Organic salt of hydrogen L-tartaric acid: a novel wide-temperature-range ferroelectrics with a reversible phase transition†
De-Hong
Wu
,
Jia-Zhen
Ge
,
Hong-Lin
Cai
,
Wen
Zhang
* and
Ren-Gen
Xiong
*
Ordered Matter Science Research Center, Southeast University, Nanjing, 211189, P. R. China. E-mail: zhangwen@seu.edu.cn; xiongrg@seu.edu.cn; Fax: (+86)-25-52090626; Tel: (+86)-25-52090626
First published on 8th September 2010
Abstract
4-Ethylanilinium hydrogen (2R,3R)-tartrate, a novel wide-temperature-range ferroelectric was synthesized. DSC measurement discloses that the homochiral organic salt undergoes an isosymmetric reversible phase transition at about 186 K with a sharply narrow heat hysteresis of 0.7 K. The heat capacity Cp obtained from the calorimetric measurement exhibits a sharp peak at 185.8 K, characteristic of a first-order phase transition. However temperature-dependence dielectric constant measurements reveal no dielectric anomaly near the phase transition point. The measurement of the unit cell parameters except for c axis versus temperature suggests that the values change abruptly and remarkably between 180 and 190 K with the cell volume doubled. The crystal structures determined at 123(2) K (a = 7.461 Å, b = 11.930 Å, c = 14.873 Å, α = 95.34°, β = 91.95°, γ = 107.92°) and 298(2) K (a = 6.078 Å, b = 7.478 Å, c = 14.951 Å, α = 87.66°, β = 82.69°, γ = 71.80°) also show that the phase transition could be a type of isosymmetric change with the same triclinic space group P1 (No. 1). Structural analysis shows that the different modes of hydrogen bonds probably affect the configurations of the phenyl rings from the cations, consequently leading to a reversible structural phase transition.
Introduction
The influence of hydrogen-bonded interactions on the crystal packing and geometry of organic molecular solids is well known.1,2 Ever since Pasteur carried out the first reported separation of crystalline enantiomers,3tartaric acid (TA) has held a firm place in the archives of ground-breaking chemical discoveries. TA has two kinds of different proton-donor centers, two carboxylic and two hydroxyl groups (pKa1 = 3.0 and pKa2 = 4.3 in H2O)4 and, therefore, is potentially capable of forming monovalent (semi-tartrate) or divalent (tartrate) anions to afford 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 proton-transfer salts with most Lewis bases. However, with stoichiometric control it is possible to selectively form 1
2 proton-transfer salts with most Lewis bases. However, with stoichiometric control it is possible to selectively form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hydrogen tartrates, and the crystal structures of a large number of these 1
1 hydrogen tartrates, and the crystal structures of a large number of these 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 salts have been reported. More recently, its utility as an agent for the introduction of chirality in achiral organic compounds for the generation of crystalline materials with potentially useful non-linear optical properties has been also explored.5–9
1 salts have been reported. More recently, its utility as an agent for the introduction of chirality in achiral organic compounds for the generation of crystalline materials with potentially useful non-linear optical properties has been also explored.5–9
(2R,3R)-tartaric acid (L-(+)-TA) was chosen for this study because a great number of potential hydrogen-bond donors and acceptors are present, and its chirality guarantees its salts crystallize in the chiral group which meets with the requirements of ferroelectric space groups or ten polar point groups (C1, Cs, C2, C2v, C3, C3v, C4, C4v, C6, C6v or 1, 2, 3, 4, 6, m, mm2, 3m, 4mm, 6mm) while the ferroelectric materials have found widespread applications in the modern high-technology field.10,11 Along this line, herein we report a novel salt composed of [EtPhNH3]+·[HOCOCH(OH)CH(OH)COO]− (1), of which is interesting to note that the homochiral organic salt undergoes a reversible isosymmetric phase transition at about 186 K. To the best of our knowledge, 1 represents the first example of a homochiral organic salt undergoing a reversible phase transition in the known homochiral compounds.12
Experimental
L-(+)-TA (Aldrich) and 4-ethylbenzenamine (Aldrich) were used as commercial products without further purification. The 4-ethylanilinium hydrogen L-(+)-tartrate was prepared by dissolving equimolar amouts of L-(+)-TA and 4-ethylbenzenamine in a methanol solution at room temperature, the mixtures were set aside to crystallize to obtain the transparently colorless single crystals within a few days. Yield: 97%. m.p. 159–161 °C with decomp. 1H NMR (DMSO-d6): δ 1.09 (t, J = 7.5 Hz, 3H, CH3), 2.42 (q, J = 7.5 Hz, 2H, CH2), 4.35 (s, 2H, CHOH), 6.54 (d, J = 8.0 Hz, 2H, C6H4), 6.86 (d, J = 8.0 Hz, 2H, C6H4), 7.57 (s, 6H, OH and NH). 13C NMR (DMSO-d6): δ 16.51 (CH3), 27.84 (CH2), 72.62 (CHOH), 115.10, 128.53 (CH in phenyl ring), 132.21, 145.62 (quaternary C in phenyl ring), 173.68 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). IR (cm−1, KBr pellet): 3325, 2907, 2597, 1714, 1575, 1513.
