Empirical modelling as an experimental approach to optimize lactone production
Nelma
Gomes
,
José A.
Teixeira
and
Isabel
Belo
*
IBB-Institute for Biotechnology and Bioengineering, Center of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057, Braga, Portugal. E-mail: ibelo@deb.uminho.pt; Fax: +351253678986; Tel: 351253604413
First published on 4th February 2011
Abstract
The biotransformation of ricinoleic acid, carried out by Yarrowia lipolytica, leads to the formation of γ-decalactone, a well-known peach-like aroma compound, interesting to produce and to use in the flavouring industry, reason why it is imperative to define the most appropriate conditions for its production. Thus, the aim of this work is the optimization of operating conditions for this lactone. However, as the accumulation of another compound, namely 3-hydroxy-γ-decalactone (the precursor of two other aromatic compounds, dec-2-enolide and dec-3-enolide), may also occur simultaneously in the biotransformation medium, and since this compound may as well be of interest for the flavouring industry, the operating conditions for its production were also a focus of attention. Therefore, a 32 level full-factorial design was used to determine the effect of pH and dissolved oxygen concentration (DO) on the production of γ-decalactone and 3-hydroxy-γ-decalactone. Since both factors were found to influence the two lactones production, a response surface methodology (RSM) analysis was also applied to identify the optimal conditions for the production of those two compounds. The statistical model pointed out pH = 6.17 and DO = 44.4% as the best conditions optimizing γ-decalactone production. Using these optimal conditions, the maximal γ-decalactone concentration achieved was 680.9 mg L−1, which was quite similar to the predicted value of 718.7 mg γ-decalactone L−1. Among the range of operating conditions tested, no optimization was possible for 3-hydroxy-γ-decalactone production, since all possible solutions corresponded to operating conditions not analyzed.
Introduction
The demand for fragrances increases yearly as a result of the increasing production of processed foods. Most fragrances are produced by chemical means and natural aromas are obtained from plant materials in small amounts, being very expensive. Chemical synthesis of flavour compounds generally requires numerous steps and often lacks stereoselectivity.1 Moreover, climates, seasonal variation, political and socioeconomic factors, often lead to a constant shortfall of product supply of natural fragrances from plant sources. In addition, due to the preferences of consumers, it is important that the flavours used can be designated as natural. Microbial fermentation is regarded as a potential means to produce natural flavour substances and has attracted a great deal of research interest.2Lactones are well-known aroma compounds found in a variety of foods and beverages, reason why the food industry has a high interest in their biotechnological production.3 The most widely biotechnologically produced lactone is γ-decalactone, a peach-like flavour that can be obtained from the biotransformation of ricinoleic acid, catalyzed by yeasts with GRAS status, since in this case, a natural label is conferred to the aroma,4 which is very important, considering the increasing health- and nutrition-conscious lifestyles.
Yarrowia lipolytica is one of the few types of yeast able to grow on hydrophobic substrates such as oils, n-alkanes, fats and fatty acids, for which it has specific metabolic pathways.5 The main metabolic degradative pathway of fatty acids, in yeasts, is through peroxisomal β-oxidation. Fatty acids are degraded through a multiple step process, involving four different enzymes. Among them, acyl-CoA oxidase is usually considered the most specific for the different substrates (Fig. 1).
 | ||
| Fig. 1 β-oxidation cycle at the C10 level, during the degradation of ricinoleoyl-CoA. Shuttle mechanisms leading to oxidation of NADH and the link with the mitochondrial respiratory chain have been simplified (adapted from Bakker et al., 2001). | ||
Several compounds (3-hydroxy-γ-decalactone, dec-2-enolide and dec-3-enolide) derived from 4-hydroxydecanoic acid, the direct precursor of γ-decalactone, may be detected in the medium. The accumulation of those compounds gives an indication about the activities of the enzymes of the pathway, namely acyl-CoA oxidase and 3-hydroxyacyl-CoA dehydrogenase. Oxygen may influence their activities since it is necessary for the regeneration of the cofactors FAD and, more indirectly, NAD+6 and therefore, influence the production of γ-decalactone and 3-hydroxy-γ-decalactone. This lactone is the precursor of two decenolides presenting aroma properties: dec-2-enolide and dec-3-enolide, with mushroom and fruity notes, respectively. Due to their flavouring properties, these two molecules arise as interesting compounds to be produced and used in the flavouring industry.7 For this reason it is imperative to define the most appropriate conditions for lactones production.
