Phase behaviour and conductivity study of electrolytes in supercritical hydrofluorocarbons†
Philip N.
Bartlett
*a,
David C.
Cook
a,
Michael W.
George
*b,
Jie
Ke
*b,
William
Levason
a,
Gillian
Reid
a,
Wenta
Su
b and
Wenjian
Zhang
a
aSchool of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: pnb@soton.ac.uk
bSchool of Chemistry, University of Nottingham, Nottingham, NG7 2RD, UK. E-mail: mike.george@nottingham.ac.uk; jie.ke@nottingham.ac.uk
First published on 12th November 2010
Abstract
The purpose of this work was to characterise supercritical hydrofluorocarbons (HFC) that can be used as solvents for electrodeposition. The phase behaviour of CHF3, CH2F2, and CH2FCF3 containing [NBun4][BF4], [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4] was studied and the conditions for forming a single supercritical phase established. Although all three HFCs are good solvents for [NBun4][BF4] the results show that the CH2F2 system has the lowest pr for dissolving a given amount of [NBun4][BF4]. The solubility of Na[B{3,5-C6H3(CF3)2}4] in CH2F2 was found to be unexpectedly high. Studies of the phase behaviour of CH2F2 containing [NBun4][BF4] and [Cu(CH3CN)4][BF4] showed that the copper complex was unstable in the absence of CH3CN. For CHF3, [Cu(hfac)2] was more soluble and more stable than [Cu(CH3CN)4][BF4] and only increased the phase-separation pressure by a moderate amount. Studies of the conductivity of [NBun4][B(C6F5)4], [NBun4][B{3,5-C6H3(CF3)2}4], [NRfBun3][B{3,5-C6H3(CF3)2}4] (Rf = (CH2)3C7F15), and Na[B{3,5-C6H3(CF3)2}4] were carried out in scCH2F2. The results show that these salts are more conducting than [NBun4][BF4] under the same conditions although the increase is much less significant than that reported in previous work in supercritical CO2 + CH3CN. Consequently, either [NBun4][BF4] or the corresponding BARF salts would be suitable background electrolytes for electrodeposition from scCH2F2.
1. Introduction
Supercritical fluids (fluids at temperatures and pressures above their critical point) are potentially attractive media for materials electrodeposition because they have low or zero surface tension enabling them to readily penetrate nanopores, they show enhanced mass transport rates,1 and, given a suitable choice of fluid/electrolyte, they can have wide electrochemical windows2 allowing the deposition of reactive materials at elevated temperatures. The properties of supercritical fluids (dielectric constant, viscosity, density, etc.) are intermediate between those of the gas and the liquid states and they can be varied by altering the temperature and pressure, making them “tuneable” solvents.3Despite these potential advantages there are very few reports in the literature of electrodeposition from supercritical fluids because they pose a number of challenges. Electrodeposition places significant demands on the supercritical fluid in terms of the solubility of the reagents and conductivity provided by the supporting electrolyte in order to achieve deposition at meaningful rates and without significant problems caused by large uncompensated solution resistance. The major obstacle to carrying out electrodeposition in many supercritical fluids is the low dielectric constant of the fluid (ε < 8) which limits the solubility and conductivity for many electrolytes.4
In a recent publication we reported the first generally applicable method for electrodeposition from a homogeneous supercritical fluid.5 In that paper we described the electrodeposition of Cu, Ag and Co from a single-phase supercritical CO2–MeCN. This was achieved by the use of appropriately designed metal complexes and a range of tailored electrolytes to give high solution conductivities.6 The inclusion of MeCN as a co-solvent limits the accessible potential range and introduces complications. The use of supercritical fluorinated hydrocarbons2,7–16 is an attractive alternative which avoids these problems.
In this paper we present results of a study of the phase behaviour of trifluoromethane (CHF3), difluoromethane (CH2F2), and 1,1,1,2-tetrafluoroethane (CH2FCF3) containing tetrabutylammonium tetrafluoroborate ([NBun4][BF4]), [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4]. In addition we present results for the phase behaviour of CH2F2 containing [NBun4][BF4] and [Cu(CH3CN)4][BF4] and for CHF3 containing [NBun4][BF4] and [Cu(hfac)2]. Finally, we report electrical conductivity data for scCH2F2 with four BARF salts (i.e.[NBun4][B(C6F5)4], [NBun4][B{3,5-C6H3(CF3)2}4], [NRfBun3][B{3,5-C6H3(CF3)2}4], and Na[B{3,5-C6H3(CF3)2}4]) and compare these results with the published data8 for [NBun4][BF4].
The purpose of this work is to characterise supercritical fluids that can be used as electrolytes for electrodeposition of a range of materials and we therefore concentrate on finding the conditions which give single supercritical phases with good electrical conductivity.
2. Experimental
2.1 Materials
Trifluoromethane (CHF3, Aldrich, 98+%), difluoromethane (CH2F2, INEOS Fluor Ltd, UK, 99.90 wt/wt%), and 1,1,1,2-tetrafluoroethane (CH2FCF3, Air Product, technical grade, 99.9 wt/wt%) were used as supplied. Tetrabutylammonium tetrafluoroborate [NBun4][BF4], Fluka, electrochemical grade, 99.0+%), tetrakis(acetonitrile)copper(I) tetrafluoroborate ([Cu(CH3CN)4][BF4], Aldrich, 97%), acetonitrile (Fisher Scientific, 99.98+%) were used without further purification. Bis(hexafluoroacetyl acetonato)copper(II) hydrate ([Cu(hfac)2]·H2O) was supplied by Aldrich. [NBun4][B(C6F5)4], Na[B{3,5-C6H3(CF3)2}4], [NBun4][B{3,5-C6H3(CF3)2}4], and [{CF3(CF2)7(CH2)3}NBun3][B{3,5-C6H3(CF3)2}4] were synthesised and purified as described elsewhere.5,62.2 Methods
The phase behaviour was studied by using a visual method in a variable-volume, view-cell. The details of the high pressure cell and the experimental procedure were described elsewhere.6,17 The conductivity measurements were performed in the same cell. Unlike our previously study on ternary systems, the conductivity study in this work was focused on binary systems. It is more convenient to measure the conductivity of the binary systems in a fixed-volume cell, in which the molar concentration of solute can be kept as a constant during the course of a series of measurements under different pressures. Therefore, the movable piston of the high-pressure cell was replaced by a stainless steel plug fitted with a double acting hydraulic type seal to minimise leaking of the samples, especially at the higher temperatures (e.g. 363 K) required to perform the measurements for the CH2F2 systems. A schematic diagram of the apparatus for measuring the conductivity of binary systems is presented in Fig. 1.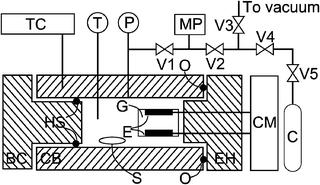 | ||
| Fig. 1 Schematic diagram of the experimental setup for measuring the conductivity of binary systems: BC, back cap; C, gas cylinder; CB, cell body; CM, conductivity meter; E, platinum electrodes; EH, electrode holder; G, glass tube; HS, hydraulic seal; MP, manual high-pressure pump; O, O-ring; P, pressure transducer, S, magnetic stirrer; T, thermocouple; TC, temperature controller; V1–V5, valves. | ||
The experimental procedure for the conductivity measurements are described briefly as follows. A known weight of a solid (e.g. supporting electrolyte) was first placed at the bottom of the cell. Then the cell was fitted with the electrode holder and purged with a suitable fluid. After heating the cell to the desired temperature, the supercritical fluid was delivered gradually into the cellvia a hand operated high-pressure pump. At each pressure step, the mixture was left with stirring to equilibrate for at least 30 min before the conductivity was recorded. The conductivity data were collected by JENWAY 4510 conductivity meter that was calibrated using the standard conductivity solution of KCl.