O). IR (cm−1, KBr pellet): 3325, 2907, 2597, 1714, 1575, 1513.
Results and discussion
Structure discussion
Complex 1 crystallizes in triclinic space group P1 (No. 1). Fig. 1 shows its molecular structure with labeling scheme of LT phase (low temperature, 123 K) and RT phase (room temperature, 298 K). Crystallographic data and structural refinement details of the LT phase and RT phase are listed in Table 1. | ||
| Fig. 1 A view of asymmetric unit of 1 with atomic numbering scheme at 298(2) K (left) and 123(2) K (right). Displacement ellipsoids were dawn at 30% probability level. Hydrogen atoms in 123(2) K were omitted for clarity. | ||
| LT (123 K) phase | RT(298 K) phase | |
|---|---|---|
| CCDC number | 773838 | 773839 |
| chemical formula | C8H12N C4H5O6 | C8H12N C4H5O6 |
| Crystal size/mm | 0.36 × 0.32 × 0.30 | 0.36 × 0.32 × 0.30 |
| Formula weight | 271.27 | 271.27 |
| T/K | 123(2) | 298(2) |
| Radiation | Mo-Kα (0.71073 Å) | Mo-Kα (0.71073 Å) |
| Crystal system | Triclinic | Triclinic |
| Space group | P1 | P1 |
| a/Å | 7.4606(12) | 6.0782(12) |
| b/Å | 11.9298(18) | 7.4777(15) |
| c/Å | 14.8726(9) | 14.9506(10) |
| α (°) | 95.338(2) | 87.655(10) |
| β (°) | 91.950(1) | 82.692(10) |
| γ (°) | 107.916(1) | 71.802(1) |
| V/Å3 | 1251.3(3) | 640.29(19) |
| Z | 4 | 2 |
| ρ c/g cm−3 | 1.440 | 1.407 |
| μ/mm−1 | 0.116 | 0.113 |
| F(000) | 576 | 288 |
| θ range (deg) | 3.11–27.49 | 3.18–27.48 |
| Reflns collected | 13753 (Rint = 0.0298) | 6591 (Rint = 0.0357) |
| Indep. reflns | 5681 | 2930 |
| Reflns obs.[I > 2σ(I)] | 4661 | 2745 |
| Data/restr./paras | 5681/3/705 | 2930/3/353 |
| GOF | 1.001 | 1.057 |
| R 1, wR2[I > 2σ(I)] | 0.0386/0.0867 | 0.0411/0.1009 |
| R 1, wR2 (all data) | 0.0495/0.0929 | 0.0440/0.1029 |
| larg. peak/hole/e Å−3 | 0.245/−0.219 | 0.235/−0.194 |
The structural determination of LT phase or RT phase reveals distinctive hydrogen bonded anion bilayer-sheets with cations being pendant from both faces of the sheet, via three N–H⋯O hydrogen bonds. Another feature of interest in the tartrate structures was the very wide range of anion substructures observed, including a variety of chains of fused rings and a variety of sheet substructures, as well as three-dimensional frameworks of anions encapsulating large voids which enclose the cations.
