers, there is no change on the layered charges as a results that Mg2+ have the same chemical valence as Cu, thereby not affecting the amount of negative-charged anions in the interlayer space. Meanwhile, after calcination, all samples have mixed oxides compositions with mesopores structures as a result of the collapse and re-aggregation of nano-particles of synthesized LDH materials. Therefore, the calcination process has a more considerable impact on catalytic performance because of the chemical state of Cu, rather than the layered structure and changes in the texture.
Scanning electron microscopy (FE-SEM) micrographs from Fig. 7 show that when Cu is incorporated in the AlMg, LDH materials form plate-like agglomerated crystals, with the size of the plates depending on the Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg molar ratio. In contrast, CuAl LDH materials that were prepared with CO32− species show a larger plate size compared with those materials synthesized using tertiary metal doping, i.e.CuAlMg LDH materials. From the SEM pictures, it was also observed that the agglomerated particles are composed of small spherical to hexagonal platelet like particles. Although it was not possible to accurately determine the particle size from the SEM results, it is still possible to infer that CuAlMg LDHs materials are composed of relatively small nano-sized particles, which is in line with the typical observation by TEM (Fig. 8A).
Mg molar ratio. In contrast, CuAl LDH materials that were prepared with CO32− species show a larger plate size compared with those materials synthesized using tertiary metal doping, i.e.CuAlMg LDH materials. From the SEM pictures, it was also observed that the agglomerated particles are composed of small spherical to hexagonal platelet like particles. Although it was not possible to accurately determine the particle size from the SEM results, it is still possible to infer that CuAlMg LDHs materials are composed of relatively small nano-sized particles, which is in line with the typical observation by TEM (Fig. 8A).
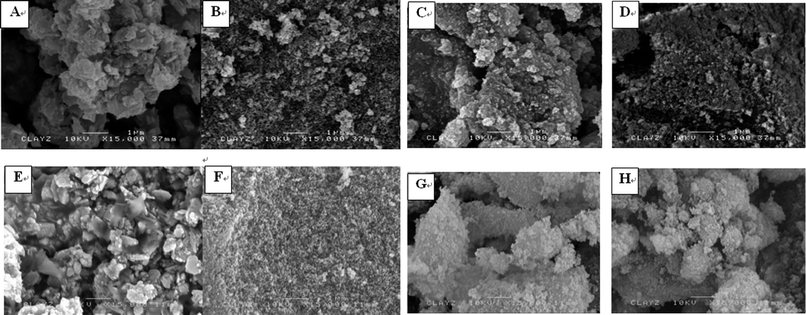 | ||
Fig. 7
FE-SEM images of samples CuAlMg at a molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg (A) (2.5 Mg (A) (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) (B) l (5 200) (B) l (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), (C) (10 200), (C) (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) (D) (20 200) (D) (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), and their corresponding CuAlMg–C samples after calcinations for 6 h at 650 °C at a molar ratio of Cu 200), and their corresponding CuAlMg–C samples after calcinations for 6 h at 650 °C at a molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg (E) (2.5 Mg (E) (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) (F) (5 200) (F) (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), (G) (10 200), (G) (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) and (H) (20 200) and (H) (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) (bar = 1 micron). 200) (bar = 1 micron). | ||
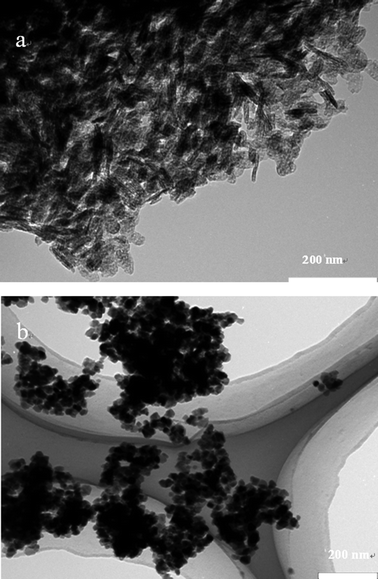 | ||
Fig. 8 Typical TEM images of samples CuAlMg at a molar ratio of (a) Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg 20 Mg 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and (b) Cu 200 and (b) Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg 20 Mg 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200. 200. | ||
The particle morphologies obtained by SEM on calcined samples are compared in Fig. 7E–H. Although the samples exhibited similar morphologies to their corresponding uncalcined counterparts, from the images it is clear that calcination treatments slightly decreased the particle size. All samples, with the exception the one of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 molar ratio, show consistent uniform crystal size distribution and shape. Moreover, there is no significant change when the Cu content is different. These results further confirm that the difference in catalytic behaviour originates from the chemical state of Cu, rather than the layered structure, texture, morphology and particle sizes (Fig. 8A and B).