The accuracy of the mixture composition is ∼±0.1% for HFCs and CH3CN, and ∼±1% for supporting electrolytes and metal precursors. The estimated uncertainties are ±0.2 K for the temperature measurement, ±0.04 MPa for the pressure measurement, ∼±0.5% for the internal volume of the cell, and ∼±1% for the conductivity.
3. Results and discussion
3.1 Phase behaviour
CHF3, CH2F2 and CH2FCF3 have a dipole moment greater than 1.6 D (see Table 1), which is comparable to low-polarity organic solvents, such as tetrahydrofuran (1.75 D) and ethyl acetate (1.78 D).18 Thus, in contrast to the CO2 system reported in our previous study,6 addition of co-solvents may not be necessary to enhance the solubility of the supporting electrolytes in the HFC systems. Additionally, the critical temperatures (Tc) of CHF3, CH2F2 and CH2FCF3 vary between 299 and 375 K, which also allows more choices of working temperatures because the experimental conditions often require that the bath temperature is not far away from the critical temperature of the media used for supercritical fluid electrodeposition. Our first aim of this study was to examine how different the three HFCs are in terms of the phase equilibrium of their mixtures with supporting electrolytes and metallic complexes.Fig. 2 illustrates the p–T phase boundary of three binary systems of [NBun4][BF4] with CHF3, CH2F2, and CH2FCF3, respectively. The mole fraction of all of the 13 mixtures and the phase boundary data can be found from Table S1 in the ESI.† The measurements were carried out in the temperature region covering both of the sub- and supercritical states, i.e. 293–328 K for the CHF3 system, 295–363 K for the CH2F2 system, and 322–83 K for the CH2FCF3 system. Clearly, the boundaries of the three binary systems ([NBun4][BF4] + CHF3, [NBun4][BF4] + CH2F2, and [NBun4][BF4] + CH2FCF3) lie in three separate regions from top left to bottom right in p, T space, which is related to the position of their critical points. The other main features of the phase diagrams may be described as follows: (i) for a mixture with a given composition, the phase transition pressure rises as the temperature rises; (ii) when the temperature is below the corresponding Tc of each HFC (indicated by the arrows in Fig. 2), the p–T boundaries are overlapped for the mixtures with different x[NBu4][BF4]. In particular, the phase transition pressures of the CH2F2 and CH2FCF3 systems are only 0.3–0.7 MPa higher than the vapour pressures21 of the corresponding HFC at T < Tc,HFC. (iii) Above Tc of each HFC, the p–T boundary shifts to high pressures with increasing xNBu4BF4, except for the mixture with the highest composition of [NBun4][BF4] in CH2F2.
![p–T phase diagram of the mixture of [NBun4][BF4] (1) + HFC (2). HFC = CHF3: □, x1 = 0.82 × 10−3; ○, x1 = 1.59 × 10−3; △, x1 = 2.44 × 10−3; ▽, x1 = 4.83 × 10−3; ◊, x1 = 6.90 × 10−3. HFC = CH2F2: +, x1 = 1.67 × 10−3; ×, x1 = 2.55 × 10−3; , x1 = 3.29 × 10−3; ⊕, x1 = 4.17 × 10−3; HFC = CHF2CF3: ■, x1 = 1.33 × 10−3; ●, x1 = 2.62 × 10−3; ▲, x1 = 3.91 × 10−3; ▼, x1 = 5.14 × 10−3. The arrows indicate the critical temperature of the HFCs.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f2.gif) | ||
Fig. 2
p–T phase diagram of the mixture of [NBun4][BF4] (1) + HFC (2). HFC = CHF3: □, x1 = 0.82 × 10−3; ○, x1 = 1.59 × 10−3; △, x1 = 2.44 × 10−3; ▽, x1 = 4.83 × 10−3; ◊, x1 = 6.90 × 10−3. HFC = CH2F2: +, x1 = 1.67 × 10−3; ×, x1 = 2.55 × 10−3;  , x1 = 3.29 × 10−3; ⊕, x1 = 4.17 × 10−3; HFC = CHF2CF3: ■, x1 = 1.33 × 10−3; ●, x1 = 2.62 × 10−3; ▲, x1 = 3.91 × 10−3; ▼, x1 = 5.14 × 10−3. The arrows indicate the critical temperature of the HFCs. , x1 = 3.29 × 10−3; ⊕, x1 = 4.17 × 10−3; HFC = CHF2CF3: ■, x1 = 1.33 × 10−3; ●, x1 = 2.62 × 10−3; ▲, x1 = 3.91 × 10−3; ▼, x1 = 5.14 × 10−3. The arrows indicate the critical temperature of the HFCs. | ||
![p–x phase diagram of the mixture of [NBun4][BF4] (1) + HFC (2) at the same reduced temperature (T/Tc,HFC = 1.01). The y-axis is shown as the reduced pressure, which is defined as pr = p/pc,HFC. The critical temperature (Tc,HFC) and the critical pressure (pc,HFC) of each HFC are listed in Table 1. □, HFC = CHF3, T = 302.3 K; ○, HFC = CH2F2, T = 354.8 K; and ◊, HFC = CHF2CF3, T = 378.0 K. Piecewise cubic Hermite polynomials were used to interpolate the data shown in Fig. 2 to the fixed temperature.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f3.gif) | ||
| Fig. 3 p–x phase diagram of the mixture of [NBun4][BF4] (1) + HFC (2) at the same reduced temperature (T/Tc,HFC = 1.01). The y-axis is shown as the reduced pressure, which is defined as pr = p/pc,HFC. The critical temperature (Tc,HFC) and the critical pressure (pc,HFC) of each HFC are listed in Table 1. □, HFC = CHF3, T = 302.3 K; ○, HFC = CH2F2, T = 354.8 K; and ◊, HFC = CHF2CF3, T = 378.0 K. Piecewise cubic Hermite polynomials were used to interpolate the data shown in Fig. 2 to the fixed temperature. | ||
It is not unexpected that the p–T boundaries are overlapped below Tc for the mixtures with the different compositions of the electrolyte. Since [NBun4][BF4] is completely miscible with the liquid HFC in the composition region studied here, the phase equilibrium established below Tc may be regarded as equilibrium between a gas phase that consists of virtually no [NBun4][BF4] and a liquid phase with dissolved [NBun4][BF4]. Therefore, such a low mole fraction (x[NBu4][BF4] < 6.9 × 10−3) of solute cannot significantly change the vapour pressure of the liquid CH2F2 or CH2FCF3. However, when the system temperature is increased above a certain temperature (often around Tc of the pure HFC), the nature of the phase transition switches from vapour–liquid‡ equilibrium to fluid–liquid§ equilibrium. Because the density of the fluid phase is more pressure dependent than the density of the liquid phase, the higher the pressure of the system, the larger the density, and hence the higher the solubility of [NBun4][BF4] in the HFC fluid phase.
