DOI:
10.1039/C0CE00190B
(Paper)
CrystEngComm, 2011,
13, 330-338
Syntheses, structures and luminescent properties of zinc(II) coordination polymers based on bis(imidazole) and dicarboxylate†
Received
9th May 2010
, Accepted 13th July 2010
First published on 8th September 2010
Abstract
Five zinc(II) coordination polymers with 5-methylisophthalic acid (H2L1), 5-methoxylisophthalic acid (H2L2) and bridging bis(imidazole) ligands, [Zn(L1)(biim-3)]n (1), [Zn(L1)(biim-6)]n (2), [Zn(L1)(biim-5)]n (3), [Zn(L2)(biim-3)]n (4) and [Zn(L2)(biim-6)]n (5) (biim-3 = 1,3-bis(imidazole)propane, biim-5 = 1,5-bis(imidazole)pentane, biim-6 = 1,6-bis(imidazole)hexane), have been hydrothermally synthesized and structurally characterized. Compound 1 shows a two-dimensional (4,4) net which contains left- and right-handed helical chains. Compounds 2 and 3 are both 3-fold parallel interpenetrated 2D → 3D architecture while 4 shows a 2-fold parallel interpenetrated 3D → 3D architecture and contains two kinds of chiral layers. Compound 5 exhibits a 2-fold parallel interpenetrated 2D → 2D (4,4) net. Furthermore, luminescent properties and thermo-gravimetric properties of these compounds were investigated. The results suggest that both carboxylate and bis(imidazole) ligands influence on the final resulting complexes.
Introduction
Metal–organic coordination polymers have been attracted considerable attention because of their potential applications as function materials as well as their structure diversity and intriguing variety of topologies.1,2 The diversity in the framework structures of such materials greatly depends on the metal centers and the structure of the spacer ligands, as well as on the reaction pathway.3 Although a large number of polycarboxylate-based coordination polymers have been synthesized, systematic investigation of external physical or chemical stimuli effects on the structures of polycarboxylatebased coordination polymers remains rare.4 It is noteworthy that 1,n-benzenedicar-boxylate (n = 2, 3, or 4), 1,3,5- or 1,2,4-benzenetricarboxylate, and 1,2,4,5-benzenetetracarboxylate have been widely used to prepare such hybrid complexes.5–7 However, there is little attention about 5-methylisophthalic acid and 5-methoxylisophthalic acid.8 These polycarboxylate are chemically pertinent and differentiated by the 5-position substituents. It is anticipated that these modified linkers may afford different supramolecular assemblies in view of their steric and/or electronic effect.
Bis(imidazole) ligands with –CH2– spacers are good candidate for N-donor bridging ligands. The flexible nature of –CH2– spacers allows the ligands to bend and rotate freely when coordinating to metal centers so as to conform to the coordination geometries of metal ions.9 The previous studies show that these ligands can exhibit special abilities to formulate interesting compounds, and the results also indicate that different organic anions play important roles in directing the final structures and topologies.10 From another point of view, coordination polymers containing metal ions with a d10 configuration, such as Zn(II), Cd(II) and Hg(II), are potential materials for optical applications, such as fluorescence probes and nonlinear optical materials.11 Therefore, systematic studies have been carried out in our laboratory by reactions of the Zn(II) salts, H2L1 and H2L2 ligands together with a series of N-donor ligands to investigate the influence of dicarboxylate and N-donor ligands in framework construction. In this work, several ternary systems containing Zn(II) ions, bis(imidazole) ligands and carboxylate anions are selected to survey the influence factors. We describe the successful syntheses of five new compounds: [Zn(L1)(biim-3)]n (1), [Zn(L1)(biim-6)]n (2), [Zn(L1)(biim-5)]n (3), [Zn(L2)(biim-3)]n (4) and [Zn(L2)(biim-6)]n (5). The crystal structures of these compounds and topological analyses, along with the systematic investigation of the modulated effect with coordination modes of L1 and L2 anions and bis(imidazole) ligands on the ultimate framework, will be represented and discussed (Scheme 1).
 |
| | Scheme 1 Structures of the carboxylate and bis(imidazole) ligands used in this work | |
Experimental
Materials and physical measurements
All reagents used in the syntheses were of analytical grade. Elemental analyses for carbon, hydrogen and nitrogen atoms were performed on a Vario EL III elemental analyzer. The infrared (IR) spectra (4000–400 cm−1) were recorded by using KBr pellet on an Avatar™360 E. S. P. IR spectrometer. Thermo-gravimetric (TG) analyses were carried out on a STA449C integration thermal analyzer. The luminescence spectra for the powdered solid samples were measured at room temperature on a Hitachi F-4500 Fluorescence Spectrophotometer.
Preparation of complexes 1–5
Synthesis of [Zn(L1)(biim-3)]n (1).
A mixture of H2L1 (0.10 mmol, 18.0 mg), biim-3 (0.10 mmol, 17.6 mg), Zn(OAc)2·2H2O (0.10 mmol, 21.9 mg) and H2O (16 mL) was placed in a Teflon-lined stainless steel vessel, heated to 130 °C for 3 d, and then cooled to room temperature over 24 h. Colourless block crystals of 1 were obtained. Yield: 52% (based on Zn). Elemental analysis (%): calcd for C18H18N4O4Zn (Mr = 419.73): C 51.51, H 4.32, N 13.35; found: C 51.49, H 4.29, N 13.32. IR (cm−1): 3434 (s), 2927 (m), 2075 (m), 1624 (s), 1387 (m), 1239 (m), 1092 (s), 954 (m), 879 (m), 772 (m), 721 (m), 656 (m).