200 molar ratio, show consistent uniform crystal size distribution and shape. Moreover, there is no significant change when the Cu content is different. These results further confirm that the difference in catalytic behaviour originates from the chemical state of Cu, rather than the layered structure, texture, morphology and particle sizes (Fig. 8A and B).
3.5 The effect of active species on reaction mechanism
FTIR spectroscopy is generally employed to identify the nature of interlayer anions present in LDH materials, and to provide information regarding the purity of the compound. All synthetic uncalcined samples shows characteristic bands in the FTIR spectra for MgAl–LDH intercalated with CO32− as the counter-anions (Fig. 9A). For example, these FTIR spectra show a broad band at 3300–3700 cm−1 for the uncalcined samples, which may be assigned to the stretching vibration mode of hydrogen-bonded hydroxyl groups from the gibbsite and brucite-like layers and from the interlayer water molecules. This broad band could overlap with the band above 3000 cm−1 assigned to υOH from water molecules hydrogen bonded to carbonate ions in the interlamellar layer.40,41 Therefore, such bands reflect to some extent that the samples contain a certain amount of carbonate ions. As the Cu content in LDHs samples increases, a change of the absorption band in the region of 3300–3700 cm−1, 1370 cm−1 and 400–1000 cm−1, respectively, was observed. Moreover, for samples after calcination, the intensity of the band at 3300–3700 cm−1 greatly decreased, perhaps as a result of the elimination of water molecules from the interlayer space. This finding is in agreement with the facts that carbonate content decreased when LDHs samples were calcined. Consequently, it proves that the layered structure were changed into mixed oxides by calcination. This assumption can be also supported by the fact that the band at about 1370 cm−1 corresponding to carbonate anions in the interlayer of the hydrotalcite-like compounds42 also changed and even was shifted to lower frequency, in particular for samples with higher copper content (see: arrow in the Fig. 9).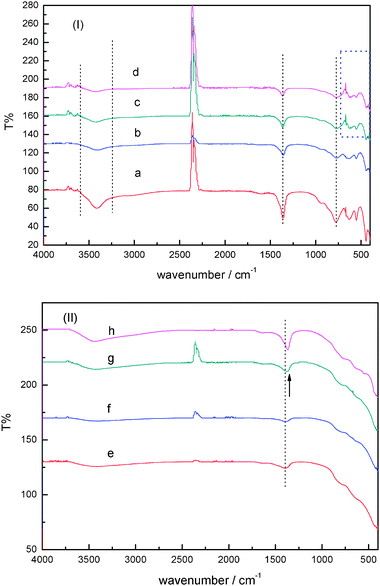 | ||
Fig. 9
FTIR spectra of CuAlMg material samples at a molar ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg (a) 2.5 Mg (a) 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, (b) 5 200, (b) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, (c) 10 200, (c) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and (d) 20 200 and (d) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 respectively. (I) Uncalcined samples; (II) samples calcined. 200 respectively. (I) Uncalcined samples; (II) samples calcined. | ||
Meanwhile, FTIR absorption bands at around 870, 1370, and 660 cm−1 can be assigned to the out-of-plane deformation, asymmetric stretching, due to the in-plane bending of CO32− ion existing in the LDH interlayer.40–42FTIR absorption bands detected at 400–1000 cm−1 are attributed to weak M–O vibration bands, which appeared more sensitive to the change of LDH phases after calcination above 600 °C. This is an indication of chemical transformation of LDHs to mixed oxides. The LDH compounds are generally described by the empirical formula:
| [(M2+)1−x(M3+)x(OH)2]x+(An−)x/n·yH2O, |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg molar ratio of 20
Mg molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, the formula and calcination reaction are as follows:
200, the formula and calcination reaction are as follows:| [Cu0.104Al0.375Mg0.521 (OH)2]0.375+[CO32−]0.1875·yH2O |
| → 0.521MgO + 0.104CuO + 0.1875Al2O3 + 0.1875CO2 + (y + 1)H2O |
Thereafter, the influence of the Cu and Al sites distribution in the structure of the calcined samples and its influence on the catalytic properties, was studied using SEM to conduct a surface survey of the chemical composition for a CuAlMg sample with (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) molar ratio. From the images in Fig. 10 we observed that Cu, Mg and Al are uniformly distributed in the structure. This results combined with the findings from PXRD and FTIR analysis, confirmed that well-distributed Cu exists in the form of amorphous copper oxide on the LDH structure.