We also note that the pressures required to form a homogeneous solution are much lower for the HFC system than for the CO2 + CH3CN system (xCO2/xCH3CN = 0.14)6 when [NBun4][BF4] is used as the supporting electrolyte. For example, the phase separation pressure is 22.2 MPa at 318.15 K for the CO2 + CH3CN mixture (x[NBu4][BF4] = 2.70 × 10−3), while the phase separation pressure is only 11.7 MPa at the same temperature for the CHF3 mixture with a much higher mole fraction of [NBun4][BF4] (x[NBu4][BF4] = 4.83 × 10−3).
Although the p–T phase boundary data shown in Fig. 2 demonstrate that all of the three HFCs are good solvents to dissolve a sufficient amount of [NBun4][BF4] for carrying out electrodeposition, it is difficult to ascertain directly from the figure which HFC out of the three has the best solvation power since the vapour–liquid equilibria of the three HFCs are very different. To overcome this problem, we present our results in a modified p–x phase diagram. Rather than plotting the phase diagrams of the three HFC systems at a constant temperature, we have plotted the diagrams at the same reduced temperature, Tr = T/Tc,HFC, in which T is the system temperature, and Tc,HFC is the critical temperature of each HFC. Moreover, the pressure on the phase diagram is replaced by the reduced pressure (pr) to eliminate the effect of different pc for the three HFCs (pr = p/pc,HFC, where p is the pressure on the phase boundary, and pc,HFC is the corresponding critical pressure of each HFC.).
Fig. 3 shows the modified p–x phase diagram for all three HFC systems at a constant Tr = 1.01, which corresponds to the commonly used working temperatures for applying HFCs to electrodeposition5 and other applications.22 The data shown in Fig. 3 have been obtained by interpolating the p–T boundary of the isopleths (Fig. 2) to a specified temperature for each HFC, i.e. 302.3 K for the CHF3 system, 354.8 K for the CH2F2 system, and 378.0 K for the CH2FCF3 system. We can see from the figure that for the CHF3 and CH2FCF3 systems the solubility (x[NBu4][BF4]) increases as the increase of pr, and that for the CH2F2 system pr does not change dramatically as a function of x[NBu4][BF4] within the composition range measured here. More importantly, it is clear that the CH2F2 system has the lowest pr for dissolving the same amount of [NBun4][BF4]. This result may be explained by the fact that CH2F2 has the highest dipole moment among the three HFCs (see Table 1), and hence it is a more polar solvent than either CHF3 or CH2FCF3.
Fig. 4 shows the p–T phase diagram of three binary mixtures of CH2F2 and the supporting electrolyte (i.e.[NBun4][BF4], [NBun4][B{3,5-C6H3(CF3)2}4], and Na[B{3,5-C6H3(CF3)2}4], respectively). The mole fractions of the test mixtures were kept virtually identical for the three electrolytes (xelectrolyte = ∼1.65 × 10−3). It can be seen from the figure that the p–T phase boundary for all of the three mixtures are almost superimposed within the temperature region between 295 to 353 K. The slight difference in the phase separation pressure can only be observed when T > 353 K. It is not surprising that two binary mixtures with [NBun4][BF4] and [NBun4][B{3,5-C6H3(CF3)2}4] show similar phase behaviour because CH2F2 is such a good solvent for the tetrabutylammonium salts. Thus the effect of introducing the BARF anion on the phase boundary is not as significant as that for the CO2 systems (see Fig. 6 in our previous paper).5 However, the behaviour of Na[B{3,5-C6H3(CF3)2}4] in CH2F2 is somewhat unexpected due to the fact that sodium salts often have low solubility in organic solvents. The BARF anion is a key component to enhance the solubility of Na[B{3,5-C6H3(CF3)2}4] in CH2F2, and the large size difference between Na+ and [B{3,5-C6H3(CF3)2}4]− may play a role on improving solubility in CH2F2.
![p–T phase diagram of the mixtures of electrolyte (1) + CH2F2 (2): ■, [NBun4][BF4], x1 = 1.67 × 10−3; ●, [NBun4][B{3,5-C6H3(CF3)2}4], x1 = 1.65 × 10−3; ◆, Na[B{3,5-C6H3(CF3)2}4], x1 = 1.62 × 10−3; and of electrolyte (1) + CHF2CF3 (2): □, [NBun4][BF4], x1 = 2.62 × 10−3; ○, [NBun4][B{3,5-C6H3(CF3)2}4], x1 = 2.58 × 10−3; ◊, Na[B{3,5-C6H3(CF3)2}4], x1 = 2.59 × 10−3.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f4.gif) | ||
| Fig. 4 p–T phase diagram of the mixtures of electrolyte (1) + CH2F2 (2): ■, [NBun4][BF4], x1 = 1.67 × 10−3; ●, [NBun4][B{3,5-C6H3(CF3)2}4], x1 = 1.65 × 10−3; ◆, Na[B{3,5-C6H3(CF3)2}4], x1 = 1.62 × 10−3; and of electrolyte (1) + CHF2CF3 (2): □, [NBun4][BF4], x1 = 2.62 × 10−3; ○, [NBun4][B{3,5-C6H3(CF3)2}4], x1 = 2.58 × 10−3; ◊, Na[B{3,5-C6H3(CF3)2}4], x1 = 2.59 × 10−3. | ||
Similarly, the phase equilibrium measurements for the same three supporting electrolytes were made in CH2FCF3 at a fixed mole fraction of ∼2.60 × 10−3, see Fig. 4. Just as for the CH2F2 + electrolyte mixtures, the p–T phase boundaries of the three CH2FCF3 systems are overlapped below the temperature around Tc,CH2FCF3, while the phase separation pressure for the Na[B{3,5-C6H3(CF3)2}4] mixture is lower than that of the other two mixtures when T > Tc,CH2FCF3. It is worth noting that Na[B{3,5-C6H3(CF3)2}4] is an intermediate in the preparation of [NBun4][B{3,5-C6H3(CF3)2}4]. The use of Na[B{3,5-C6H3(CF3)2}4] may minimise impurities introduced from the metathesis step and make the supporting electrolyte less expensive.