Synthesis of [Zn(L1)(biim-6)]n (2).
The preparation of 2 was similar to that of 1 except that biim-3 was replaced by biim-6 (0.10 mmol, 21.8 mg), Colourless block crystals of 2 were obtained. Yield: 43% (based on Zn). Elemental analysis (%): calcd for C21H24N4O4Zn (Mr = 461.81): C 54.62, H 5.24, N 12.13; found: C 54.60, H 5.21, N 12.10. IR (cm−1): 3433 (s), 2924 (m), 2071 (m), 1625 (s), 1338 (s), 1240 (m), 1112 (s), 952 (m), 840 (m), 779 (s), 724 (m), 654 (m).
Synthesis of [Zn(L1)(biim-5)]n (3).
The preparation of 3 was similar to that of 1 except that biim-3 was replaced by biim-5 (0.10 mmol, 20.4 mg), Colourless block crystals of 3 were obtained. Yield: 31% (based on Zn). Elemental analysis (%): calcd for C20H22N4O4Zn (Mr = 447.79): C 53.65, H 4.95, N 12.51; found: C 53.62, H 4.93, N 12.48. IR (cm−1): 3428 (s), 2931 (m), 2070 (m), 1632 (s), 1580 (s), 1335 (s), 1111 (s), 953 (m), 841 (m), 786 (m), 724 (m), 656 (m).
Synthesis of [Zn(L2)(biim-3)]n (4).
The preparation of 4 was similar to that of 1 except that H2L1 was replaced by H2L2 (0.10 mmol, 19.6 mg), Colourless block crystals of 4 were obtained. Yield: 29% (based on Zn). Elemental analysis (%): calcd for C18H18N4O5Zn (Mr = 435.73): C 49.62, H 4.16, N 12.86; found: C 49.60, H 4.12, N 12.83. IR (cm−1): 3435 (s), 2927 (m), 2356 (m), 2072 (m), 1632 (m), 1593 (m), 1347 (m), 1122 (s), 951 (m), 778 (m), 729 (m), 655 (m).
Synthesis of [Zn(L2)(biim-6)]n (5).
The preparation of 5 was similar to that of 4 except that biim-3 was replaced by biim-6 (0.10 mmol, 21.8 mg). Colourless block crystals of 5 were obtained. Yield: 37% (based on Zn). Elemental analysis (%): calcd for C21H24N4O5Zn (Mr = 477.81): C 52.79, H 5.06, N 11.73; found: C 52.77, H 5.02, N 11.70. IR (cm−1): 3428 (s), 2926 (m), 2075 (m), 1621 (s), 1581 (s), 1348 (s), 1108 (s), 1051 (m), 851 (m), 781 (m), 730 (m), 658 (m).
Single crystals suitable for X-ray analyses were used on a Bruker SMART APEX II CCD diffractometer using graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) by using a ϕ/ω scan technique at room temperature. Lorentz polarization and absorption corrections were applied. The structures were solved by direct methods and refined with the full-matrix least-squares technique using the SHELXS-97 and SHELXL-97 programs.12 The hydrogen atoms were assigned with common isotropic displacement factors and included in the final refinement by use of geometrical restrains, while the non-hydrogen atoms were treated with common anisotropic displacement factors and included in the final refinement with geometrical restrains. The crystallographic data and details of the structure determinations for complexes 1–5 are listed in Table 1. Selected bond lengths and angles for compounds 1–5 are given in the ESI, Table S1.†
| Compounds |
1
|
2
|
3
|
4
|
5
|
| Empirical formula |
C18H18N4O4Zn
|
C20H22N4O4Zn
|
C21H24N4O4Zn
|
C18H18N4O5Zn
|
C21H24N4O5Zn
|
| Formula weight |
419.73 |
447.79 |
461.81 |
435.73 |
477.81 |
|
T/K |
296(2) |
296(2) |
296(2) |
296(2) |
296(2) |
|
λ/Å |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
| Crystal system |
Monoclinic |
Monoclinic |
Monoclinic |
Orthorhombic |
Triclinic |
| Space group |
P21/c |
P21/c |
P21/n |
Pbca
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
|
a/Å |
8.1512(7) |
9.4664(10) |
9.6105(10) |
16.4763(14) |
9.7754(10) |
|
b/Å |
15.0605(13) |
14.5654(15) |
15.2014(16) |
13.2934(11) |
10.1743(11) |
|
c/Å |
14.8508(12) |
15.1276(16) |
14.4610(15) |
17.2155(15) |
11.4600(12) |
|
α/° |
— |
— |
— |
— |
94.8700(10) |
|
β/° |
93.7260(10) |
102.2470(10) |
90.6450(10) |
90 |
100.6930(10) |
|
γ/° |
— |
— |
— |
— |
105.2020(10) |
|
Z
|
4 |
4 |
4 |
8 |
2 |
|
V/Å 3 |
1819.2(3) |
2038.4(4) |
2112.5(4) |
3770.6(6) |
1070.03(19) |
| Calculated density (Kg m−3) |
1.532 |
1.459 |
1.452 |
1.535 |
1.483 |
|
μ/mm−1 |
1.382 |
1.239 |
1.198 |
1.341 |
1.188 |
|
F(000) |
864 |
928 |
960 |
1792 |
496 |
| Independent reflections |
4160 [Rint = 0.0227] |
3794 [Rint = 0.0392] |
3934 [Rint = 0.0410] |
3501 [Rint = 0.0523] |
3951 [Rint = 0.0175] |
| Final R indices [I > 2σ(I)] |
R
1 = 0.0270, wR2 = 0.0676 |
R
1 = 0.0330 wR2 = 0.0724 |
R
1 = 0.0434, wR2 = 0.1036 |
R
1 = 0.0336 wR2 = 0.0760 |
R
1 = 0.0300, wR2 = 0.0747 |
|
R indices (all data) |
R
1 = 0.0351, wR2 = 0.0714 |
R
1 = 0.0508 wR2 = 0.0804 |
R
1 = 0.0664, wR2 = 0.1156 |
R
1 = 0.0520 wR2 = 0.0857 |
R
1 = 0.0366, wR2 = 0.0791 |
|
ρ
max/ρmin/Å−3 |
0.294/−0.216 |
0.281/−0.218 |
0.426/−0.332 |
0.596/−0.304 |
0.470/−0.207 |
Results and discussion
Description of crystal structures
[Zn(L1)(biim-3)]n (1).