200) molar ratio. From the images in Fig. 10 we observed that Cu, Mg and Al are uniformly distributed in the structure. This results combined with the findings from PXRD and FTIR analysis, confirmed that well-distributed Cu exists in the form of amorphous copper oxide on the LDH structure.
 | ||
Fig. 10 Surface survey of chemical composition on SEM for sample CuAlMg (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200)-C. 200)-C. | ||
Knowing that the hydrotalcite structure is based upon the brucite structure, in this case the Mg atoms were partly replaced by Cu and Al atoms, where Mg(OH)2·xH2O becomes [Cu0.104Al0.375Mg0.521 (OH)2]0.375+[CO32−]0.1875·yH2O. As only copper increases in the reactants during the synthesis of LDH samples, and Cu2+ and Mg2+ are with the chemical valence, so the variation of copper does not significantly change the layered charge, which concurs with the no significant change of carbonate ions and water content in the interlayer space as shown in FTIR. Therefore, the layered charge did not play an essential role in improving catalytic performance. Consequently, it could reasonable to deduce that Cu is the active species in the LDHs structure and in the calcined mixed oxides. Identification of metal species in the synthetic LDH was checked by X-ray photoelectron spectroscopy (XPS). The LDHs samples synthesized in the presence of Cu2+ solution led to a XPS spectrum typically obtained from copper hydroxides, showing the characteristic Cu 2p bind energy around 93.4 eV. It could be ascribed to the existence of Cu in the layered in the form similar to copper hydroxides.43 After calcination, Cu 2p bind energy decrease a bit to around 93.5 eV and this can be caused by the formation of CuO. The results of XPS analyses are consistent with the above PXRD, TG-DTA results to indicate the substitution of brucite layers by partly copper hydroxides and after calcination the transformation of copper hydroxides to copper oxides. It reflected that Cu2+ exists in the form of Cu(OH)2 in the LDHs is less active than Cu2+ present in the calcined mixed oxides.
Considering that over Cu-containing LDHs-based catalysts, glyceric acid and hydroxyethanoic acid are the main products obtained, it can be postulated the following possible reaction mechanism (Scheme 1).
 | ||
| Scheme 1 Possible catalytic active sites and postulated reaction mechanism. | ||
Under the reaction conditions, glycerol molecules were readily adsorbed on the catalyst surface through contact between –OH and catalyst surface. In the case of Cu species on the catalyst surface, primary –OH groups of glycerol molecules were oxidized into –CHO groups, yielding the intermediates of glyceraldehyde. As findings in the literature, the –CHO group in glyceraldehyde readily react with Cu species perhaps as the mechanism taking place in the oxidation of glucose.44 Then glyceraldehyde could act as intermediate, which is more active than glycerol, thus glyceraldehyde was further oxidized to yield glyceric acid. Meanwhile, if one C–C bond is broken on the surface of the catalyst in the presence of active oxygen species, then hydroxyehthanoic acid is yielded along with some formic acid. This suggested that over Cu-containing LDHs-based catalysts, glycerol oxidation reaction took place by an route of the oxidation of the primary alcohol functions, with glyceraldehyde as intermediates, and then selectively yield glyceric acid. For detailed mechanisms, further research is under investigation.
4. Conclusion
Ternary hydrotalcite-like LDH precursors containing Zn, Co, Ni, Cu and Mg Al in the brucite layers were prepared by co-precipitation with hydrothermal treatment. These synthetic LDHs were tested as catalysts for the catalytic oxidation reaction of glycerol. Under reaction condition at 60 °C for 3 h in the presence of oxygen, the products consisted of oxalic acid, glyceric acid, hydroxyehthanoic acid (i.e. glycolic acid), and formic acid. Formic acid was mostly the main product from the over-oxidation of glycerol over these catalysts. However, Cu-containing LDHs exhibit distinct catalytic behaviour with higher yields of glyceric acid.Characterization results from PXRD, TG-DTA, SEM and FTIR showed that synthetic LDHs with hexagonal morphology were successfully synthesized using an Al and Mg precursors and introducing a third metal element such as Zn, Co, Ni or Cu. The materials consisted of a pure LDH phase with a homogeneous crystal size distribution. As to Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg LDHs, with increasing the Cu content in LDHs sample, the percentage of glyceric acid in the products distribution increased. Calcination of Cu
Mg LDHs, with increasing the Cu content in LDHs sample, the percentage of glyceric acid in the products distribution increased. Calcination of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg LDH led to the formation of amorphous mixed oxides and the calcined catalyst with a Cu
Mg LDH led to the formation of amorphous mixed oxides and the calcined catalyst with a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al
Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg molar ratio of 20
Mg molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 exhibited the best results with a 97.3% conversion of glycerol and a 70% of glyceric acid in the products distribution.