Moreover, although Y. lipolytica can regulate a relatively steady intracellular pH in different environmental pH,8 this parameter could modify some intracellular fluxes and, indirectly, β-oxidation enzyme activities, reason why it is necessary to define the optimal value for operation.
There are several works in literature describing the effect of operating conditions in the production of lactones from the biotransformation of methyl ricinoleate carried out by Y. lipolytica.7,9–12 However, there are some discrepancies between the results obtained in those works, especially in what concerns the effect of oxygen, and for that reason the present work was conducted in order to clarify those differences observed. Therefore, a 32 full factorial design was employed to determine the influence of pH and dissolved oxygen concentration in the biotransformation medium on the production of lactones from methyl ricinoleate, by the aerobic yeast Yarrowia lipolytica, and to optimize the process in bioreactor. This design is an empirical modelling technique used to evaluate, at the same time, the relationship between a set of controllable experimental factors and their observed results.
The main goal of this work is to optimize the operating conditions for γ-decalactone production. However, as 3-hydroxy-γ-decalactone is produced simultaneously in the same fermentation, it was also analyzed, in the hope of providing new insights for its production.
Materials and methods
Microorganism, media and culture conditions
Yarrowia lipolytica W29 (ATCC20460) was cultured for 48 h on YPDA medium (30 g L−1 agar, 20 g L−1glucose, 20 g L−1peptone, 10 g L−1 yeast extract) at 27 °C and used to inoculate (to reach an optical density at 600 nm (OD600) of 1) a 3.7-L bioreactor (type RALF PLUS SOLO, Bioengineering, Wald, Switzerland) containing 1.7 L of glucose medium (YPD medium: 20 g L−1glucose, 20 g L−1peptone, 10 g L−1 yeast extract). Cellular growth occurred at 27 °C, 500 rpm and 1.76 vvm (volume of air per volume of reactor per minute) for 19 h until the culture reached the late logarithmic growth phase, with a final OD600 of 4, and the glucose was completely consumed. The biotransformation medium components were then added as a solution, in order to start the biotransformation stage. The source of ricinoleic acid used was methyl ricinoleate (MR). The composition of the biotransformation medium was 6.7 g L−1 Yeast Nitrogen Base (YNB) with amino acids, 2.5 g L−1NH4Cl, 30 g L−1 MR and 3 g L−1 Tween 80.All chemicals were purchased from Sigma-Aldrich (Sintra, Portugal), except for methyl ricinoleate that was kindly supplied by Stéarinerie Dubois (Bourgogne, France).
Cell concentration and viability
Cell concentration was estimated using a Neubauer-improved counting chamber (Paul Marienfeld GmbH & Co, Lauda-Königshofen, Germany).13 For viability, the methylene blue method was used.14Lactones extraction and quantification
For the quantification of lactones, 2 mL medium samples were removed and their pH was lowered to 2 with HCl. The extraction of lactones was performed with 2 mL of diethyl ether by 60 gentle shaking, by hand, after addition of γ-undecalactone as internal standard. After the complete separation of the liquid phases, the ether phase was separated and analyzed by gas chromatography (Varian 3800 instrument, Varian, Inc., Palo Alto, CA, USA) with a TR-WAX capillary column (30 m × 0.32 mm × 0.25 μm) with He as a carrier gas. The temperatures of the split injector and the detector were set to 250 °C and 300 °C, respectively. The oven temperature was programmed to increase from 60 °C to 145 °C at a rate of 5 °C min−1 and then to 180 °C at a rate of 2 °C min−1.Full factorial design experiments for maximum lactone production
In order to investigate the effect of important variables such as pH and dissolved oxygen concentration (DO) in the biotransformation medium, on the production of γ-decalactone and 3-hydroxy-γ-decalactone, a full factorial design with two factors at three levels (32), with an additional central point to estimate the intrinsic variability (pure error) in the data, was performed. A series of ten experiments was carried out in random order.The pH and DO values analyzed were: 4.5, 5.6 and 6.7 for pH; and 10%, 30% and 50% for dissolved oxygen concentration in the biotransformation medium.
The set point values for pH and DO were automatically controlled by a control unit coupled to the bioreactor. The pH control was performed by addition of 5N KOH or 21% (v/v) orthophosphoric acid, through Peripex peristaltic pumps (Bioengineering, Wald, Switzerland). The DO concentration was controlled by manipulating the agitation and aeration rates, through a cascade control mode.