As shown in Fig. 1a, the asymmetric unit of 1 contains one Zn(II) ion, one L1 anion and one biim-3 ligand. The crystallographically unique Zn(II) atom is four-coordinated by two oxygen atoms from two L1 anions (Zn(1)–O(1) = 1.9169(13) Å, Zn(1)–O(3A) = 1.9261(12) Å) and two nitrogen atoms from two biim-3 ligands (Zn(1)–N(4B) = 2.0188(15) Å, Zn(1)–N(1) = 2.0254(15) Å), showing a distorted tetrahedral coordination geometry (ZnO2N2). Each L1 anion acts as a bis-monodentate bridging mode to link adjacent Zn(II) atoms to yield a wavelike chain (Fig. 1b), which are further linked by biim-3 ligands to form a 2D layer (Fig. 1c). And the biim-3 ligand links Zn cation to form a left- and right-handed helical chain (Fig. 1d) with a pitch of 15.061(13) Å. From the topological point of view, the 2D plane of 1 can be reduced to a bimodal (4,4) net (Fig. 1e).
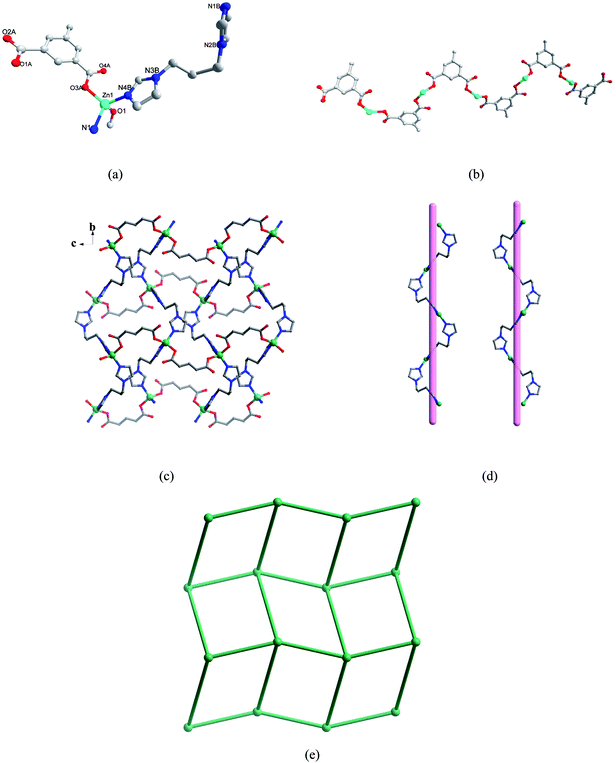 |
| | Fig. 1 (a) Coordination environment of Zn(II) ion in 1. The hydrogen atoms are omitted for clarity. A: x, −y + 5/2, z + 1/2 B: −x + 1, y + 1/2, −z + 1/2. (b) The 1D chain constructed by L1 anions and Zn(II) atoms along the c axis. (c) 2D layer of 1. (d) View of left- and right-handed helical chains. The H2L1 groups are omitted for clarity. (e) Schematic representation of the (4,4) net topology. | |
[Zn(L1)(biim-6)]n (2).
The fundamental building unit of 2 contains one Zn(II) ion, one L1 anion and one biim-6 ligand. The Zn(II) ion adopts a tetrahedral coordination geometry, coordinating to two carboxylate O atoms from two L1 anions (Zn(1)–O(2) = 1.946(2) Å and Zn(1)–O(4A) = 1.953(2) Å) and two N atoms from two biim-6 ligands (Zn(1)–N(6B) = 2.008(3) Å and Zn(1)–N(1) = 2.014(3) Å), as shown in Fig. 2a. Each L1 ligand adopts a bis-monodentate bridging mode to link neighbouring Zn(II) ions to form a 1D chain (Fig. 2b). The adjacent chains are connected by biim-6 ligands to form a puckered 2D (4,4) sheet (Fig. 2c). The identical 2D single nets are interlocked with each other and form an interesting 3-fold parallel interpenetrated 2D → 3D architecture (Fig. 2d).
 |
| | Fig. 2 (a) Coordination environment of Zn(II) ion in 2. The hydrogen atoms are omitted for clarity. A: x − 1, y, z B: −x + 1/2, y + 1/2, −z + 1/2. (b) The 1D chain constructed by L1 anions and Zn(II) atoms along the a axis. (c) 2D layer of 2. (d) A schematic view of the 3-fold interpenetrating architecture of 2. | |
[Zn(L1)(biim-5)]n (3).
