Surfactant-assisted hydrothermal synthesis and electrochemical properties of nanoplate-assembled 3D flower-like Cu3V2O7(OH)2·2H2O microstructures†
Xiujuan
Sun
a,
Jiawei
Wang
a,
Yan
Xing
*a,
Ying
Zhao
b,
Xianchun
Liu
a,
Bo
Liu
a and
Suying
Hou
a
aCollege of Chemistry, Northeast Normal University, Changchun, 130024, P. R. China. E-mail: xingyan69cn@yahoo.com.cn; Tel: +86-431-86105529
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry of Jilin University, Changchun, 130012, P. R. China
First published on 13th September 2010
Abstract
Micrometer-scale hierarchical flower-like structures of Cu3V2O7(OH)2·2H2O assembled by nanoplates were, for the first time, fabricated in a high yield via a simple hydrothermal process in the presence of cetyltrimethylammonium bromide (CTAB). X-Ray diffraction, Fourier-transform infrared spectroscopy (FTIR) spectrum, scanning electron microscopy and transmission electron microscopy were used to characterize the samples. The results demonstrated that CTAB played an important role as a soft template in the formation of the 3D Cu3V2O7(OH)2·2H2O architecture. The formation mechanism was preliminarily studied based on XRD studies and SEM observations by arresting the growth at a series of intermediate stages in the formation of the flower-like structures. Some factors influencing the morphologies of the Cu3V2O7(OH)2·2H2O flower-like micro/nanocomposite structures were systematically investigated. The electrochemical measurements revealed that the Cu3V2O7(OH)2·2H2O microflowers displayed a high discharge capacity.
Introduction
In recent years, the hierarchical assembly of 1D or 2D nanoscale building blocks into 3D arrayed superstructures or complex architectures has attracted intensive attention in materials chemistry and nanotechnology due to their important role in the systematic study of structure–property relationships and their novel collective optical, magnetic, and electronic properties.1–11 Despite several methods having been attempted for the synthesis of inorganic materials with complex patterns, it is highly desirable that the materials can be produced with low-cost and large-scale production capability. The hydrothermal method, a solution-phase chemical method, is one of the most promising routes due to its low growth temperature, potential for scaled-up fabrication, and many adjustable parameters that allow control of the final size and morphology of the products.12–14 In most hydrothermal routes in the synthesis of well structured materials with controlled morphology, various types of surfactants have been widely used due to their efficient self-assembly properties.15–17Transition metal vanadates have long been studied as potential battery materials for primary or secondary lithium battery applications.18–20 Among them, copper vanadate is a very promising cathode candidate for primary lithium batteries used in long-term implantable cardioverter defibrillators (ICD) due to its layered structure and multistep reductions during the insertion/intercalation of lithium. Some recent efforts have been dedicated to the synthesis of copper vanadate nanocrystals with various morphologies via different ways. For example, α-CuV2O6 nanowires have been successfully synthesized hydrothermally and the nanowires displayed high discharge capacities as well as excellent high-rate capability.21 Recently Zhang's group prepared Cu3V2O7(OH)2·2H2O nanowires, nanoflakes, and nanoparticles through a simple template-free solution method.22 Moreover, Melghit et al. reported the soft chemistry synthesis of MxV2O5+ε·nH2O (M = Co, Ni, Cu, Zn) nanowires.23Very recently, single-crystalline nanowires β′-CuxV2O5nanowires have been synthesized by the hydrothermal reduction of bulk CuV2O6 using small-molecule aliphatic alcohols as reducing agents.24 However, to the best knowledge, there has been no report on the synthesis of 3D copper vanadates micro/nanocomposite structure. Herein, we for the first time report the synthesis of Cu3V2O7(OH)2·2H2O 3D uniform hierarchical architecture microflowers by a simple hydrothermal process with the assistance of CTAB, their electrochemical properties are also investigated in detail.