200 exhibited the best results with a 97.3% conversion of glycerol and a 70% of glyceric acid in the products distribution.
The nature of active components, in combination with the chemical state in calcined and uncalcined Cu LDHs-based materials would significantly affect the catalytic performance of the synthesized catalysts during the liquid phase oxidation of glycerol in terms of activity and distribution of products. This meant that the difference in catalytic behaviour mainly originated from the type of metal element, chemical state of Cu, rather than the layered structure, texture, morphology and particle size. Cu2+ existing as Cu(OH)2 in the LDH structures was less active than Cu2+ present in the calcined mixed oxides. A most likely reaction mechanism was proposed, in which over Cu-containing LDH-based catalysts, glycerol oxidation reaction might take place by an oxidation of the primary alcohol functions, given glyceraldehyde as intermediate, and finally selectively yield to high glyceric acid.
Acknowledgements
The authors wish to acknowledge the financial support from the ARC Centre of Excellence for Functional Nanomaterials and the financial support for visiting academic CH Zhou by the China Scholarship Council (CSC) and National Natural Scientific Foundation (NSF) of China (no. 20773110, 20541002), the Natural Scientific Foundation of Zhejiang Province (no. Y405064, R4100436) and Project of Zhejiang “151” talents project.References
- Y. X. Fan, C. H. Zhou and X. H. Zhu, Catal. Rev. Sci. Eng., 2009, 51, 293 CrossRef CAS.
- A. Tanksale, C. H. Zhou, J. Beltramini and G. Q. Lu, J. Inclusion Phenom. Macrocyclic Chem., 2009, 65, 83 CrossRef CAS.
- C. S. Gong, J. X. Du, N. J. Cao and G. T. Tsao, Appl. Biochem. Biotechnol., 2000, 84–86, 543 CrossRef CAS.
- P. L. Rogers, Y. J. Jeon and C. J. Svenson, Process Saf. Environ. Prot., 2005, 83, 499 Search PubMed.
- J. A. Onwudili and P. T. Williams, Fuel, 2010, 89, 501 CrossRef CAS.
- M. Mccoy, Glycerin surplus. Chem. Eng. News, 2006, 84, 7 Search PubMed.
- M. Sankar, N. Dimitratos, D. W. Knight, A. F. Carley, R. Tiruvalam, C. J. Kiely, D. Thomas and G. J. Hutchings, ChemSusChem, 2009, 2, 1145 CrossRef CAS.
- M. Aresta, A. Dibenedetto, M. Carone, T. Colonna and C. Fragale, Environ. Chem. Lett., 2005, 3, 136 Search PubMed.
- C. A. Ramirez-Lopez, J. R. Ochoa-Gomez, M. Fernandez-Santos, O. Gomez-Jimenez-Aberasturi, A. Aonso-Vicario and J. Torrecilla-Soria, Ind. Eng. Chem. Res., 2010, 49, 6270 CrossRef CAS.
- J. C. Ye, S. Tu and Y. Sha, Bioresour. Technol., 2010, 101, 7368 CrossRef CAS.
- C.-H. Zhou, J. N. Beltramini, Y.-X. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527 RSC.
- H. Kimura, K. Tsuto, T. Wakisaka, Y. Kazumi and Y. Inaya, Appl. Catal., A, 1993, 96, 217 CrossRef CAS.
- H. Kimura, Appl. Catal., A, 1993, 105, 147 CrossRef CAS.
- A. Abbadi and H. van Bekkum, Appl. Catal., A, 1996, 148, 113 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311(5759), 362–365 CrossRef CAS.
- S. Carrettin, P. McMorn, P. Johnston, K. Griffin and G. J. Huthchings, Chem. Commun., 2002, 696 RSC.