For statistical calculation, the factors were coded as xi, at three levels starting from −1, 0 and 1, defined by eqn (1):
| xi = (Xi − X0)/(ΔXi) | (1) |
A multiple regression analysis of the data was carried out using the Design Expert v.8 software (Stat-Ease, Inc., Minneapolis, MN, USA). The effect of each variable and their interactions on lactones production was studied. The significance of the regression coefficients was tested by a F-test. A probability (p-value) less than 0.05 for a given factor was considered as significant.
To predict the yield of lactones production, the data were fitted to a second order polynomial equation, which includes all the terms, regardless of their significance (eqn (2)):
| Y = β0 + β1x1 + β11x12 + β2x2 + β22x22 + β12x1x2 + β112x12x2 + β122x1x22 + β1122x12x22 | (2) |
The validity of the polynomial models obtained for each lactone was checked by analysis of the residual values, analysis of variance (ANOVA) and by the correlation coefficient, R2. The significance of the regression coefficients was checked by the F-test, where a p-value less than 0.05 indicated significance of model terms.
Results and discussion
The present work is based upon the optimization of operating conditions for lactones production. Biotransformations were carried out in a 3.7 L bioreactor and the optimization was achieved varying pH and dissolved oxygen concentration in the medium.Table 1 shows the experimental matrix for each assay and the respective responses of interest: concentration of γ-decalactone (Y1) and 3-hydroxy-γ-decalactone (Y2), both expressed as mg L−1. According to results, the highest γ-decalactone and 3-hydroxy-γ-decalactone concentrations were obtained in the runs no. 5 (pH 5.6; 50% O2) and 8 (pH 5.6; 30% O2), respectively.
| Run no. | Experimental responses | |||
|---|---|---|---|---|
| x 1 (pH) | x 2 (%O2) | Y 1, γ-decalactone/mg L−1 | Y 2, 3-OH-γ-decalactone/mg L−1 | |
| 1 | −1 | 1 | 197 | 2267 |
| 2 | −1 | −1 | 164 | 3127 |
| 3 | 0 | −1 | 424 | 1198 |
| 4 | 1 | 1 | 588 | 1673 |
| 5 | 0 | 1 | 1284 | 3929 |
| 6 | −1 | 0 | 311 | 5079 |
| 7 | 1 | −1 | 415 | 539 |
| 8 | 0 | 0 | 326 | 8016 |
| 9 | 1 | 0 | 1192 | 389 |
| 10 | 0 | 0 | 352 | 8137 |
| Level-1 | 4.5 | 10 | ||
| Level 0 | 5.6 | 30 | ||
| Level 1 | 6.7 | 50 | ||
The biphasic system used to produce γ-decalactone is very complex, usually presenting low reproducibility, thus becoming a significant problem. However, in this series of experiments, it is possible to conclude that the reproducibility was quite high, since relative standard deviations (RSD) of 5.42% and 1.06% for γ-decalactone and 3-OH-γ-decalactone, respectively, were determined.
Statistical analysis of these data (p-values less than 0.05) indicated that pH (x1) and dissolved oxygen concentration (x2) have significant effects on the production of both lactones (Tables 2 and 3).
| Source | Estimates | Sum of squares | Degrees of freedom (df) | Mean square | F-value | p-value (Prob > F) |
|---|---|---|---|---|---|---|
| R 2 = 99.98%; R2adj (adjusted for df) = 99.78%; CV = 3.50%. | ||||||
| Model | 1.40 × 106 | 8 | 1.75 × 105 | 517.80 | 0.034 | |
| x 1: pH | 253.83 | 4.18 × 105 | 2 | 2.09 × 105 | 618.61 | 0.28 |
| x 2: O2 | 177.67 | 1.90 × 105 | 2 | 9.49 × 104 | 280.83 | 0.042 |
| x 1·x2 | 35.00 | 7.90 × 105 | 4 | 1.98 × 105 | 584.25 | 0.031 |
| Pure error | 338.00 | 1 | 338.00 | |||
| Cor. Total | 1.40 × 106 | 9 | ||||
| Source | Estimates | Sum of squares | Degrees of freedom (df) | Mean square | F-value | p-value (Prob > F) |
|---|---|---|---|---|---|---|
| R 2 = 99.98%; R2adj (adjusted for df) = 99.91%; CV = 2.49%. | ||||||
| Model | 7.33 × 107 | 8 | 9.16 × 106 | 1251.05 | 0.022 | |
| x 1: pH | −1312.00 | 2.69 × 107 | 2 | 1.35 × 107 | 1839.79 | 0.016 |
| x 2: O2 | 500.83 | 2.03 × 107 | 2 | 1.02 × 107 | 1386.78 | 0.019 |
| x 1·x2 | 498.50 | 1.90 × 107 | 4 | 4.74 × 106 | 647.38 | 0.030 |
| Pure error | 7320.50 | 1 | 7320.50 | |||
| Cor. total | 7.33 × 107 | 9 | ||||
The use of the main response plot allows determining which of the factors influence the response and to compare the relative strength of the effects. The results show that there is a positive relationship between γ-decalactone production and both factors: pH and DO (Fig. 2A and B). The higher the pH and DO values are (in the studied range), the greater the production of γ-decalactone will be. The higher slope of the line corresponding to pH indicates that the effect of this factor on γ-decalactone production is stronger than the effect of dissolved oxygen, which is confirmed by the estimate values presented on Table 2 (253.83 for pH and 177.67 for dissolved oxygen).