Experimental section
Synthesis
All chemical reagents were of analytical grade and used as received without further purification. In a typical procedure, 0.5 mmol of CTAB was dissolved in 4 mL deionized water at room temperature, followed by the addition of 0.1 mmol of Cu2(OH)2CO3 under stirring. And 0.3 mmol of NH4VO3 was dissolved in another 4 mL of deionized water at 80 °C. Then the NH4VO3 solution was added slowly to the Cu2(OH)2CO3 solution under strong magnetic stirring. Finally, the resulting mixture was transferred into a 15 mL Teflon-lined stainless steel autoclave and heated at 80 °C for a given time. After the autoclave was cooled to room temperature naturally, the resulting yellow precipitates were collected, washed with distilled water and absolute ethanol. Then the final products were dried under vacuum at 60 °C for 4 h.Characterization
X-Ray powder diffraction (XRD) analysis was measured on a Siemens D5005 Diffractometer with CuKα radiation (λ = 0.15418 nm) and 2θ ranging from 10° to 70°. Fourier-transform infrared spectroscopy (FT-IR) spectra was recorded within the 500–4000 cm−1 region on a Nicolet Impact 410 FTIR spectrometer using KBr pellets. Field-emission scanning electron microscopy (FESEM) images were obtained with a JSM-6700F microscope. Transmission electron microscopy (TEM) images, high-resolution transmission electron microscopy (HRTEM) images were obtained on a JEM-2100F microscope with an accelerating voltage of 200 kV.The working electrode was fabricated by dispersing 80 wt% active materials (namely, the as-prepared Cu3V2O7(OH)2·2H2O microflowers), 10 wt% carbon black, and 10 wt% polyvinylidene fluoride (PVDF) binder in the N-methylpyrrolidone (NMP) solvent to form a homogeneous slurry. The formed slurry was coated onto an aluminium foil. After solvent evaporation at room temperature and drying in a vaccuum oven at 120 °C for 12 h, the electrodes were assembled into coin-like cells (CR2025) with lithium metal as the counter and reference electrode. The electrolyte solution was 1.0 mol L−1LiPF6 dissolved in a mixture of ethylene carbonate (EC) and diethyl carbonate (DEC) solution with the volumetric ratio of EC/DEC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cells were constructed and handled in an Ar (99.999%) filled glovebox. The capacity of the electrode was measured by a galvanostatic discharged method at different current densities to the cutoff voltage of 1.5 V at room temperature (25 °C) on the electrochemical test instrument (CT2001A, Wuhan Land Electronic Co. Ltd., China). The cyclic voltammogram (CV) was obtained using a CHI1120A potentiostat analyzer at a scanning rate of 5 mV s−1 at room temperature.
1. The cells were constructed and handled in an Ar (99.999%) filled glovebox. The capacity of the electrode was measured by a galvanostatic discharged method at different current densities to the cutoff voltage of 1.5 V at room temperature (25 °C) on the electrochemical test instrument (CT2001A, Wuhan Land Electronic Co. Ltd., China). The cyclic voltammogram (CV) was obtained using a CHI1120A potentiostat analyzer at a scanning rate of 5 mV s−1 at room temperature.
Results and discussion
Characterization of Cu3V2O7(OH)2·2H2O microflowers
The phase compositions and phase structures of the samples were examined by X-ray powder diffraction (XRD). As shown in Fig. 1(a), all diffraction peaks can be indexed to monoclinic structured Cu3V2O7(OH)2·2H2O with lattice constants a = 1.061 nm, b = 0.5860 nm, c = 0.7205 nm, and β = 94.86° (JCPDS card No. 46-1443), and no impurity phase can be detected. The strong and sharp diffraction peaks indicate that the as-obtained products are well-crystalline.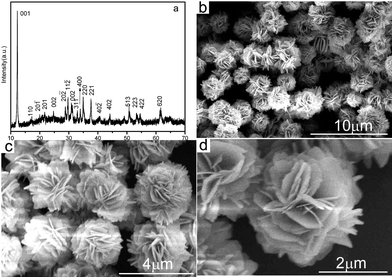 | ||
| Fig. 1 XRD pattern (a) and FE-SEM images with different magnifications (b–d) of the Cu3V2O7(OH)2·2H2O microflowers. | ||
The morphology of the as-prepared products under hydrothermal treatment at 80 °C for 24 h was examined by field-emission scanning electron microscopy (FE-SEM). A large number of uniform Cu3V2O7(OH)2·2H2O flower-like microstructures with an average size of 2 μm were formed, as shown in Fig. 1(b). The as-obtained flower-like microstructures could not be destroyed and broken into discrete individual nanoplates even by subjecting their aqueous suspension to ultrasonication for a much longer time indicating that the microflowers were not a random aggregate of nanoplates but the ordered self-assemble of themselves. More detailed morphologies, as displayed in Fig. 1(c) and 1(d), show that the flower-like structure was composed of abundant 2D nanoplates with thickness of 40 to 80 nm. More importantly, many pores with different diameter sizes, which may improve the physicochemical properties, were found among the nanoplates in the superstructures.