As illustrated in Fig. 3a, the structure of 3 contains one Zn(II) atom, one L1 anion and one biim-5 ligand. The Zn(II) ion is four-coordinated by two carboxylate oxygen atoms from two L1 anions and two nitrogen atoms from two biim-5 ligands in a distorted tetrahedral environment (ZnO2N2). The Zn–O bond distances are 1.9326(16), 1.9434(16) Å and Zn–N bond distances are 1.999(2), 2.003(2) Å, respectively, falling into the normal range.13 The L1 ligand shows a bis-monodentate mode to bridge the Zn(II) ions to form a 1D chain. These chains are across-linked by bridging biim-5 ligands along the a axis to generate a 2D (4,4) rectangular grid layer (Fig. 3b). Then a pair of identical 2D single nets is interlocked with each other, forming a 3-fold parallel interpenetrated 2D → 3D architecture.
 |
| | Fig. 3 (a) Coordination environment of Zn(II) ion in 3. The hydrogen atoms are omitted for clarity. A: x + 1, y, z B: x, −y + 1/2, z + 1/2. (b) View of the 2D layer of 3. | |
[Zn(L2)(biim-3)]n (4).
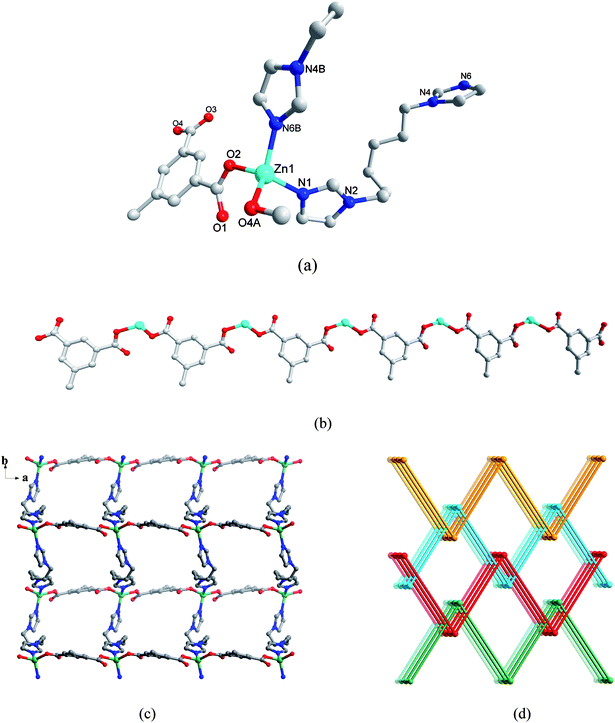
The asymmetric unit of 4 consists of one Zn(II) ion, one L2 anion and one biim-3 ligand. Each Zn(II) center is in a distorted tetrahedral geometry. The four atoms coordinated to Zn(II) ion come from two oxygen atoms of two L2 anions, (Zn(1)–O(2) = 1.964(2) and Zn(1)–O(4A) = 1.979(2) Å), two nitrogen atoms of two biim-3 ligands (Zn(1)–N(1) = 1.997(2) and Zn(1)–N(4B) = 2.022(2) Å). Each L2 anion and biim-3 bridges the adjacent Zn(II) atoms to form two kinds of helical chains. Thus an overall 4-connected net is formed which has the diamond topology. A feature of the diamond net is the hexagonal channels are quite crowded due to the ligand conformations, however the square channels are more apparent (Fig. 4d). As for the diamond net, adjoining square channels have alternating chirality (Fig. 4b and 4c). And a pair of identical 3D single nets is interlocked with each other to form a 2-fold parallel interpenetrated 3D → 3D network (Fig. 4e).
 |
| | Fig. 4 (a) Coordination environment of Zn(II) ion in 4. The hydrogen atoms are omitted for clarity. A: x + 1/2, −y + 1/2, −z; B: −x + 1, y − 1/2, −z + 1/2. (b) View of left- and right-handed helical chains. The imidazole groups are omitted for clarity. (c) View of left- and right-handed helical chains. The L1 groups are omitted for clarity. (d) The framework viewed along the a direction, showing two types of helical channels along the a axis (L: left-handed helical channel, R: right-handed helical channel). (e) A schematic view of the 2-fold interpenetrating network of 4. | |
[Zn(L2)(biim-6)]n (5).
The structure of 5 exhibits a 2D (4,4) net. As shown in Fig. 5a, the structure of 5 contains one Zn(II) ion, one L2 anion and one biim-6 ligand. The Zn(II) ion adopts a tetrahedral coordination geometry, coordinating to two carboxylate O atoms from two L2 anions (Zn(1)–O(1) = 2.0009(14) and Zn(1)–O(4A) = 1.9837(13) Å) and two N atoms from two biim-6 ligands (Zn(1)–N(1) = 1.9982(17) and Zn(1)–N(3) = 2.0174(17) Å). Each L2 acts as a bis-monodentate mode to bridge adjacent Zn(II) ions to form a 1D chain (Fig. 5b). These chains are further linked by bridging biim-6 ligands along the c axis to generate a 2D rectangular (4,4) grid layer (Fig. 5c). Then a pair of identical 2D single nets is interlocked with each other to form a 2-fold parallel interpenetrated 2D → 2D architecture (Fig. 5d).
 |
| | Fig. 5 (a) Coordination environment of Zn(II) ion in 5. The hydrogen atoms are omitted for clarity. A: x, y + 1, z. (b) The 1D chain constructed by L2 anions and Zn(II) atoms along the b axis. (c) 2D layer of 5. (d) A schematic view of the 2-fold interpenetrating along the c axis in 5. | |
Structural diversity and synthesis chemistry.