 | ||
| Fig. 2 Main response plot of the two factors (pH and dissolved oxygen) on the production of γ-decalactone (A, B) and 3-hydroxy-γ-decalactone (C, D). | ||
Fig. 2C and D indicate that, among the experimental conditions tested, the highest 3-hydroxy-γ-decalactone accumulation will occur when intermediate levels of each factor are used: with a pH of 5.6 (5320 mg L−1) and a DO of 30% (5405 mg L−1).
Table 3 shows a negative effect of pH on the response, an effect which is only valid for pH values higher than 5.6, according to Fig. 2C. There is a positive effect of oxygen in the accumulation of 3-hydroxy-γ-decalactone when the concentrations of dissolved oxygen are comprised between 10% and 30%.
Significant interactions (x1·x2) between pH and oxygen were observed, so the interaction plots were also constituted (Fig. 3). The maximum accumulation of γ-decalactone was observed when a pH of 5.6 was used simultaneously with 50% DO (1284 mg L−1) and when a pH of 6.7 was used together with 30% DO (1192 mg L−1). These results are better (in terms of higher aroma concentration obtained) than those observed previously when the individual factor effects were analyzed and the highest γ-decalactone production was obtained using the highest levels of each factor (among the range of values tested). This suggests that there is a synergistic effect of pH and dissolved oxygen in the biotransformation medium, meaning that the effect of the two factors on γ-decalactone production is greater than the effect of each factor individually. One factor enhances the effects of the second, leading to a greater γ-decalactone production.
 | ||
| Fig. 3 Interaction plot of the two factors (pH and dissolved oxygen) on the production of γ-decalactone (A) and 3-hydroxy-γ-decalactone (B). | ||
The interaction plot displayed on Fig. 3B, shows that the maximum 3-hydroxy-γ-decalactone concentration was obtained when a pH of 5.6 was used in the presence of 30% DO (8076 mg L−1). A synergistic effect of both factors is also evidenced in this case.
In order to obtain an empirical relationship between the lactones production and the experimental variables, which will allow predicting the yield of lactones, the data were fitted to eqn (2), based on the calculated regression coefficients and significance of the variables. As result, the following models were obtained, to predict the lactones production, as a function of the coded values of the factors:
| Yγ-decalactone = 546 + 253.8x1 − 68.2x12 + 177.7x2 − 34x22 + 35x1x2 − 126.2x12x2 − 93.3x1x22 − 102.8x12x22 | (3) |
| Y3-OH-γ-decalactone = 2919.7 − 1312x1 − 740.7x12 + 500.8x2 − 797.6x22 + 498.5x1x2 − 432.3x12x2 + 516.5x1x22 + 520.1x12x22 | (4) |
The statistical models proposed for γ-decalactone and 3-hydroxy-γ-decalactone productions are significant since the corresponding F-values were 517.80 and 1251.05, respectively (Tables 2 and 3). These values mean that there is only a 3.4% and 2.2% chance, respectively, that “Model F-Values” that large could occur due to noise.
The calculated coefficient of determination (R2) for the model corresponding to γ-decalactone production was 0.9998 (Table 2). This high value ensures a good adjustment of the model to the experimental data, indicating that 99.98% of the variability in the response could be explained by the model. The observed low differences (0.002) of the adjusted R2 value (0.9978) and the R2 value, further confirm the data accuracy. At the same time, a relatively low value of the coefficient of variation (R2 = 3.50%) indicated a better precision and reliability of the performed experiments.