- S. Carrettin, P. McMorn, P. Johnston, K. Griffin, G. J. Hutchings and C. J. Kielly, Phys. Chem. Chem. Phys., 2003, 5, 1329 RSC.
- F. Porta, L. Prati. J. Catal., 2004, 224, 397 Search PubMed.
- A. Villa, G. M. Veith and L. Prati, Angew. Chem., Int. Ed., 2010, 49, 4499 CrossRef CAS.
- M. Besson and P. Gallezot, Catal. Today, 2000, 57, 127 CrossRef.
- T. Mallat and A. Baiker, Catal. Today, 1994, 19, 247 CrossRef CAS.
- P. McMorn, G. Roberts and G. J. Hutchings, Catal. Lett., 1999, 63, 193 CrossRef CAS.
- M. Y. Li, C. H. Zhou, J. Beltramini, W. H. Yu and Y. X. Fan, Progress in Chemistry, 2008, 20, 1474 Search PubMed.
- C. H. Zhou, Appl. Clay Sci., 2010, 48, 1 CrossRef CAS.
- S. Guggenheim and R. T. Martin, Clays Clay Miner., 1995, 43, 255 CrossRef CAS.
- C. H. Zhou, H. L. Xie, Z. X. Du, Y. X. Fan, Z. H. Ge and X. N. Li, J Chem. Eng. Chin. Univ., 2009, 23, 516 Search PubMed.
- D. S. Tong, C. H. Zhou, M. Y. Li, W. H. Yu, J. Beltramini, C. X. Lin and Z. P. Xu, Appl. Clay Sci., 2010, 48, 569 CrossRef CAS.
- A. Alejandre, F. Medina, X. Rodriguez, P. Salagre and J. E. Sueiras, J. Catal., 1999, 188, 311 CrossRef CAS.
- C.-X. Chen, C.-H. Xu, L.-R. Feng, F.-L. Qiu and J.-S. Suo, J. Mol. Catal. A: Chem., 2006, 252, 171 CrossRef CAS.
- D. Sachdev, M. A. Naik, A. Dubey and B. G. Mishra, Catal. Commun., 2010, 11, 684 CrossRef CAS.
- C. Gennequin, S. Kouassi, L. Tidahy, R. Cousin, J.-F. Lamonier, G. Garcon, P. Shirali, F. Cazier, A. Aboukaïs and S. Siffert, C. R. Chim., 2010, 13, 494 CrossRef CAS.
- F. Cavani, F. Trifiro and A. Vaccari, Catal. Today, 1991, 11, 173 CrossRef CAS.
- M. R. Weir, J. Moore and R. A. Kydd, Chem. Mater., 1997, 9, 1686 CrossRef CAS.
- H. S. Kim and H. W. Kim, Acta Physica Polonica A, 2009, 116, 58 Search PubMed.
- R. L. Frost, Z. Ding, W. N. Martens and T. E. Johnson, Thermochim. Acta, 2003, 398, 167 CrossRef CAS.
- S. Velu, K. Suzuki, S. Hashimoto, N. Satoh, F. Ohashi and S. Tomura, J. Mater. Chem., 2001, 11, 2049 RSC.
- S. J. Gregg and K. S. W. Sing, Adsorption, Surface Area and Porosity, Academic Press, London, 1982 Search PubMed.
- H. P. Lin, S. T. Wong, C. Y. Mou and C. Y. Tang, J. Phys. Chem. B, 2000, 104, 8967 CrossRef CAS.
- Z. Q. Yang, K.-M. Choi, N. Z. Jiang and S.-Eon Park, Bull. Korean Chem. Soc., 2007, 28, 2029 CrossRef CAS.
- M. M. V. M. Souza, K. A. Ferreira, O. R. de Macedo Neto, N. F. P. Ribeiro and M. Schmal, Catal. Today, 2008, 133–135, 750 CrossRef CAS.
- F. Kooli, V. Rives and M. A. Ulibarri, Inorg. Chem., 1995, 34, 5122 CrossRef CAS.
- T. Yamaoka, M. Abe and M. Tsuji, Mater. Res. Bull., 1989, 24, 1183 CrossRef CAS.
- M. L. Farquhar, J. M. Charnock, K. E. R. England and D. J. Vaughan, J. Colloid Interface Sci., 1996, 177, 561 CrossRef CAS.
- N. Torto, Bioelectrochemistry, 2009, 76, 195 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