Further structural characterization of the Cu3V2O7(OH)2·2H2O nanostructures was conducted using transmission electron microscopy (TEM) and high-resolution TEM (HRTEM). Fig. 2(a) shows a typical TEM image of 3D flower-like structure, revealing that the entire flower is built from thin nanoplates, which is in accordance with the SEM images. A representative HRTEM image at the edge of an individual Cu3V2O7(OH)2·2H2O nanoplate was shown in Fig. 2(b), which further supports the claim of crystallinity for Cu3V2O7(OH)2·2H2O flowers. The lattice fringes are clearly visible with a spacing of 0.333 nm, which agrees well with the lattice spacing of (002) of monoclinic Cu3V2O7(OH)2·2H2O.
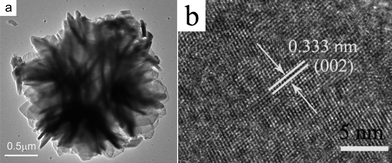 | ||
| Fig. 2 TEM image (a) and HRTEM image (b) of the Cu3V2O7(OH)2·2H2O micro/nanocomposite structure. | ||
Formation mechanism of the as-obtained Cu3V2O7(OH)2·2H2O microflowers
The integration of FESEM images and the corresponding XRD patterns of the different intermediate samples at different reaction stages at 80 °C clearly reveal the growth process of the hierarchical flower-like structures of Cu3V2O7(OH)2·2H2O. Initially, a light green suspension was obtained after hydrothermal treatment for 0.5 h, all of the diffraction peaks in Fig. 3(a) can be indexed to a monoclinic phase of Cu2(OH)2CO3 (JCPDS No. 41-1390) at this stage, suggesting that Cu2(OH)2CO3 did not begin to react with NH4VO3 at the early stage. The FE-SEM image (Fig. 4(a)) shows this sample was mainly composed of 2D thin nanosheets. With increase of the reaction time to 2 h, the products were the mixture of light green and yellow precipitates. The corresponding XRD pattern in Fig. 3(b) shows that monoclinic Cu3V2O7(OH)2·2H2O (JCPDS card No. 46-1443) crystalline phase starts to form, whereas there is still monoclinic phase Cu2(OH)2CO3 in existence too. The FE-SEM image (Fig. 4(b)) shows that many sphere-like shapes consisted of sheets formed after hydrothermal treatment for 2 h. When the reaction time was prolonged to 8 h, only a yellow precipitate was obtained, and underdeveloped flower-like microstructures were observed in the products as shown in Fig. 4(c), meanwhile highly pure phase Cu3V2O7(OH)2·2H2O could be obtained as shown in Fig. 3(c). When the reaction time was increased to 16 h or longer, well-defined microflowers with narrowly distributed diameters were obtained, as shown in Fig. 4(d). The results reveal that reaction time is the main factor to form nanoplate-based microflowers, that is, the Ostwald ripening process is indispensable. Such a similar process has occurred in the preparation of Fe2O3 and CeO2 microflowers.25,26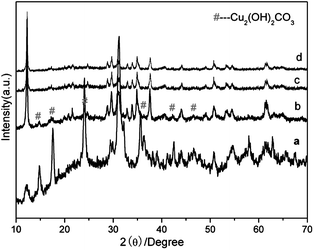 | ||
| Fig. 3 XRD patterns of the samples obtained by hydrothermal treatment at 80 °C for different time intervals: (a) 0.5 h, (b) 2 h, (c) 8 h, (d) 16 h. | ||
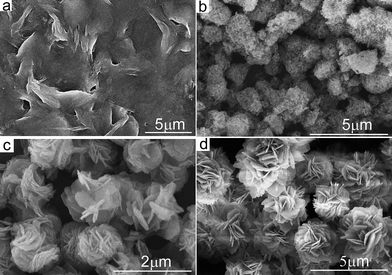 | ||
| Fig. 4 SEM images of the samples obtained by hydrothermal treatment at 80 °C for different time intervals. (a) 0.5 h, (b) 2 h, (c) 8 h and (d) 16 h. | ||
In a control experiment, for the products prepared in the absence of CTAB with the other parameters kept the same, most of the petals could not assemble into a 3D flower-like structure (see ESI Fig. S1a†). Therefore, it can be assumed that CTAB molecules facilitate the assembly of petals into the 3D flower-like structure through the interactions between the organic molecules CTAB and the inorganic petals, similar to the cases in the reported literature.1,27,28 Another experiment was carried out by using an appropriate dosage of cetyltrimethyl chloride (CPC), which has unique structural properties in common with CTAB as the soft template. Larger microspheres composed of thick plates were obtained (see ESI Fig. S1b†) under similar conditions. If sodium dodecyl sulfate (SDS) was used instead of CTAB, the products were only the aggregation of nanofibers (see ESI Fig. S1c†). While the use of a triblock copolymer of P123 (EO20PO70EO20) resulted in irregular nanoplates (see ESI Fig. S1d†).