Concerning the model proposed for 3-hydroxy-γ-decalactone accumulation, a similar high significance of the model was observed since the R2 value was 0.9999 (Table 3). Therefore, 99.99% of the variability in the response can be explained by the model. Moreover, the low differences observed (0.0008) of the adjusted R2 value (0.9991) and the R2 value, also confirm the data accuracy. The small value of the coefficient of variation (R2 = 2.49%) indicated, as well, a good precision and reliability of the essays.
Three dimensional response surfaces (the graphical representation of the regression equations) were plotted, using MATLAB v. 7.7.0 (The MathWorks, Inc., Natick, MA, USA), to provide a visual interpretation of the interaction between the two factors and facilitate the location of the optimal experimental conditions.
Fig. 4 illustrates the response surface describing γ-decalactone and 3-hydroxy-γ-decalactone productions, as function of pH and dissolved oxygen concentration.
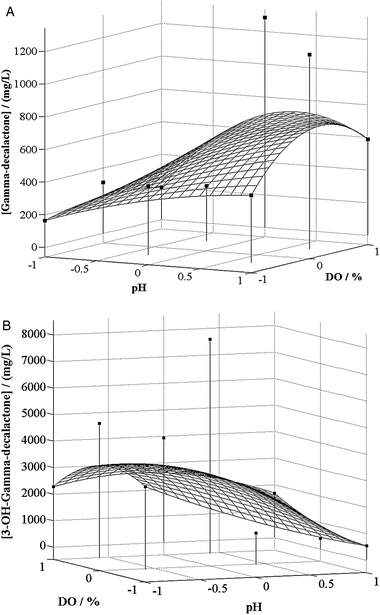 | ||
| Fig. 4 (A) Response surface curve for lactones (γ-decalactone-A; 3-hydroxy-γ-decalactone-B) production by Yarrowia lipolytica as a function of pH and dissolved oxygen concentration in the biotransformation medium. (B) Response surface curve for lactones (γ-decalactone-A; 3-hydroxy-γ-decalactone-B) production by Yarrowia lipolytica as a function of pH and dissolved oxygen concentration in the biotransformation medium. | ||
Regarding γ-decalactone accumulation, the trend observed in Fig. 4A reveals a progressive increase in the response variable with increasing both independent variables up to a threshold value (DO and pH values comprised between 30%–47% and 5.8–6.3, respectively), beyond which it decreased. These values are in accordance to those pointed out by Garcia et al.,7 who refers an optimal pH of 6 for γ-decalactone accumulation and a dissolved oxygen concentration around 40% at the maximum point of production. Gomes et al.12 also concluded that the accumulation of γ-decalactone was directly dependent on the oxygen transfer to the medium, in the range of the operating conditions analyzed (between 400 rpm, 0.6 vvm and 600 rpm, 3 vvm), meaning that higher oxygen levels would improve this aroma production.
According to Aguedo et al.9 and Garcia et al.,11 and contrarily to the results obtained in the present work, the maximum γ-decalactone accumulation would occur using low aeration rates. Aguedo et al.9 used higher oxygen concentrations, under hyperbaric conditions, which may had toxic effects on the yeast (oxidative stress), limiting the production of the aroma.
Equations derived by differentiation of the polynomial model given by eqn (3), allowed estimating an optimum γ-decalactone concentration of 718.7 mg L−1, from which it was possible to conclude that the experimental conditions able to simultaneously maximize these parameters would be: pH = 6.17 and DO = 44.4%.
In order to confirm the model adequacy in predicting maximum γ-decalactone concentration, one additional experiment under the optimal conditions was performed. The resulting maximum γ-decalactone accumulation was 680.9 mg L−1, in 7 h, which was very close to the estimated concentration (718.7 mg γ-decalactone L−1). The good agreement between these two results verified the validity of the model and the existence of an optimal point.
By estimating the 3-hydroxy-γ-decalactone maximum concentration using the equations derived by differentiation of the model represented by eqn (4), all possible solutions obtained corresponded to factors values out of the range tested. Therefore, no optimization was possible for this compound, among the range of operating conditions analyzed. However, Fig. 4B suggests that 3-hydroxy-γ-decalactone production is higher when pH values are low and DO is around 25%.