Furthermore, the crystal structure of 4-ethylanilinium hydrogen L-(+)-tartrate consists of two L-(+)-tartrate and two 4-ethylanilinium cations in the asymmetric unit at RT phase. In comparison, four L-(+)-tartrate and four 4-ethylanilinium ions in the asymmetric unit were found at LT phase. Both the structure of the RT phase and that of the LT phase are characterized by an extensive hydrogen bonding network. There are infinite chains of hydrogen bonds of the type COO−⋯HO2C, involving the usually found short O–H⋯O (∼2.6 Å) contacts between the monoanionic L-(+)-tartrate in a “head-to-tail” fashion. 4-Ethylanilinium cations are antiparallel to each other. At the same time, the two adjacent infinite chains of anions are interlinked by N–H⋯O types of hydrogen bonds provided by the cations. When 4-ethylbenzenamine was co-crystallized with L-(+)-TA, the salt, 1 was produced showing the presence of complete single-proton transfer from L-(+)-TA to the N atom of 4-ethylbenzenamine. The hydrogen tartrate anions then form a three-dimensional hydrogen-bonded substructure through carboxylate interactions with other tartrate carboxylic acid and hydroxyl groups (Table 2).
| D–H⋯A | H⋯A | D⋯A | ∠DHA | |
|---|---|---|---|---|
| a Symmetry codes: (i) x + 1, y, z; (ii) x − 1, y, z; (iii) x, y + 1, z; (iv) x − 1, y, z + 1; (v) x + 1, y + 1, z; (vi) x, y + 1, z + 1; (vii) x − 1, y + 1, z; (viii) x − 1, y, z; (ix) x − 1, y − 1, z; (x) x, y − 1, z; (xi) x + 1, y, z. | ||||
| (A) Intra-anion hydrogen bonds | ||||
| LT phase | O3–H3⋯O2 | 2.07 | 2.580(3) | 120.3 |
| O4–H4D⋯O5 | 2.13 | 2.625(3) | 118.9 | |
| O9–H9⋯O8 | 2.06 | 2.573(3) | 120.0 | |
| O10–H10⋯O12 | 2.15 | 2.638(3) | 118.1 | |
| O15–H15A⋯O14 | 2.08 | 2.578(2) | 119.3 | |
| O15–H15A⋯O23 | 2.10 | 2.727(3) | 133.7 | |
| O16–H16A⋯O15 | 2.58 | 2.927(2) | 107.4 | |
| O21–H21A⋯O20 | 2.08 | 2.586(2) | 119.3 | |
| O22–H22⋯O21 | 2.59 | 2.922(3) | 105.8 | |
| RT phase | O3–H3⋯O1 | 2.15 | 2.640(3) | 118.1 |
| O4–H4A⋯O6 | 2.07 | 2.578(3) | 119.4 | |
| O10–H10⋯O12 | 2.08 | 2.583(2) | 119.0 | |
| O9–H9⋯O10 | 2.54 | 2.898(3) | 108.0 | |
| (B) Inter-anion hydrogen bonds | ||||
| LT phase | O6–H6⋯O1ii | 1.78 | 2.587(3) | 167.5 |
| O11–H11⋯O7ii | 1.78 | 2.588(3) | 170.0 | |
| O16–H16A⋯O7iv | 2.05 | 2.728(3) | 140.2 | |
| O18–H18⋯O13ii | 1.74 | 2.551(2) | 170.8 | |
| O21–H21A⋯O17v | 2.09 | 2.713(3) | 132.3 | |
| O22–H22⋯O1vi | 2.06 | 2.739(3) | 139.2 | |
| O24–H24A⋯O19ii | 1.73 | 2.538(3) | 169.8 | |
| RT phase | O2–H2⋯O5x | 1.80 | 2.601(3) | 165.9 |
| O7–H7A⋯O11x | 1.75 | 2.553(3) | 167.5 | |
| O9–H9⋯O5xi | 2.07 | 2.755(3) | 140.8 | |
| O10–H10⋯O8xi | 2.14 | 2.757(3) | 132.1 | |
| (C) Cation-anion hydrogen bond | ||||
| LT phase | N1–H1D⋯O14 | 1.84 | 2.712(3) | 165.3 |
| N1–H1E⋯O1 | 1.89 | 2.775(3) | 170.7 | |
| N1–H1F⋯O15i | 1.92 | 2.753(3) | 154.6 | |
| N2–H2C⋯O3 | 1.98 | 2.862(3) | 174.0 | |
| N2–H2D⋯O7ii | 2.41 | 3.260(3) | 159.5 | |
| N2–H2D⋯O10ii | 2.48 | 2.939(3) | 112.6 | |
| N2–H2E⋯O2ii | 1.89 | 2.762(3) | 165.7 | |