The Cu source in our experiment also plays a vital role in the formation of the as-prepared flower-like microstructures. Parallel experiments were carried out by substituting Cu2(OH)2CO3 with CuSO4·5H2O, CuCl2·2H2O and Cu(NO3)2·5H2O, respectively. As shown in Fig. S2†, only irregular nanocrystals can be formed under these conditions. Moreover, when the reaction temperature was increased to 180 °C, keeping other reaction conditions constant, only nanobelts were obtained (see ESI Fig. S3†).
Electrochemical properties
Fig. 5 shows the initial discharge curves of the electrodes with the as-prepared 3D Cu3V2O7(OH)2·2H2O microflowers at different current densities at room temperature (25 °C). All the discharge curves exhibit a voltage plateau around 1.8 V. To the cutoff voltage of 1.5 V, the discharge capacity of Cu3V2O7(OH)2·2H2O microflowers is as high as 620 mAh g−1 at 20 mAh g−1. In comparison, the discharge capacities of Cu3V2O7(OH)2·2H2O microflowers at current densities of 25 mAh g−1 and 40 mAh g−1 were 575 mAh g−1 and 525 mAh g−1 at 25 °C, respectively, which are still much higher than that for either the as-synthesized bulk particles (326 mAh g−1) or the previously reported bulk CuV2O629 and Ag2V4O11.30 It is known that the discharge capacity of the cell increased as the operating temperature was raised.31,32 Therefore, the as-prepared Cu3V2O7(OH)2·2H2O microflowers would exhibit better electrochemical performance when this material discharged at 37 °C.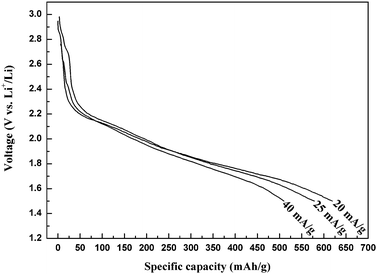 | ||
| Fig. 5 Discharge curves of the electrodes made from the as-prepared Cu3V2O7(OH)2·2H2O microflowers at different current densities at 25 °C. | ||
Fig. 6 displays the cyclic voltammetry (CV) curve of the electrode fabricated from Cu3V2O7(OH)2·2H2O microflowers in the first cycle at a scan rate of 5 mV s−1 at 25 °C with the potential range of 4.0–1.5 V vs. Li+/Li. As shown in Fig. 6, there is a cathodic peak at about 1.8 V, which is in good agreement with the initial discharge curves.
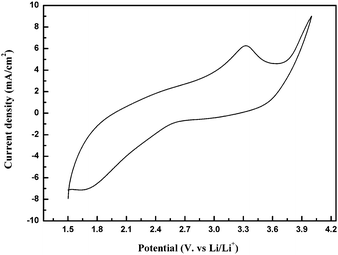 | ||
| Fig. 6 CV of the electrodes made from the Cu3V2O7(OH)2·2H2O microflowers at a scan rate of 5 mV s−1 and the temperature of 25 °C. | ||
Conclusions
In summary, 3D flower-like Cu3V2O7(OH)2·2H2O microflowers with a diameter of 2 μm, which were assembled by nanoplates with a thickness of 40 to 80 nm can be prepared by the surfactant CTAB assisted hydrothermal method. CTAB played a crucial role in directing the formation of 3D Cu3V2O7(OH)2·2H2O microflowers in the present reaction system. This simple synthetic route will offer great opportunities for the scale-up preparation of novel-shape transition metal vanadate micro/nanostructures.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21073032), Jilin Province Foundation (20090518), Department of Science and Technology of Jilin Province (20082103), Training Fund of NENU'S Scientific Innovation Project (No.NENU-STC07004) and Opening Fund of State Key Laboratory of Inorganic Synthesis and Preparative Chemistry of Jilin University.Notes and references
- H. Cölfen and M. Antonietti, Angew. Chem., Int. Ed., 2005, 44, 5576 CrossRef.
- S. Mann, Angew. Chem., Int. Ed., 2000, 39, 3392 CrossRef CAS.
- B. Liu and H. C. Zeng, J. Am. Chem. Soc., 2004, 126, 8124 CrossRef CAS.
- S. Y. Song, Y. Zhang, J. Feng, Y. Xing, Y. Q. Lei, W. Q. Fan and H. J. Zhang, Cryst. Growth Des., 2009, 9, 848 CrossRef CAS.
- X. J. Sun, Y. Xing, X. C. Liu, B. Liu and S. Y. Hou, Dalton Trans., 2010, 39, 1985 RSC.