The low pH values as the optimum for 3-hydroxy-γ-decalactone accumulation are corroborated by the works of Garcia et al.7,11 Concerning the effect of oxygen on the production of this lactone, the results herein obtained conflict with the results of Aguedo et al.,9 Garcia et al.11 and Gomes et al.,12 who concluded that high oxygen transfer rates improved the accumulation of 3-hydroxy-γ-decalactone. On the contrary, in another work, Garcia et al.7 describe a quite low dissolved oxygen concentration in the biotransformation medium (5%) as the optimal oxygen condition for 3-hydroxy-γ-decalactone production. Nevertheless, it is worth highlight once more that it was not our primary goal to optimize the operating conditions for this lactone. Moreover, the accumulation of this compound indicates a deviation in the metabolic pathway of γ-decalactone production, decreasing its yields.
Conclusions
This work demonstrated the feasibility of using experimental design tools to screen significant factors with influence in the responses and optimize biotransformation conditions for lactones production.pH and dissolved oxygen revealed to be significant factors for the production of both lactones.
The response surface methodology optimized operating conditions for γ-decalactone production were pH 6.17 and dissolved oxygen concentration in the medium of 44.4%. Applying these conditions, γ-decalactone production reached 680.9 mg L−1, which was very similar to the RSM model predicted values of 718.7 mg γ-decalactone L−1.
It was not possible to optimize 3-hydroxy-γ-decalactone production, among the range of operating conditions tested, and there are still some discrepancies among the data available in literature on this matter. Therefore, further studies will be focused on the optimization of the production of this compound, by enlarging the range of operating conditions, namely lowering the pH values.
Acknowledgements
The authors aknowledge Fundação para a Ciência e Tecnologia (FCT) for the financial support provided (SFRH/BD/28039/2006) and Héctor Ruíz for the help provided with MATLAB.References
- M. Gopinath, L. Vijayakumar, R. Dhanasekar and T. Viruthagiri, Global J. Biotech. & Biochem., 2008, 3(2), 60–68 Search PubMed.
- M. A. Longo and M. A. Sanromán, Food Technol. Biotechnol., 2006, 335–353 Search PubMed.
- I. L. Gatfield, in Advances in Biochemical Engineering/Biotechnology, ed. T. Scheper, Springer-Verlag, Berlin, 1997, vol. 55, pp. 221–238 Search PubMed.
- J. Schrader, M. M. W. Etschmann, D. Sell, J.-M. Hilmer and J. Rabenhorst, Biotechnol. Lett., 2004, 26, 463–472 CrossRef CAS.
- P. Fickers, P.-H. Benetti, Y. Waché, A. Marty, S. Mauersberger, M. S. Smit and J.-M. Nicaud, FEMS Yeast Res., 2005, 5, 527–543 CrossRef CAS.
- B. M. Bakker, K. M. Overkamp, A. J. A. van Maris, P. Kötter, M. A. H. Luttik, J. P. van Dijken and J. T. Pronk, FEMS Microbiol. Rev., 2001, 25, 15–37 CrossRef CAS.
- E. E. Garcia, M. Aguedo, N. Gomes, A. Choquet, I. Belo, J. A. Teixeira, J.-M. Belin and Y. Waché, J. Mol. Catal. B: Enzym., 2009, 57, 22–26 CrossRef CAS.
- M. Aguedo, PhD Thesis, Université de Bourgogne, 2002 Search PubMed.
- M. Aguedo, N. Gomes, E. E. Garcia, Y. Waché, M. Mota, J. A. Teixeira and I. Belo, Biotechnol. Lett., 2005, 27, 1617–1621 CrossRef CAS.
- N. Gomes, M. Aguedo, J. A. Teixeira and I. Belo, Biochem. Eng. J., 2007, 35, 380–386 CrossRef CAS.
- E. E. Garcia, J.-M. Belin and Y. Waché, J. Appl. Microbiol., 2007, 103, 1508–1515 CrossRef CAS.
- N. Gomes, J. A. Teixeira and I. Belo, Biocatal. Biotransform., 2010, 28(4), 227–234 CrossRef CAS.
- J. P. Mather and P. E. Roberts, in Introduction to Cell and Tissue Culture: Theory and Technique, Plenum Press, New York, 1998 Search PubMed.
- A. Bonora and D. Mares, Curr. Microbiol., 1982, 7, 217–221 CrossRef.
| This journal is © The Royal Society of Chemistry 2011 |