Our initial experiments on a binary mixture of [Cu(CH3CN)4][BF4] and CH2F2 showed that [Cu(CH3CN)4][BF4] was not stable enough for use in electrodeposition from pure CH2F2 in near- or supercritical conditions. In these experiments some blue solid (rather than white powder for pure [Cu(CH3CN)4][BF4]) precipitated on the inner surface of the windows of the view cell when the system temperature was ∼333 K indicating decomposition of the copper complex. Consequently, a small amount of CH3CN was added to the CH2F2 not only to increase the solubility of [Cu(CH3CN)4][BF4], but also to stabilise it. Fig. 5 shows the p–T phase boundary of a ternary mixture of [Cu(CH3CN)4][BF4] + CH3CN + CH2F2, together, for comparison, with the phase boundaries of a ternary mixture of [NBun4][BF4] + CH3CN + CH2F2, and a quaternary mixture of [Cu(CH3CN)4][BF4] + [NBun4][BF4] + CH3CN + CH2F2. The mole fraction of CH3CN was kept at ∼0.034 for all of the three mixtures, and the mole fractions of [Cu(CH3CN)4][BF4] (∼3.0 × 10−4) and [NBun4][BF4] (∼1.2 × 10−3) in the quaternary mixture were chosen to match the values of the two corresponding ternary systems, respectively.
![p–T phase diagram of the [Cu(CH3CN)4][BF4] mixtures in CH2F2: ◊, [NBun4][BF4] (1) + CH3CN (2) + CH2F2 (3), x1 = 1.17 × 10−3, x2 = 0.0312; □, [Cu(CH3CN)4][BF4] (1) + CH3CN (2) + CH2F2 (3), x1 = 3.0 × 10−4, x2 = 0.0375; ○, [Cu(CH3CN)4][BF4] (1) + [NBun4][BF4] (2) + CH3CN (3) + CH2F2 (4), x1 = 3.0 × 10−4, x2 = 1.22 × 10−3, x3 = 0.0324.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f5.gif) | ||
| Fig. 5 p–T phase diagram of the [Cu(CH3CN)4][BF4] mixtures in CH2F2: ◊, [NBun4][BF4] (1) + CH3CN (2) + CH2F2 (3), x1 = 1.17 × 10−3, x2 = 0.0312; □, [Cu(CH3CN)4][BF4] (1) + CH3CN (2) + CH2F2 (3), x1 = 3.0 × 10−4, x2 = 0.0375; ○, [Cu(CH3CN)4][BF4] (1) + [NBun4][BF4] (2) + CH3CN (3) + CH2F2 (4), x1 = 3.0 × 10−4, x2 = 1.22 × 10−3, x3 = 0.0324. | ||
One can see from Fig. 5 that below 353 K the p–T boundaries overlap each other for all of the three mixtures, and that in the temperature region 353–368 K, the difference in the phase-separation pressure is still small between the two ternary systems with either [Cu(CH3CN)4][BF4] or [NBun4][BF4]. Surprisingly, the p–T boundary of the quaternary mixture (with both [Cu(CH3CN)4][BF4] and [NBun4][BF4]) exhibits a steep rise between 353 and 363 K, indicating the change of the nature of the phase transition. In fact, we observed some white particles precipitated out of the solution at both 363 and 368 K when the system pressure was below the phase separation pressure. This is in contrast to the typical vapour–liquid phase transition observed for the same mixture at temperatures below 353 K. Therefore, it is clear that addition of [NBun4][BF4] to the [Cu(CH3CN)4][BF4] system drastically shifts the phase boundary to high pressures at T > Tc,CH2F2. This phenomenon may be understood as follows. Our measured data on the ternary mixture with [Cu(CH3CN)4][BF4] indicate that 3.75% of CH3CN should be adequate to stabilise [Cu(CH3CN)4][BF4] in scCH2F2 at temperatures between 333 and 368 K. However, when [NBun4][BF4] is also present in the mixture at ∼4 times as much as [Cu(CH3CN)4][BF4], there may not be enough free CH3CN molecules in CH2F2 available to stabilise the copper–acetonitrile complex because it is known that co-solvent molecules (e.g.CH3CN) form clusters around the solute molecules and dissociated ions (e.g.[NBun4][BF4], [NBun4]+, and [BF4]−) in supercritical fluids with enhanced local concentration of co-solvents.24–27 In order to compensate the depletion of the CH3CN molecules in the bulk phase, the density, and hence the pressure of the system, needs to be increased considerably for the quaternary mixture to form a homogenous phase.
Similar measurements were made for the [Cu(hfac)2] system in CHF3. Since [Cu(hfac)2] is very soluble and stable in CHF3 at the required temperatures for electrodeposition (<330 K), no co-solvents were added to our test mixtures. Fig. 6 illustrates the p–T phase diagram of a ternary mixture of [Cu(hfac)2] + [NBun4][BF4] + CHF3, together with the phase diagrams of two corresponding binary mixtures: [NBun4][BF4] + CHF3, and [Cu(hfac)2] + CHF3. Again, the mole fractions of [Cu(hfac)2] (∼3.1 × 10−4) and [NBun4][BF4] (∼8.1 × 10−4) in the ternary mixture were chosen to match the values of the two corresponding binary systems, respectively.