- J. Wu, F. Duan, Y. Zheng and Y. Xie, J. Phys. Chem. C, 2007, 111, 12866 CrossRef CAS.
- J. Y. Liu, T. Luo, S. Mouli, F. L. Meng, B. Sun, M. Q. Li and J. H. Liu, Chem. Commun., 2010, 46, 472 RSC.
- D. B. Zhang, L. M. Qi, J. M. Ma and H. M. Chen, CrystEngComm, 2002, 4, 536 RSC.
- G. C. Li, K. Chao, C. Q. Zhang, Q. S. Zhang, H. R. Peng and K. Z. Chen, Inorg. Chem., 2009, 48, 1168 CrossRef CAS.
- Y. Xing, H. J. Zhang, S. Y. Song, J. Feng, Y. Q. Lei, L. J. Zhao and M. Y. Li, Chem. Commun., 2008, 1476 RSC.
- W. T. Yao, S. H. Yu, J. Jiang and L. Zhang, Chem.–Eur. J., 2006, 12, 2066 CrossRef CAS.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 5627 CrossRef CAS.
- M. Yang, H. P. You, Y. H. Zheng, K. Liu, G. Jia, Y. H. Song, Y. J. Huang, L. H. Zhang and H. J. Zhang, Inorg. Chem., 2009, 48, 11559 CrossRef CAS.
- Y. P. Fang, A. W. Xu, R. Q. Song, H. X. Zhang, L. P. You, J. C. Yu and H. Q. Liu, J. Am. Chem. Soc., 2003, 125, 16025 CrossRef CAS.
- Z. P. Liu, S. Li, Y. Yang, S. Peng, Z. K. Hu and Y. T. Qian, Adv. Mater., 2003, 15, 1946 CrossRef CAS.
- H. Zhang, D. R. Yang, Y. J. Ji, X. Y. Ma, J. Xu and D. L. Que, J. Phys. Chem. B, 2004, 108, 3955 CrossRef CAS.
- N. N. Zhao and L. M. Qi, Adv. Mater., 2006, 18, 359 CrossRef CAS.
- P. M. Skarstad, J. Power Sources, 2004, 136, 263 CrossRef CAS.
- R. A. Leising and E. S. Takeuchi, Chem. Mater., 1994, 6, 489 CrossRef CAS.
- Y. Piffard, F. Leroux, D. Guyomard, J. L. Mansot and M. Tournoux, J. Power Sources, 1997, 68, 698 CrossRef CAS.
- H. Ma, S. Y. Zhang, W. Q. Ji, Z. L. Tao and J. Chen, J. Am. Chem. Soc., 2008, 130, 5361 CrossRef CAS.
- S. Y. Zhang, L. J. Ci and H. R. Liu, J. Phys. Chem. C, 2009, 113, 8624 CrossRef CAS.
- K. Melghit and M. Al-belushi, Mater. Lett., 2008, 62, 3358 CrossRef CAS.
- C. J. Patridge, C. Jaye, H. S. Zhang, A. C. Marschilok, D. A. Fischer, E. S. Takeuchi and S. Banerjee, Inorg. Chem., 2009, 48, 3145 CrossRef CAS.
- L. S. Zhong, J. S. Hu, H. P. Liang, A. M. Cao, W. G. Song and L. J. Wan, Adv. Mater., 2006, 18, 2426 CrossRef CAS.
- L. S. Zhong, J. S. Hu, A. M. Cao, Q. Liu, W. G. Song and L. J. Wan, Chem. Mater., 2007, 19, 1648 CrossRef CAS.
- H. Cölfen and S. Mann, Angew. Chem., Int. Ed., 2003, 42, 2350 CrossRef.
- L. S. Zhong, J. S. Hu, A. M. Cao, Q. Liu, W. G. Song and L. J. Wan, Chem. Mater., 2007, 19, 1648 CrossRef CAS.
- X. Y. Cao, J. G. Xie, H. Zhan and Y. H. Zhou, Mater. Chem. Phys., 2006, 98, 71 CrossRef CAS.
- S. Beninati, M. Fantuzzi, M. Mastragostino and F. Soavi, J. Power Sources, 2006, 157, 483 CrossRef CAS.
- M. Takahashi, S. Tobishima, K. J. Takei and Y. J. Sakurai, J. Power Sources, 2001, 97–98, 508.
- M. Takahashi, S. Tobishima, K. J. Takei and Y. J. Sakurai, Solid State Ionics, 2002, 148, 283 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: More SEM images of the products. See DOI: 10.1039/c0ce00083c |
| This journal is © The Royal Society of Chemistry 2011 |