| N3–H3A⋯O9 | 2.00 | 2.883(3) | 169.2 | |
| N3–H3B⋯O1iii | 2.38 | 3.225(3) | 159.3 | |
| N3–H3C⋯O8ii | 1.87 | 2.736(3) | 165.0 | |
| N4–H4A⋯O20ii | 1.81 | 2.679(3) | 163.6 | |
| N4–H4B⋯O13iii | 1.90 | 2.783(3) | 171.6 | |
| N4–H4C⋯O21 | 1.94 | 2.784(3) | 157.6 | |
| RT phase | N1–H1D⋯O4vii | 2.00 | 2.880(3) | 169.6 |
| N1–H1E⋯O5 | 2.49 | 3.350(4) | 161.6 | |
| N1–H1E⋯O3 | 2.57 | 3.048(3) | 114.6 | |
| N1–H1F⋯O6vii | 1.88 | 2.749(3) | 164.9 | |
| N2–H2C⋯O12ix | 1.83 | 2.704(3) | 165.8 | |
| N2–H2D⋯O11x | 1.92 | 2.798(3) | 170.8 | |
| N2–H2E⋯O10vii | 1.95 | 2.792(3) | 156.9 | |
In the structure of the RT phase, the hydrogen tartrate ions are connected in infinite two-dimensional bilayer-sheets via relatively short O–H⋯O hydrogen bonds between hydroxyl, carboxyl, and carboxylate groups. The hydroxyl O3, O4, O10 and O9 atoms act as donors to the carboxylate O1, O6, O12 and the hydroxyl O10 atoms in an intra-anion hydrogen bond, respectively. The carboxyl O3 and O7 atoms act as donors to the carboxylate O5x and O11x atoms in an inter-anion hydrogen bond, respectively. Also the hydroxyl O9 and O10 atoms act as donors to the carboxylate O5i and O8i atoms in an inter-anion hydrogen bond, respectively. Furthermore the two layer sheets are interlocked by inter-anion O–H⋯O hydrogen bonds. The N atom of the cation is linked to the O atoms of the anions, acting as a threefold donor in N2–H2C⋯O12ix, N2–H2D⋯O11x, N2–H2E⋯O10viihydrogen bonds or a fourfold donor in N1–H1D⋯O4vii, N1–H1E⋯O5, N1–H1E⋯O3, N1–H1F⋯O6vii ones (Table 2). The majority of these hydrogen bonds are of a two-centre type and the minority a three-centre type of N1–H1E⋯(O)2, O9–H⋯(O)2 and O10–H⋯(O)2.
In the structure of the LT phase, the hydrogen tartrate ions are connected in infinite two-dimensional bilayer-sheets via relatively short O–H⋯O hydrogen bonds between the hydroxyl, carboxyl, and carboxylate groups. The hydroxyl O3, O4, O9, O10, O21, O16 and O22 atoms act as donors to the carboxylate O2, O5, O8, O12, O20, and the hydroxyl O15 and O21 atoms in an intra-anion hydrogen bond, respectively. The hydroxyl O15 atom acts as a donor to the carboxylate O14 and O23 atoms in an intra-anion with a three-centre type of hydrogen bond O15–H⋯(O)2. The carboxyl O6, O11, O18 and O24 atoms act as donors to the carboxylate O1ii, O7ii, O13ii and O19ii atoms in an inter-anion hydrogen bond, respectively. The hydroxyl O16, O21 and O22 atoms act as donors to the carboxylate O7iv, O17v and O1vi atoms in an inter-anion hydrogen bond, respectively. Furthermore the two layer sheets are interlocked by inter-anion O–H⋯O hydrogen bonds. The N atom of the cation is linked to the O atoms of the anions, acting as a threefold donor in N1–H⋯O, N3–H⋯O, N4–H⋯O hydrogen bonds or as a fourfold donor in N2–H⋯O (Table 2). The majority of these hydrogen bonds are of a two-centre type and the minority a three-centre type of N2–H2D⋯(O)2, O15–H⋯(O)2, O16–H⋯(O)2, O21–H⋯(O)2 and O22–H⋯(O)2.