![p–T phase diagram of the Cu(hfac)2 mixtures in CH2F2: ◊, [NBun4][BF4] (1) + CHF3 (2), x1 = 8.23 × 10−4; □, Cu(hfac)2 (1) + CHF3 (2), x1 = 3.0 × 10−4; ○, [Cu(hfac)2](1) + [NBun4][BF4](2) + CHF3(3), x1 = 3.1 × 10−4, x2 = 8.03 × 10−4.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f6.gif) | ||
| Fig. 6 p–T phase diagram of the Cu(hfac)2 mixtures in CH2F2: ◊, [NBun4][BF4] (1) + CHF3 (2), x1 = 8.23 × 10−4; □, Cu(hfac)2 (1) + CHF3 (2), x1 = 3.0 × 10−4; ○, [Cu(hfac)2](1) + [NBun4][BF4](2) + CHF3(3), x1 = 3.1 × 10−4, x2 = 8.03 × 10−4. | ||
In Fig. 6 the binary mixture of [Cu(hfac)2] + CHF3 exhibits the lowest phase-separation pressure between 298 and 328 K among the three mixtures. As can also be seen from the figure, the pressure of this mixture increases with increasing temperature, and then gradually levels off when the temperature approaches 328 K. This is in contrast to the behaviours of the mixtures of [NBun4][BF4] + CHF3 and [Cu(hfac)2] + [NBun4][BF4] + CHF3, the boundaries of which are approximately two straight lines in parallel. Furthermore, when a small amount of [Cu(hfac)2] (xCu(hfac)2 = 3.1 × 10−4) is added to the binary mixture of [NBun4][BF4] + CHF3, the p–T boundary is shifted to higher pressure by only ∼0.5 MPa. This result is consistent with our previous report6 on the mixtures of [Cu(hfac)2] and [NBun4][BF4] in a solution of CH3CN + CO2. Returning to the phase diagrams of the [Cu(CH3CN)4][BF4] mixtures in Fig. 5, one can see the significant difference in the shift of the p–T boundary by adding different copper precursors to the solution of HFC + [NBun4][BF4]. Clearly, [Cu(hfac)2] is more soluble and stable than [Cu(CH3CN)4][BF4] in HFCs, and therefore it only increases the phase-separation pressure by a moderate amount (0.5 MPa in the example shown above) when added to the CHF3 solution with the supporting electrolyte. This result may be understood by the fact that [Cu(hfac)2] is a neutral complex with fluorinated, bidentate ligands, while [Cu(CH3CN)4][BF4] is an ionic compound and the coordination bond between CH3CN and Cu is rather weak.
3.2 Conductivity of BARF salts in HFCs
We have reported in our previous paper6 the conductivity of a variety of BARF electrolytes in a mixed fluid of CO2 and CH3CN, indicating that the three electrolytes [NBun4][B{3,5-C6H3(CF3)2}4], [NRfMe3][B{3,5-C6H3(CF3)2}4], and [NRfBun3][B{3,5-C6H3(CF3)2}4] (Rf = CF3(CF2)7(CH2)3) have the highest molar conductivities (22–26 S cm2 mol−1) at 328.15 K and 20 MPa. In this work, we extend our conductivity measurements to scCH2F2. The supporting electrolytes employed in this study were four BARF salts (i.e.[NBun4][B(C6F5)4], [NBun4][B{3,5-C6H3(CF3)2}4], [NRfBun3][B{3,5-C6H3(CF3)2}4], and Na[B{3,5-C6H3(CF3)2}4]), and [NBun4][BF4], which has been measured by Abbott and Eardley8 in both liquid and scCH2F2. Na[B{3,5-C6H3(CF3)2}4] has been included as a candidate mainly because the phase behaviour described in the previous section of this paper demonstrates that it is surprisingly soluble in scCH2F2.Fig. 7 illustrates the molar conductivity of the five electrolytes in scCH2F2 as a function of pressure at a constant temperature of 363 K and a fixed molar concentration of ∼9.0 mol m−3. One can see that, as the system pressure increases, the molar conductivity of all of the five electrolytes increases within the pressure range between 10–32 MPa. In particular, the [NBun4][BF4] mixture shows the most pronounced pressure effect on the molar conductivity, which is consistent with the trend reported in the literature8 at the same temperature, but for a mixture with a higher concentration (cNBu4BF4 = 20 mol m−3). Moreover, the molar conductivity of [NBun4][BF4] obtained in our measurement is reasonably close to data reported in the literature. For example, Abbott and Eardley reported a molar conductivity of 0.013 S m2 mol−1 at 26.0 MPa and cNBu4BF4 = 10.2 mol m−3 (estimated from Fig. 6 in ref. 8); our result is 0.0144 S m2 mol−1 at cNBu4BF4 = 9.0 mol m−3, obtained by interpolating the data shown in Fig. 7 to 26.0 MPa.
![Molar conductivity (Λ) of the supporting electrolytes in CH2F2 at 363.15 K. The molar concentration of the electrolytes is ∼9.0 mol m−3. ■, [NBun4][BF4]; ●, [NRfBun3][B{3,5-C6H3(CF3)2}4]; ▲, [NBun4][B(C6F5)4]; ▼, [NBun4][B{3,5-C6H3(CF3)2}4]; ◆, Na[B{3,5-C6H3(CF3)2}4].](/image/article/2011/CP/c0cp01202e/c0cp01202e-f7.gif) | ||
| Fig. 7 Molar conductivity (Λ) of the supporting electrolytes in CH2F2 at 363.15 K. The molar concentration of the electrolytes is ∼9.0 mol m−3. ■, [NBun4][BF4]; ●, [NRfBun3][B{3,5-C6H3(CF3)2}4]; ▲, [NBun4][B(C6F5)4]; ▼, [NBun4][B{3,5-C6H3(CF3)2}4]; ◆, Na[B{3,5-C6H3(CF3)2}4]. | ||
It can also be seen from Fig. 7 that when the system pressure is below 20 MPa, the molar conductivity of all of the five electrolytes increases considerably with increasing pressure, reflecting the fact that the dielectric constant of CH2F2 increases from 7.39 at 10.0 MPa to 8.99 at 20.1 MPa at a constant temperature of 363 K.28 However, when the system pressure is above 20 MPa, the molar conductivity is almost unchanged with pressure for the three electrolytes having [B{3,5-C6H3(CF3)2}4]− as the anion. Although at 363 K the dielectric constant of CH2F2 increases from 8.99 at 20.1 MPa to 9.72 at 28.1 MPa,28 such a small change in the dielectric constant may have less significant effect on association/dissociation of the [B{3,5-C6H3(CF3)2}4]− salts than on [NBun4][BF4], and its effect on the molar conductivity may be cancelled out by the change in the viscosity of the fluid mixtures.9 Nevertheless, the pressure effect on the molar conductivity of the five electrolytes largely depends on the anions. Comparing the results for [NBun4][BF4], [NBun4][B(C6F5)4] and [NBun4][B{3,5-C6H3(CF3)2}4] in Fig. 7, as the anion gets bigger the molar conductivity of the electrolyte increases and the molar conductivity shows less pressure dependence within the pressure range measured in this study (10–32 MPa).