Our aim is to investigate the effects of the different carboxylate and N-donor bridging ligands on the self-assembly of supra-molecules and coordination polymers. The synthetic strategy for the Zn(II)-H2L1,H2L2/biim-3, biim-5, biim-6 system is schematically depicted in Scheme 2. From these results, When the carboxylate ligands was H2L2, three MOFs were formed under different N-donor ligands. We got polymers of different structures because the different lengths of the C chains, 2D plane structure of 1, an interesting 3-fold parallel interpenetrated 2D → 3D net architecture of 2 and a 3-fold parallel interpenetrated 2D → 3D architecture of 3. A similar change also occurs in 4 and 5. We got a 2-fold parallel interpenetrated 3D → 3D network structure of 4 and a 2-fold parallel interpenetrated 2D → 2D architecture of 5. While if the N-donor ligand was biim-5, we did not obtain the crystal structures. In comparison with compounds 1 and 4, 2 and 5, 3 and 6, using the same N-donor ligand, it has been shown that the carboxylate ligands have a critical impact on the construction of different MOFs because of different supramolecular assemblies in view of their steric and/or electronic effect.
 |
| | Scheme 2 Synthesis of complexes 1–5. | |
PXRD and thermal analysis
In order to check the phase purity of these compounds, the X-ray powder diffraction (XRPD) patterns of compounds 1–5 were checked at room temperature. As shown in Fig. S1,† the peak positions of the simulated and experimental XRPD patterns are in agreement with each other, demonstrating the good phase purity of the compounds.
The thermal behaviour of 1–5 were studied by TGA. The experiments were performed on samples consisting of numerous single crystals under N2 atmosphere with a heating rate of 10 °C min−1, as shown in Fig. S2.† The TGA curve for 1–5 suggested that no mass loss until ∼342 °C, ∼352 °C, ∼326 °C, ∼317 °C and ∼221 °C, respectively, at which point a series of mass losses ensued. Compounds 1–5 can keep stabilization in high temperature because they do not include guest solvent molecule, and the remaining weight corresponds to the formation of ZnO (observed, 19.42%, 18.24%, 17.68%, 18.77% and 17.09%, respectively; calculated, 19.39%, 18.17%, 17.62%, 18.68% and 17.03%, respectively).
Fluorescent properties
Luminescent properties of compounds which contain zinc as the metal centers have been attracting more interest because of their potential applications in chemical sensors, photochemistry and electroluminescent display.14 These crystalline solids usually display and photoluminescence properties.15 In this paper, the solid-state photo-luminescent properties of compounds 1–5 have been investigated in the solid state at room temperature cures are shown in Fig. 6. The photo-luminescent spectra of compounds 1–5 show the emission maxima at 453 nm for 1 (λex = 390 nm), 412 nm for 2 (λex = 328 nm), 426 nm for 3 (λex = 312 nm), 406 nm for 4 (λex = 355 nm) and 390 nm for 5 (λex = 357 nm), respectively. In order to understand the nature of free ligands were measured, upon excitation at ca. 280 nm for H2L1 and 295 nm for H2L2, which show the similar emissions at ca. 380 nm for H2L1 and 416 nm H2L2. In comparison to the free dicarboxylate ligands, the emission maximums of compounds 1–5 have changed and show blue or red shifts. It is possible that a combination of several factors together,16 including a change in the highest occupied molecular orbital and lowest unoccupied molecular orbital energy levels of deprotonated L12− and L22− anions and neutral ligands coordinating to metal centers, a charge-transfer transition between ligands and metal centers, and a joint contribution of the intraligand transitions or charge-transfer transitions between the coordinated ligands and the metal centers. However, since the ZnII ions are difficult to oxidize or reduce, these bands should also be assigned to the intraligand fluorescent emissions17 that are tuned by the metal–ligand interactions and deprotonated effect of the tricarboxyl ligands.
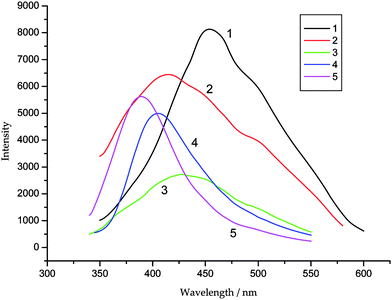 |
| | Fig. 6 The fluorescent emission spectra of 1–5 in solid state at room temperature. | |
Conclusion
Assembly of zinc(II) salt with 5-methyisophthalic acid (H2L1), 5-methoxyisophthalic acid (H2L2) and a series of rationally selected N-donor ligands, results in the formation of five coordination polymers with three diverse 2D layer structures, a 2-fold parallel interpenetrating 2D → 2D network, one 3-fold parallel interpenetrated 2D → 3D architecture and one 3-fold parallel interpenetrated 3D → 3D network. In addition, photoluminescence of the complexes were studied in the solid state at room temperature. The results revealed that both the dicarboxylate ligands and the flexibility of the bis(imidazole) ligands play an important role in governing the final structures of 1–5. It is believed that the preliminary results presented in this paper provide a promising access to the rational design and synthesis of metal–organic coordination polymers with specific structures and properties.