The hydrogen tartrate ions in both the RT phase and LT phase are interlinked viahydrogen bonds into infinite two-dimensional bilayer-sheets. And some other non-classic intra- and inter-molecular C–H⋯O weak hydrogen-bonding interactions are present in the LT and RT phase.
In the structure of the LT phase, the phenyl rings (C3 to C8) and (C19 to C24) are nearly antiparallel to the phenyl rings (C11 to C16) and (C27 to C32), respectively, with dihedral angles of 3.6° and 0.9°. But the phenyl ring (C3 to C8) is un-parallel to the phenyl ring (C19 to C24) with a dihedral angle of 24.0°. Also, the phenyl ring (C11 to C16) is un-parallel to (C27 to C32) with a dihedral angle of 20.7°. As a result, in the LT phase, there are two sets of parallel phenyl rings with a dihedral angle of about 22.3°. In the structure of the RT phase, the phenyl ring (C3 to C8) is nearly antiparallel to the phenyl ring (C11 to C16) with a dihedral angle of 1.5°. Thus, in RT phase, all the phenyl rings are parallel (Fig. 2 and 3).
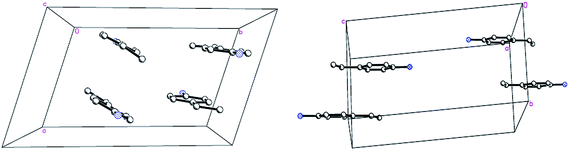 | ||
| Fig. 2 Packing diagrams of 4-ethylanilinium cation moieties in 1 at 123(2) K (left) and 298(2) K (right). The hydrogen tartrate anions and all hydrogen atoms were omitted for clarity. | ||
 | ||
| Fig. 3 Packing diagrams of 4-ethylanilinium cation moieties in 1 at 123(2) K (left) and 298(2) K (right) viewed along the b axis and a axis, respectively. The hydrogen tartrate anions and all hydrogen atoms were omitted for clarity. | ||
X-ray crystal structures of 1 under different temperatures at 373 K, 298 K, 233 K, 223 K, 213 K, 203 K, 193 K, 183 K, 173 K and 123 K were measured. The cell parameters of 1 measured at the temperature from 373 K to 193 K show very small differences with those measured at room temperature when the temperature influence on the cell parameters, i.e., the thermal expansion and contraction, is excluded. Thus above 193 K, no phase transition occurred in 1. Interestingly, the cell parameters of 1 at 183 K change abruptly and the cell volume is nearly doubled at 183 K vs. 193 K, indicating a first-order phase transition.13 The cell parameters of 1 measured at the temperature from 183 K to 93 K show very small differences, indicating no phase transition from 183 K to 93 K (Fig. 4).