We have interpolated the molar conductivity data shown in Fig. 7 to a fixed pressure of 20.0 MPa by using piecewise cubic Hermite polynomials, and the results are listed in Table 2. In Table 2, the molar conductivity of all of the five electrolytes lies between 95 and 255 S cm2 mol−1 for the mixtures with c = ∼9.0 mol m−3, which is at least 3.6 times as high as that of the same electrolyte in the mixed fluid of CO2 + CH3CN (see Table 2 in ref. 6). Thus, the CH2F2 system provides a higher conductivity than that of the CO2 + CH3CN system (xCH3CN/xCO2 = 0.12).
| Electrolyte | Λ/S cm2 mol−1 |
|---|---|
| a The concentration of all of the electrolytes is ∼9.0 mol m−3. Piecewise cubic Hermite polynomials were used to interpolate the data shown in Fig. 7 to the fixed pressure (20 MPa). b Rf = CF3(CF2)7(CH2)3. | |
| [NBun4][BF4] | 124 |
| [NRfBun3][B{3,5-C6H3(CF3)2}4] b | 95 |
| [NBun4][B(C6F5)4] | 170 |
| [NBun4][B{3,5-C6H3(CF3)2}4] | 197 |
| Na[B{3,5-C6H3(CF3)2}4] | 255 |
Fig. 7 also shows that within the pressure range between 10–32 MPa, the general trend of the molar conductivity for the four BARF electrolytes is as follows:
| [NRfBun3][B{3,5-C6H3(CF3)2}4] < [NBun4][B(C6F5)4] < [NBun4][B{3,5-C6H3(CF3)2}4] < Na[B{3,5-C6H3(CF3)2}4]. |
The radii of the cations are in the order Na+ < [NBun4]+ < [NRfBun3]+. Thus, for the three electrolytes with the same anion ([B{3,5-C6H3(CF3)2}4]−) the molar conductivities increase as the radius of the cation decreases. We also note that Na[B{3,5-C6H3(CF3)2}4] has a much higher molar conductivity than [NBun4][B{3,5-C6H3(CF3)2}4], i.e. 255 S cm2 mol−1 for Na[B{3,5-C6H3(CF3)2}4], and 197 S cm2 mol−1 for [NBun4][B{3,5-C6H3(CF3)2}4]), respectively, at 363 K and 20 MPa (see Table 2). This may also be related to the greater dissociation of Na[B{3,5-C6H3(CF3)2}4] in CH2F2.
Since Na[B{3,5-C6H3(CF3)2}4] and [NBun4][B{3,5-C6H3(CF3)2}4] have the highest conductivity among the four BARF salts, these two compounds were chosen for a detailed conductivity study at various pressures and concentrations. Fig. 8 and 9 present results, respectively, for [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4] at 363 K and the molar concentration up to 60 mol m−3. All of the conductivity data shown in the figures were recorded under the conditions corresponding to a homogeneous single phase present in the high-pressure cell. Two main features of the conductivity diagrams can be summarised as follows: (i) the higher the molar concentration of the supporting electrolyte, the larger the conductivity the mixture has at a given pressure for both of the [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4] systems; (ii) the system pressure has a moderate effect on the conductivity for all of the mixtures with a molar concentration ranging from 0.8 to 60 mol m−3 (See also the previous discussion on the pressure effect on molar conductivity using the mixtures with c = 9.0 mol m−3 as examples (Fig. 7)). We stress here that the considerable increase of conductivity with pressure, which has been observed between 10–20 MPa from a variety of mixtures cannot be seen in Fig. 8 for the mixture with 60 mol m−3 of [NBun4][B{3,5-C6H3(CF3)2}4], partially because the conductivity must be measured in a homogeneous, single-phase solution, resulting in the unavailability of the conductivity data below 18 MPa.
![Conductivity (κ) of [NBun4][B{3,5-C6H3(CF3)2}4] in scCH2F2 at 363.15 K. The molar concentrations of [NBun4][B{3,5-C6H3(CF3)2}4] are: , 0.78 mol m−3; ×, 1.04 mol m−3; +, 1.35 mol m−3; ◊, 2.54 mol m−3; ▽, 4.93 mol m−3; △, 8.98 mol m−3; ○, 30.6 mol m−3; □, 60.2 mol m−3.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f8.gif) | ||
Fig. 8 Conductivity (κ) of [NBun4][B{3,5-C6H3(CF3)2}4] in scCH2F2 at 363.15 K. The molar concentrations of [NBun4][B{3,5-C6H3(CF3)2}4] are:  , 0.78 mol m−3; ×, 1.04 mol m−3; +, 1.35 mol m−3; ◊, 2.54 mol m−3; ▽, 4.93 mol m−3; △, 8.98 mol m−3; ○, 30.6 mol m−3; □, 60.2 mol m−3. , 0.78 mol m−3; ×, 1.04 mol m−3; +, 1.35 mol m−3; ◊, 2.54 mol m−3; ▽, 4.93 mol m−3; △, 8.98 mol m−3; ○, 30.6 mol m−3; □, 60.2 mol m−3. | ||
![Conductivity (κ) of Na[B{3,5-C6H3(CF3)2}4] in scCH2F2 at 363.15 K. The molar concentrations of Na[B{3,5-C6H3(CF3)2}4] are: ◊, 1.05 mol m−3; ▽, 2.59 mol m−3; △, 9.04 mol m−3; ○, 15.0 mol m−3; □, 20.2 mol m−3.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f9.gif) | ||
| Fig. 9 Conductivity (κ) of Na[B{3,5-C6H3(CF3)2}4] in scCH2F2 at 363.15 K. The molar concentrations of Na[B{3,5-C6H3(CF3)2}4] are: ◊, 1.05 mol m−3; ▽, 2.59 mol m−3; △, 9.04 mol m−3; ○, 15.0 mol m−3; □, 20.2 mol m−3. | ||
To understand the different behaviour in conductivity between [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4], the data shown in Fig. 8 and 9 were interpolated to a fixed pressure of 20 MPa using piecewise cubic Hermite polynomials, and the results are plotted against the square root of the concentration in Fig. 10, together with the data for the [NBun4][BF4] system reported by Abbott and Eardley8 at the same temperature (363 K) but a slightly higher pressure of 22 MPa. It can be seen from the figure that the molar conductivity decreases with increasing √c for both [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4], and no minimum can be seen in the concentration range of 0.7–60 mol m−3 for [NBun4][B{3,5-C6H3(CF3)2}4], or 1–20 mol m−3 for Na[B{3,5-C6H3(CF3)2}4]. In addition, the molar conductivity of [NBun4][B{3,5-C6H3(CF3)2}4] starts to level off when c approaches 30 mol m−3, indicating the significant contribution of triple ions to the conductivity at the high concentrations, while the Λ−√c curve for the Na[B{3,5-C6H3(CF3)2}4] system shows a linear relationship within the concentration region given above. The limiting molar conductivity of Na[B{3,5-C6H3(CF3)2}4] cannot be calculated from the curve because molar conductivity data are not available at low concentrations (<1 mol m−3) from this study.