Acknowledgements
We thank the Natural Science Foundation of China (no. 21071074, 21073082 and 20771054) and 2009GGJS-104.
References
-
(a) L. Carlucci, G. Ciani, D. M. Proserpio and A. Sironi, J. Chem. Soc., Dalton Trans., 1997, 1801 RSC;
(b) A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouga, N. V. Zyk and M. Schröer, Coord. Chem. Rev., 2001, 222, 155 CrossRef CAS;
(c) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS;
(d) M. J. Zaworotko, Chem. Commun., 2001, 1 RSC;
(e) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae and M. Eddaoudi, Nature, 2003, 423, 705 CrossRef CAS;
(f) N. W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2005, 38, 176 CrossRef CAS;
(g) F. Fu, D. S. Li, X. M. Gao, M. Du, Y. P. Wu, X. N. Zhang and C. X. Meng, CrystEngComm, 2010, 12, 1227 RSC;
(h) D. S. Li, Y. P. Wu, P. Zhang, M. Du, J. Zhao and C. P. Li, Cryst. Growth Des, 2010, 10, 2037–2040 CrossRef CAS.
-
(a) J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. Jeon and K. Kim, Nature, 2000, 404, 982 CrossRef CAS;
(b) G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS;
(c) S. Takamizawa, E.-I. Nakata, H. Yokoyama, K. Mochizuki and W. Mori, Angew. Chem., Int. Ed., 2003, 42, 4331 CrossRef CAS;
(d) D. N. Dybtsev, H. Chun, S. H. Yoon, D. Kim and K. Kim, J. Am. Chem. Soc., 2004, 126, 32 CrossRef CAS;
(e) B. Kesanli, Y. Cui, M. R. Smith, E. W. Bittner, B. C. Bockrath and W. B. Lin, Angew. Chem., Int. Ed., 2005, 44, 72 CrossRef CAS;
(f) X. M. Gao, D. S. Li, J. J. Wang, F. Fu, Y. P. Wu, H. M. Hu and J. W. Wang, CrystEngComm, 2008, 10, 479 RSC;
(g) M. L. Zhang, D. S. Li, J. J. Wang, F. Fu, M. Du and Y. P. Wu, Dalton Trans., 2009, 5355 RSC.
-
(a) D. M. J. Dobe, C. H. Benison, A. J. Blake, D. Fenske, M. S. Jackson, R. D. Kay, W.-S. Li and M. Schröder, Angew. Chem., Int. Ed., 1999, 38, 1915 CrossRef CAS;
(b) M.-L. Tong, B.-H. Ye, J.-W. Cai, X.-M. Chen and S. W. Ng, Inorg. Chem., 1998, 37, 2645 CrossRef CAS;
(c) J.-W. Cheng, S.-T. Zheng and G.-Y. Yang, Dalton Trans., 2007, 4059 RSC;
(d) Y.-Q. Huang, Z.-L. Shen, Taka-aki. Okamura, Y. Wang, X.-F. Wang, W.-Y. Sun, J.-Q. Yu and N. Ueyama, Dalton Trans., 2008, 204 RSC;
(e) H. Gudbjartson, K. Biradha, K. M. Poirier and M. J. Zaworotko, J. Am. Chem. Soc., 1999, 121, 2599 CrossRef CAS;
(f) S.-I. Noro, S. Kitagawa, M. Kondo and K. Seki, Angew. Chem., Int. Ed., 2000, 39, 2081 CrossRef;
(g) D. M. Shin, I. S. Lee, Y. K. Chung and M. S. Lah, Chem. Commun., 2003, 1036 RSC;
(h) X.-H. Bu, W. Chen, W.-F. Hou, M. Du, R.-H. Zhang and F. Brisse, Inorg. Chem., 2002, 41, 3477 CrossRef CAS;
(i) M. Du, X.-J. Jiang and X.-J. Zhao, Inorg. Chem., 2006, 45, 3998 CrossRef CAS;
(j) S. Lopez and S. W. Keller, Inorg. Chem., 1999, 38, 1883 CrossRef CAS;
(k) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS.
-
(a) R. Q. Fang and X. M. Zhang, Inorg. Chem., 2006, 45, 4801 CrossRef CAS;
(b) W. X. Chen, S. T. Wu, L. S. Long, R. B. Huang and L. S. Zheng, Cryst. Growth Des., 2007, 7, 1171 CrossRef CAS;
(c) J. Yang, G. D. Li, J. J. Cao, Q. Yue, G. H. Li and J. S. Chen, Chem.–Eur. J., 2007, 13, 3248 CrossRef CAS;
(d) W. G. Lu, L. Jiang, X. L. Feng and T. B. Lu, Cryst. Growth Des., 2008, 8, 986 CrossRef CAS;
(e) Y. B. Go, X. Q. Wang, E. V. Anokhina and A. J. Jacobson, Inorg. Chem., 2005, 44, 8265 CrossRef CAS;
(f) Z. Z. Lin, F. L. Jiang, L. Chen, D. Q. Yuan, Y. F. Zhou and M. C. Hong, Eur. J. Inorg. Chem., 2005, 77 CrossRef CAS.