 | ||
| Fig. 4 Temperature dependence of unit-cell parameters of 1: (left) unit-cell lengths and (right) cell volume. | ||
DSC measurement
The DSC measurement was performed to detect whether compound 1 undergoes a phase transition triggered by temperature or not. Upon heating and cooling, the crystalline sample undergoes a single phase transition at ca. 186 K, showing an exothermic peak at 186.4 K and an endothermic peak at 185.7 K (Fig. 5). The two observed peaks demonstrate a reversible phase transition with a heat hysteresis of 0.7 K. Both the narrow thermal hysteresis and the shape of the peaks testify the character of a first-order phase transition, in good agreement with that found in variable-temperature X-ray single crystal diffraction. | ||
| Fig. 5 DSC curves obtained on heating-cooling for 1 (scanning rate 10 K min−1, sample mass 16.28 mg). | ||
Heat capacity
The calorimetric measurement exhibits a sharp peak at 185.8 K, being characteristic of a first-order phase transition (Fig. 6). From the curve, a heat change of ΔQ ≈ 0.61 J g−1 and an entropy change of ΔS ≈ 3.28 × 10−3 J g−1 K−1 = 0.89 J K−1 mol−1 are obtained. Given that ΔS = RlnN, where N represents possible configurations, it is found that N ≈ 1.17, indicating that the phase transition of the system is not simply a displacive type but an order-disorder type.14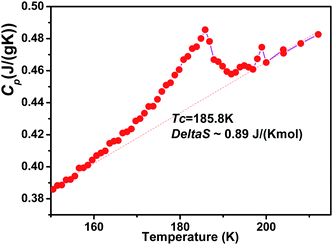 | ||
| Fig. 6 The temperature dependence of the heat capacity Cp of 1. The dotted base line was used for the evaluation of the entropy change (ΔS). | ||
Dielectric constant measurement
It is very useful for us to search for phase transitions through the variable-temperature dielectric constant response, especially in the relatively high frequency range. Owing to failure to get large crystals, the powder-pressed pellet of 1 was used in dielectric constant measurements. The temperature dependence of the dielectric constant measured at 10, 90 and 1000 kHz is shown in Fig. 7. Unexpectedly, no observable dielectric anomaly was observed. The dielectric constant is nearly equal to 4 under the three different frequencies from 86 K to 280 K, and shows a temperature-independent feature. The dielectric anomaly may be too weak to be observable in the specific system with narrow heat hysteresis and minor structural changes across the Tc. Further super-low frequency dielectric constant studies are in progress. According to D = εE + P (D = electric displacement, E = electric field, ε = dielectric constant, P = polarization), there is no dielectric anomaly at relative high frequency and the P ∼ D diagram should definitely be linear, i.e.1 should be a dielectric not ferroelectric. However, 1 definitely displays a ferroelectric space group with phase transition (Tc) and its properties are a typical feature for molecular ferroelectrics. Consequently, 1 is still a ferroelectric at very low frequency because the super-low frequency should be needed for the inversion of molecules with large size. The phase transition feature of 1 looks like BaTiO3 in which a ferroelectric-to-ferroelectric transition occurs at ca. 0 °C, and is different from Rochelle salt in which a paraelectric-to-ferroelectric phase transition occurs with the decrease of the temperature to 24 °C and a ferroelectric-to-paraelectric phase transition occurs with the decrease of the temperature to −18 °C.12 So, at relative high frequency, it is reasonable for one to fail to observe the dielectric anomaly.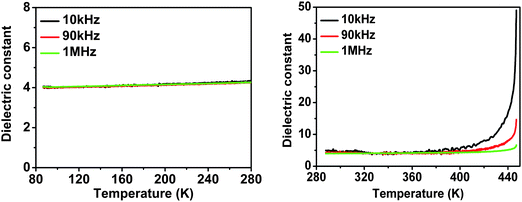 | ||
| Fig. 7 Temperature-dependent dielectric constants of 1 at 10, 90 and 1000 kHz: (left) 86 K to 280 K and (right) 286 K to 446 K. | ||
On the other hand, similar to the behavior of KTaO3 and SrTiO3, since the soft-mode is non-polar whose freezing wouldn't cause the order of ferroelectric or antiferroelectric phase, no dielectric anomaly could be observed around phase transition temperature. However, detailed measurements such as Raman and optical birefringence still need to be carried out to better understand its phase transformation.
Conclusion
In summary, the combined DSC, heat capacity measurement and structural analysis reveal that the 1 undergoes a reversible isosymmetric phase transition at ca. 186 K. The phase transition shows no observable dielectric anomaly. The hydrogen bonding interactions are very complicated for the two structures at 123 K and 298 K, maybe resulting from the configurations of the phenyl rings changing significantly with the dihedral angle of ca. 22°. This value indicates that the phase transition of the system is not simply a displacive type but a configurational order-disorder type.Acknowledgements
This work was supported by the China National Natural Science Foundations (20931002, 90922005) and Jiangsu province NSF (BK2008029). De-Hong Wu thanks China Postdoctoral Science Foundation funded project (20090451147), Jiangsu Planned Projects for Postdoctoral Research Funds (0802003B) and SEU Major Postdoctoral Research Funds (3212000901).References
- G. R. Desiraju, Crystal Engineering. The Design of Organic Solids, Elsevier,Amsterdam, 1989 Search PubMed.
- L. Pauling. The Nature of the Chemical Bond, Cornell University Press, New York, 3rd ed., 1960 Search PubMed.