![Molar conductivity (Λ) of three supporting electrolytes in scCH2F2 at 363.15 K: ■, [NBun4][B{3,5-C6H3(CF3)2}4], 20 MPa; ●, Na[B{3,5-C6H3(CF3)2}4], 20 MPa; and ◆, [NBun4][BF4], 22 MPa, estimated from Fig. 6 of ref. 8. The molar conductivity of the two BARF salts were obtained by interpolating the data shown in Fig. 8 and 9 to the fixed pressure (20 MPa) using piecewise cubic Hermite polynomials. The lines are merely to guide the eye. The error bars represent the estimated experimental error.](/image/article/2011/CP/c0cp01202e/c0cp01202e-f10.gif) | ||
| Fig. 10 Molar conductivity (Λ) of three supporting electrolytes in scCH2F2 at 363.15 K: ■, [NBun4][B{3,5-C6H3(CF3)2}4], 20 MPa; ●, Na[B{3,5-C6H3(CF3)2}4], 20 MPa; and ◆, [NBun4][BF4], 22 MPa, estimated from Fig. 6 of ref. 8. The molar conductivity of the two BARF salts were obtained by interpolating the data shown in Fig. 8 and 9 to the fixed pressure (20 MPa) using piecewise cubic Hermite polynomials. The lines are merely to guide the eye. The error bars represent the estimated experimental error. | ||
Compared with the [NBun4][BF4] system, we can see that the molar conductivities of the [NBun4][B{3,5-C6H3(CF3)2}4] system are higher at all concentrations. Furthermore, the molar conductivity of [NBun4][BF4] decreases rapidly with the increase of √c when c < 8 mol m−3 and then it starts to level off at ∼16 mol m−3, that is lower than the corresponding concentration (30 mol m−3) for the [NBun4][B{3,5-C6H3(CF3)2}4] system. Thus [NBun4][B{3,5-C6H3(CF3)2}4] can produce more free ions in scCH2F2 than [NBun4][BF4], making it a better choice of a supporting electrolyte than [NBun4][BF4] in terms of conductivity. However, this effect is less significant for the scCH2F2 system than for the CO2 + CH3CN system when replacing [NBun4][BF4] with the BARF salts: the molar conductivity increases by 60% in scCH2F2 when replacing [NBun4][BF4] with [NBun4][B{3,5-C6H3(CF3)2}4] (Table 2), whereas in CO2 + CH3CN, the conductivity increases by at least 900%6 when using [NBun4][B{3,5-C6H3(CF3)2}4]. Thus [NBun4][BF4] is a satisfactory electrolyte for the use in scCH2F2, particularly for those applications that do not require passage of large currents.
Although the difference in the conductivity of the three electrolytes is mainly ascribed to the association/dissociation ability of the electrolytes in scCH2F2, the viscosity has an effect on the mobility of the ions and hence on the conductivity. The viscosity of CH2F2 can be increased by a factor of 8–32 at 363 K when adding 5–30 mol m−3[NBun4][BF4].9 It is expected that both [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4] will increase the viscosity of the CH2F2 even more because [B{3,5-C6H3(CF3)2}4]− is a much larger anion than [BF4]−. Nevertheless, as the concentration increases, the viscosity increases for all three electrolytes, resulting in the decrease in the molar conductivity, which is consistent with the general trend shown in Fig. 10.
4. Conclusions
In this paper we have presented results of a study of the phase behaviour of CHF3, CH2F2, and CH2FCF3 containing tetrabutylammonium tetrafluoroborate ([NBun4][BF4]), [NBun4][B{3,5-C6H3(CF3)2}4] and Na[B{3,5-C6H3(CF3)2}4]. For all three HFCs we find that when the temperature is below the corresponding Tc the p–T boundaries are overlapped for the mixtures with different composition of [NBun4][BF4] but at higher temperature the solubility of [NBun4][BF4] in the HFC fluid phase increases with increasing pressure. Compared to the CO2 + CH3CN system (xCO2/xCH3CN = 0.14) studied previuously,6 when [NBun4][BF4] is used as the supporting electrolyte, the pressures required to form a homogeneous solution are much lower for the HFCs.Although all three HFCs are good solvents to dissolve [NBun4][BF4], our results show that the CH2F2 system has the lowest pr for dissolving the same amount of [NBun4][BF4]. This may be explained by the fact that CH2F2 has the highest dipole moment among the three HFCs. The behaviour of the Na[B{3,5-C6H3(CF3)2}4] in CH2F2 is somewhat unexpected since sodium salts often have low solubility in organic solvents. Here the BARF anion appears to be responsible for the enhanced solubility of the Na[B{3,5-C6H3(CF3)2}4] in CH2F2, possibly due to the large mismatch in the size of the two ions.
Studies of the phase behaviour of CH2F2 containing [NBun4][BF4] and [Cu(CH3CN)4][BF4] showed that the copper complex was unstable in the absence of CH3CN. Our results show that the addition of [NBun4][BF4] to the [Cu(CH3CN)4][BF4] system drastically shifts the phase boundary to high pressures at T > Tc,CH2F2. We believe that this is caused by selective solvation of the [NBun4]+ and [BF4]− ions by CH3CN, depleting the CH3CN molecules in the bulk phase, so that the density, and hence the pressure of the system, needs to be increased considerably for the quaternary mixture to form a homogenous phase.