-
(a) D. F. Sun, R. Cao, Y. C. Liang, Q. Shi and M. C. Hong, J. Chem. Soc., Dalton Trans., 2002, 1847 RSC;
(b) X.-M. Zhang, M.-L. Tong and X.-M. Chen, Angew. Chem., Int. Ed., 2002, 41, 1029 CrossRef CAS;
(c) T. Ohmura, W. Mori, M. Hasegawa, T. Takei and A. Yoshizawa, Chem. Lett., 2003, 32, 34 CrossRef CAS;
(d) M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469 CrossRef;
(e) L. Hou, Y.-Y. Lin and X.-M. Chen, Inorg. Chem., 2008, 47, 1346 CrossRef CAS;
(f) T.-B. Lu, H. Xiang, R. L. Luck, L. Jiang, Z.-W. Mao and L. N. Ji, New J. Chem., 2002, 26, 969 RSC;
(g) W.-G. Lu, C.-Y. Su, T.-B. Lu, L. Jiang and J.-M. Chen, J. Am. Chem. Soc., 2006, 128, 34 CrossRef CAS;
(h) L. Pan, H.-M. Liu, X.-G. Lei, X.-Y. Huang, D. H. Olson, N. J. Turro and J. Li, Angew. Chem., Int. Ed., 2003, 42, 542 CrossRef CAS;
(i) S. K. Ghosh, R. Joan and P. K. Bharadwaj, Cryst. Growth Des., 2005, 5, 623 CrossRef CAS;
(j) L. Xu, S.-H. Yan, E. Y. Choi, J. Y. Lee and Y. U. Kwon, Chem. Commun., 2009, 3431 RSC.
-
(a) Z.-F. Tian, J.-G. Lin, Y. - Su, L.-L. Wen, Y.-M. Liu, H.-Z. Zhu and Q.-J. Meng, Cryst. Growth Des., 2007, 7, 1863 CrossRef CAS;
(b) M. Du, X.-J. Jiang and X. J. Zhao, Inorg. Chem., 2007, 46, 3984 CrossRef CAS;
(c) G.-B. Che, C.-B. Liu, B. Liu, Q.-W. Wang and Z.-L. Xu, CrystEngComm, 2008, 10, 184 RSC;
(d) D.-R. Xiao, E.-B. Wang, H.-Y. An, Y.-G. Li and L. Xu, Cryst. Growth Des., 2007, 7, 506 CrossRef CAS;
(e) Y.-Y. Liu, J.-F. Ma, J. Yang and Z.-M. Su, Inorg. Chem., 2007, 46, 3027 CrossRef CAS;
(f) K. Barthelet, D. Riou, M. Nogues and G. Férey, Inorg. Chem., 2003, 42, 1739 CrossRef CAS;
(g) A. M. Kirillov, M. N. Kopylovich, M. V. Kirillova, M. Haukka, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Angew. Chem., Int. Ed., 2005, 44, 4345 CrossRef CAS;
(h) M. Du, X.-J. Jiang and X.-J. Zhao, Chem. Commun., 2005, 5521 RSC;
(i) W.-G. Lu, J.-Z. Gu, L. Jiang, M.-Y. Tan and T.-B. Lu, Cryst. Growth Des., 2008, 8, 192 CrossRef CAS;
(j) H. Chun and H. Jung, Inorg. Chem., 2009, 48, 417 CrossRef CAS.
-
(a) L.-F. Ma, L.-Y. Wang, Y.-Y. Wang, S. R. Batten and J.-G. Wang, Inorg. Chem., 2009, 48, 915 CrossRef CAS;
(b) L.-F. Ma, L.-Y. Wang, D.-H. Lu, S. R. Batten and J.-G. Wang, Cryst. Growth Des., 2009, 9, 1741 CrossRef CAS;
(c) L.-F. Ma, Y.-Y. Wang, L.-Y. Wang, D.-H. Lu, S. R. Batten and J. G. Wang, Cryst. Growth Des., 2009, 9, 2036 CrossRef CAS;
(d) L.-F. Ma, L.-Y. Wang, Y.-Y. Wang, M. Du and J.-G. Wang, CrystEngComm, 2009, 11, 109 RSC;
(e) L.-F. Ma, L.-Y. Wang, J.-L. Hu, Y.-Y. Wang, S. R. Batten and J.-G. Wang, CrystEngComm, 2009, 11, 777 RSC;
(f) L.-F. Ma, Y.-Y. Wang, J.-Q. Liu, G.-P. Yang, M. Du and L.-Y. Wang, CrystEngComm, 2009, 11, 1800 RSC.
-
(a) H. Chun, H. Jung and J. Seo, Inorg. Chem., 2009, 48, 2043 CrossRef CAS;
(b) H. Chun and H. Jung, Inorg. Chem., 2009, 48, 417 CrossRef CAS;
(c) G. J. McManus, Z. Q. Wang and M. J. Zaworotko, Cryst. Growth Des., 2004, 4, 11 CrossRef CAS;
(d) L.-F. Ma, B. Liu and L.- Y. Wang,
et al.
, Dalton Trans., 2010, 39, 2301 RSC.