- L. Pasteur, Ann. Chim. Phys., 1848, 24, 442 Search PubMed.
- E. P. Serjeant, B. Dempsey, Ionization Constants of Organic Acids in Aqueous Solutions, Pergamon Press, Oxford, 1979 Search PubMed.
- C. B. Aakeröy, P. B. Hitchcock and K. R. Seddon, J. Chem. Soc., Chem. Commun., 1992, 553 RSC.
- J. Fuller, R. T. Carlin, L. J. Simpson and T. E. Furtak, Chem. Mater., 1995, 7, 909 CrossRef CAS.
- M. K. Marchewska, S. Debrus, A. Pietraszko, A. J. Barnes and H. Ratajczak, J. Mol. Struct., 2003, 656, 265 CrossRef.
- S. Debrus, M. K. Marchewska, J. Baran, M. Drozd, A. Pietraszko and H. Ratajczak, J. Solid State Chem., 2005, 178, 2880 CrossRef CAS.
- S. Manivannan, S. Dhanuskodi, K. Kirchbaum and S. K. Tiwari, Cryst. Growth Des., 2006, 6, 1285 CrossRef CAS.
- (a) L.-Z. Chen, H. Zhao, J.-Z. Ge, R.-G. Xiong and H.-W. Hu, Cryst. Growth Des., 2009, 9, 3828 CrossRef CAS; (b) W. Zhang, L.-Z. Chen, R.-G. Xiong, T. Nakamura and S. D. Huang, J. Am. Chem. Soc., 2009, 131, 12544 CrossRef CAS; (c) W. Zhang, H.-Y. Ye and R.-G. Xiong, Coord. Chem. Rev., 2009, 253, 2980 CrossRef CAS.
- (a) R.-G. Xiong, J.-L. Zuo, X.-Z. You, H.-K. Fun and S. S. S. Raj, New J. Chem., 1999, 23, 1051 RSC; (b) H.-Y. Ye, D.-W. Fu, Y. Zhang, W. Zhang, R.-G. Xiong and S. D. Huang, J. Am. Chem. Soc., 2009, 131, 42 CrossRef CAS; (c) Y. Tajima, T. Matsuo and H. Suga, Nature, 1982, 299, 810 CrossRef CAS; (d) T. Kimura, T. Goto, H. Shintani, K. Ishizaka, T. Arima and Y. Tokura, Nature, 2003, 426, 55 CrossRef CAS.
- F. Jona, G. Shirane, Ferroelectric Crystals; Pergamon Press: New York, 1962 Search PubMed.
- (a) H.-Y. Ye, L.-Z. Chen and R.-G. Xiong, Acta Crystallogr., Sect. B: Struct. Sci., 2010, 66, 387 CrossRef; (b) W. Zhang, H.-Y. Ye, H.-L. Cai, J.-Z. Ge, R.-G. Xiong and S. D. Huang, J. Am. Chem. Soc., 2010, 132, 7300 CrossRef CAS; (c) H.-Y. Ye, J.-Z. Ge, F. Chen and R.-G. Xiong, CrystEngComm, 2010, 12, 1705 RSC; (d) D.-W. Fu, Y.-M. Song, G.-X. Wang, Q. Ye, R.-G. Xiong, T. Akutagawa, T. Nakamura, P. W. H. Chan and S. D. Huang, J. Am. Chem. Soc., 2007, 129, 5346 CrossRef CAS; (e) H. Zhao, Z.-R. Qu, H.-Y. Ye and R.-G. Xiong, Chem. Soc. Rev., 2008, 37, 84 RSC; (f) Q. Ye, X.-S. Wang, H. Zhao and R.-G. Xiong, Chem. Soc. Rev., 2005, 34, 208 RSC.
- P. Jain, V. Ramachandran, R. J. Clark, H. D. Zhou, B. H. Toby, N. S. Dalal, H. W. Kroto and A. K. Cheetham, J. Am. Chem. Soc., 2009, 131, 13625 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: IR and XRD for 1. CCDC reference numbers 773838 (LT (123 K) phase) and 773839 (RT (298 K) phase). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ce00109k |
| This journal is © The Royal Society of Chemistry 2011 |