For CHF3 and [Cu(hfac)2] we find that [Cu(hfac)2] is more soluble and more stable than [Cu(CH3CN)4][BF4] in HFCs and that it only increases the phase-separation pressure by a moderate amount when added to the CHF3 solution with [NBun4][BF4] as the supporting electrolyte. An example of using [Cu(hfac)2] as a precursor for the electrodeposition of copper from scCHF3 has been reported in our recent work.29
Since CH2F2 was found to have the lowest pr for dissolving the same amount of [NBun4][BF4] studies of the conductivity for four different BARF salts, [NBun4][B(C6F5)4], [NBun4][B{3,5-C6H3(CF3)2}4], [NRfBun3][B{3,5-C6H3(CF3)2}4], and Na[B{3,5-C6H3(CF3)2}4], were carried out. Our results show that the BARF salts are more conducting than [NBun4][BF4] under the same conditions although the effect is much less significant than for our previous work in supercritical CO2 + CH3CN (a factor of 1.6 increase in molar conductivity as opposed to 9).
The main purpose of this work was to characterise supercritical fluids that can be used as electrolytes for electrodeposition. Our results show that scCH2F2 is a suitable system for electrodeposition using either [NBun4][BF4] or the corresponding BARF salts as electrolytes and identify the condition under which a single supercritical phase is formed. The results also confirm that the visual method used in this study can provide crucial information on the stability of metal precursors under supercritical conditions, and is a powerful method for the acquisition of information on phase equilibria.
Acknowledgements
This work was supported by RCUK via a Basic Technology Grant. We are grateful to Prof. M. Poliakoff and Dr D. C. Smith for useful advice and Messrs. M. Guyler, R. Wilson, P. Fields, D. Litchfield, and J. Warren for technical support. We are grateful to Dr R. E. Low (INEOS Fluor Ltd, UK) for supplying R32. Royal Society Wolfson Merit Award to MWG is gratefully acknowledged. JK wish to thank Drs S. E. Davies, F. Qiu, D. Walsh, and J. Yang for helpful discussion.References
- I. Souvignet and S. V. Olesik, Anal. Chem., 1998, 70, 2783–2788 CrossRef.
- A. P. Abbott, C. A. Eardley, J. C. Harper and E. G. Hope, J. Electroanal. Chem., 1998, 457, 1–4 CrossRef CAS.
- T. Clifford, Fundamentals of Supercritical Fluids, Oxford University Press, Oxford, 1999 Search PubMed.
- A. P. Abbott and J. C. Harper, J. Chem. Soc., Faraday Trans., 1996, 92, 3895–3898 RSC.
- J. Ke, W. Su, S. M. Howdle, M. W. George, D. Cook, M. Perdjon-Abel, P. N. Bartlett, W. Zhang, F. Cheng, W. Levason, G. Reid, J. Hyde, J. Wilson, D. Smith, K. Mallik and P. Sazio, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 14768–14772 CrossRef CAS.
- P. N. Bartlett, D. C. Cook, M. W. George, J. Ke, W. Levason, G. Reid, W. Su and W. Zhang, Phys. Chem. Chem. Phys., 2010, 12, 492–501 RSC.
- A. P. Abbott and N. E. Durling, Phys. Chem. Chem. Phys., 2001, 3, 579–582 RSC.
- A. P. Abbott and C. A. Eardley, J. Phys. Chem. B, 2000, 104, 9351–9355 CrossRef CAS.
- A. P. Abbott, E. G. Hope and D. J. Palmer, Anal. Chem., 2005, 77, 6702–6708 CrossRef CAS.
- D. L. Goldfarb and H. R. Corti, Electrochem. Commun., 2000, 2, 663–670 CrossRef.
- D. L. Goldfarb and H. R. Corti, J. Phys. Chem. B, 2004, 108, 3368–3375 CrossRef CAS.
- D. L. Goldfarb and H. R. Corti, J. Phys. Chem. B, 2004, 108, 3358–3367 CrossRef CAS.
- S. A. Olsen and D. E. Tallman, Anal. Chem., 1994, 66, 503–509 CrossRef CAS.
- S. A. Olsen and D. E. Tallman, Anal. Chem., 1996, 68, 2054–2061 CrossRef CAS.
- M. Atobe, S. Iizuka, T. Fuchigami and H. Yamamoto, Chem. Lett., 2007, 36, 1448–1449 CrossRef CAS.
- M. Atobe, H. Ohsuka and T. Fuchigami, Chem. Lett., 2004, 33, 618–619 CrossRef CAS.
- P. Licence, M. P. Dellar, R. G. M. Wilson, P. A. Fields, D. Litchfield, H. M. Woods, M. Poliakoff and S. M. Howdle, Rev. Sci. Instrum., 2004, 75, 3233 CrossRef CAS.
- R. L. David, CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 89th edn, 2009 Search PubMed.
- K. Fukne-Kokot, A. Konig, Z. Knez and M. Skerget, Fluid Phase Equilib., 2000, 173, 297–310 CrossRef CAS.
- A. M. Scurto, E. Newton, R. R. Weikel, L. Draucker, J. Hallett, C. L. Liotta, W. Leitner and C. A. Eckert, Ind. Eng. Chem. Res., 2008, 47, 493–501 CrossRef CAS.
- E. W. Lemmon, M. L. Huber and M. O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties—REFPROP, Version 8.0, National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg, 2007 Search PubMed.
- D. R. Brannegan, M. Ashraf-Khorassani and L. T. Taylor, Chromatographia, 2001, 54, 399–401 CAS.
- A. Shadrin, V. Kamachev, A. Murzin and D. Shafikov, J. Supercrit. Fluids, 2007, 42, 347–350 CrossRef CAS.
- S. W. Kim and K. P. Johnston, AIChE J., 1987, 33, 1603–1611 CAS.
- Y. P. Sun, G. Bennett, K. P. Johnston and M. A. Fox, J. Phys. Chem., 1992, 96, 10001–10007 CrossRef CAS.
- I. Skarmoutsos, D. Dellis and J. Samios, J. Chem. Phys., 2007, 126, 10.
- J. W. Ford, M. E. Janakat, J. Lu, C. L. Liotta and C. A. Eckert, J. Org. Chem., 2008, 73, 3364–3368 CrossRef CAS.
- A. P. Abbott, C. A. Eardley and R. Tooth, J. Chem. Eng. Data, 1999, 44, 112–115 CrossRef CAS.
- D. Cook, P. N. Bartlett, W. Zhang, W. Levason, G. Reid, J. Ke, W. Su, M. W. George, J. Wilson, D. Smith, K. Mallik, E. Barrett and P. Sazio, Phys. Chem. Chem. Phys., 2010, 12, 11744–11752 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental phase equilibrium data of the binary systems of electrolytes and hydrofluorocarbons. See DOI: 10.1039/c0cp01202e |
| ‡ The liquid phase mainly consists of CH2F2 or CHF2CF3. |
| § The liquid phase consists of a large amount of [NBun4][BF4]. |
| This journal is © the Owner Societies 2011 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)