-
(a) B. F. Hoskins, R. Robson and D. A. Slizys, J. Am. Chem. Soc., 1997, 119, 2952 CrossRef CAS;
(b) E.-Q. Gao, Y.-X. Xu and C.-H. Yan, CrystEngComm, 2004, 6, 298 RSC;
(c) J. Fan, C. Slebodnick, R. Angel and B. E. Hanson, Inorg. Chem., 2005, 44, 552 CrossRef CAS;
(d) L. Zhang, X.-Q. Lü, C.-L. Chen, H.-Y. Tan, H.-X. Zhang and B.-S. Kang, Cryst. Growth Des., 2005, 5, 283 CrossRef CAS;
(e) L.-L. Wen, Y.-Z. Li, Z.-D. Lu, J.-G. Lin, C.-Y. Duan and Q.-J. Meng, Cryst. Growth Des., 2006, 6, 530 CrossRef CAS;
(f) P. C. M. Ducan, D. M. L. Goodgame, S. Menzer and D. J. Williams, Chem. Commun., 1996, 2127 RSC;
(g) X.-J. Li, R. Cao, W.-H. Bi, Y.-Q. Wang, Y.-L. Wang, X. Li and Z.-G. Guo, Cryst. Growth Des., 2005, 5, 1651 CrossRef CAS;
(h) G.-H. Cui, J.-R. Li, J.-L. Tian, X.-H. Bu and S. R. Batten, Cryst. Growth Des., 2005, 5, 1775 CrossRef CAS.
-
(a) J.-F. Ma, J. Yang, G.-L. Zheng, L. Li and J.-F. Liu, Inorg. Chem., 2003, 42, 7531 CrossRef CAS;
(b) J.-F. Ma, J. Yang, G.-L. Zheng, L. Li, Y.-M. Zhang, F.-F. Li and J.-F. Liu, Polyhedron, 2004, 23, 553 CrossRef CAS;
(c) J. Yang, J.-F. Ma, Y.-Y. Liu, J.-C. Ma, H.-Q. Jia and N.-H. Hu, Eur. J. Inorg. Chem., 2006, 1208 CrossRef CAS;
(d) J. Yang, J.-F. Ma, Y.-Y. Liu, S.-L. Li and G.-L. Zheng, Eur. J. Inorg. Chem., 2005, 2174 CrossRef CAS.
-
(a) W.-Y. Yang, H. Schmider, Q.-G. Wu, Y.-S. Zhang and S.-N. Wang, Inorg. Chem., 2000, 39, 2397 CrossRef CAS;
(b) Y.-Q. Gong, R.-H. Wang, Y.-F. Zhou, D.-Q. Yuan and M.-C. Hong, J. Mol. Struct., 2004, 705, 29 CrossRef CAS;
(c) W.-B. Lin, O. R. Evans, R.-G. Xiong and Z.-Y. Wang, J. Am. Chem. Soc., 1998, 120, 13272 CrossRef CAS;
(d) W.-B. Lin, Z.-Y. Wang and L. Ma, J. Am. Chem. Soc., 1999, 121, 11249 CrossRef CAS.
-
(a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 467 CrossRef;
G. M. Sheldrick, SHELXS97, Program for the Solution of Crystal Structures, University of Göttingen, Germany 1997 Search PubMed;
(b)
G. M. Sheldrick, SHELXS97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997 Search PubMed.
-
(a) Z. Shi, G.-H. Li, L. Wang, L. Gao, X.-B. Chen, J. Hua and S.-H. Feng, Cryst. Growth Des., 2004, 4, 25 CrossRef CAS;
(b) A. Majumder, S. Shit, C. R. Choudhury, S. R. Batten, G. Pilet, D. Luneau, N. Daro, J.-P. Sutter, N. Chattopadhyay and S. Mitra, Inorg. Chim. Acta, 2005, 358, 3855 CrossRef CAS.
-
(a) Q.-G. Wu, M. Esteghamatian, N.-X. Hu, Z. Popovic, G. Enright, Y. Tao, M. D'Iorio and S.-N. Wang, Chem. Mater., 2000, 12, 79 CrossRef CAS;
(b) J. E. McGarrah, Y.-J. Kim, M. Hissler and R. Eisenberg, Inorg. Chem., 2001, 40, 4510 CrossRef CAS;
(c) G. D. Santis, L. Fabbrizzi, M. Licchelli, A. Poggi and A. Taglietti, Angew. Chem., Int. Ed. Engl., 1996, 35, 202 CrossRef.
-
(a) S.-L. Zheng and X.-M. Chen, Aust. J. Chem., 2004, 57, 703 CrossRef CAS;
(b) S.-L. Zheng, J.-H. Yang, X.-L. Yu, X.-M. Chen and W.-T. Wong, Inorg. Chem., 2004, 43, 830 CrossRef CAS.
-
(a) S.-L. Li, Y.-Q. Lan, J.-F. Ma, J. Yang, G.-H. Wei, L.-P. Zhang and Z.-M. Su, Cryst. Growth Des., 2008, 8, 675 CrossRef CAS;
(b) S.-L. Li, Y.-Q. Lan, J.-F. Ma, Y.-M. Fu, J. Yang, G.-J. Ping, J. Liu and Z.-M. Su, Cryst. Growth Des., 2008, 8, 1610 CrossRef CAS.
-
(a) Y. Su, S.-Q. Zang, Y.-Z. Li, H.-Z. Zhu and Q.-J. Meng, Cryst. Growth Des., 2007, 7, 1277 CrossRef CAS;
(b) L.-L. Wen, D.-B. Dang, C.-Y. Duan, Y.-Z. Li, Z.-F. Tian and Q.-J. Meng, Inorg. Chem., 2005, 44, 7161 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)







