Rapid and reversible formation of a crystalline hydrate of a metal–organic framework containing a tube of hydrogen-bonded water†
Nathalie
Guillou
a,
Franck
Millange
a and
Richard I.
Walton
*b
aInstitut Lavoisier Versailles, Université de Versailles St-Quentin en Yvelines (UMR CNRS 8180), 45 Avenue des Etats-Unis, 78035, Versailles Cedex, France
bDepartment of Chemistry, The University of Warwick, Coventry, CV4 7AL, UK. E-mail: r.i.walton@warwick.ac.uk
First published on 11th November 2010
Abstract
The flexible metal–organic framework MIL-53(Cr) undergoes a dramatic volume expansion upon immersion in water at room temperature to form a crystalline hydrate in which water is held as a hydrogen-bonded tube: the hydration is readily reversible under ambient conditions as shown by time-resolved powder X-ray diffraction.
The encapsulation of water in solid hosts results in a variety of bonded motifs including clusters, layers and chains.1,2 Such systems have been the focus of some considerable attention as they are often proposed as fundamental models for understanding the behaviour of water in many situations ranging from biology and geology through to chemistry and nanoscience.3 Examples of hosts for water include organic molecular crystals,1,2 biological molecules4 and nanoporous inorganic framework materials.5 In these situations, both water–water and water–host hydrogen bonds, as well as the geometry of confinement provided by the host, play a role in defining the shape and size of the encapsulated water clusters.
In the field of porous materials, metal–organic frameworks (MOFs) are a current focus of much attention as novel hosts for the inclusion of a wide variety of guest species.6 MOFs are solid-state materials whose extended structures are made up of metal-centred polyhedral units linked by polydentate, organic ligands. They make use of the well-established coordination chemistry of a variety of metals with a range of polydentate ligands to offer the possibilities of designing new frameworks whose porosity and framework chemistry might be tuned to offer novel solid hosts with applications in areas such as gas storage, molecular sieving and heterogeneous catalysis.7 The uptake of a wide range of guest molecules in MOFs is now being studied and this work is revealing how the materials may offer advantages over conventional purely inorganic hosts, such as zeolites. One distinctive feature of the chemistry of metal–organic frameworks is their structural flexibility, where reversible atomic displacements, in some cases of up to several Ångströms, may occur whilst maintaining crystallinity.8 This offers the prospect for novel host–guest chemistry and potential application as tuneable or responsive molecular sieves.
In this communication we describe the hydration behaviour of the flexible MOF MIL-53 in its chromium(III) form. This material, Cr(OH)(O2C–C6H4–CO2), was first described as a desolvated form: its structure consists of infinite chains of {CrO4(OH)2} octahedra trans-linked by μ2-bridging hydroxides and crosslinked by bis-bidentate terephthalate (1,4-benzenedicarboxylate) ligands whose oxygen donors complete the coordination about chromium.9 Other analogues of the MIL-53 structure are known that contain the metals Fe, Al, Ga and In, all in the +3 oxidation state,10 and this family of materials has been the focus of some considerable attention with respect to the sorption properties towards a variety of molecules from small gases such as CO2, CH4 and H2S to a range of organic molecules, where it has been shown that considerable expansion of the structure may take place with insertion of guest molecules.11−13
The hydrated form of MIL-53(Cr), Cr(OH)(O2C–C6H4–CO2)·(H2O), that is isolated as a green polycrystalline powder at room temperature contains one molecule of water per chromium. In the current work, high resolution powder X-ray diffraction‡ has allowed accurate location of the guest water molecules in the solid, not previously described, Fig. 1.
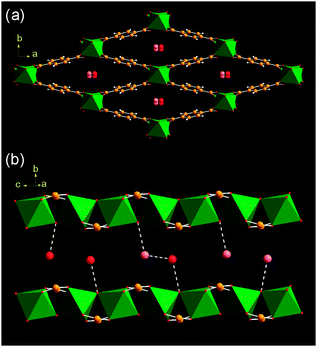 | ||
| Fig. 1 Two views of the structure of hydrated MIL-53(Cr)(H2O) (a) parallel to the lozenge-shaped channels and (b) perpendicular view of a single channel. The green octahedra are Cr(III)-centred building units and the oxygen atoms of occluded water are shown as red or pink spheres. Although there is one crystallographic site for water, it is 50% occupied and in (b) the scenarios of water hydrogen bonding are shown. | ||
The water molecules in hydrated MIL-53(Cr) reside in the one-dimensional channels and interact with hydrogen bonds to the hydroxide anions that form the backbone of the inorganic chain (closest O1⋯Ow1 distance of 2.800(6) Å), or to form a water dimer (Ow⋯Ow = 2.671(7) Å) (Fig. 1b). It is established that dehydration occurs at 80 °C and this results in a dramatic unit cell volume expansion (from ∼995 to ∼1496 Å3) involving displacement of the framework atoms of up to 5.2 Å.11 We now present evidence for the expansion of MIL-53(Cr) in the presence of excesswater. In order to obtain structural information about this process, high resolution powder X-ray diffraction from solid immersed in water within a capillary was recorded (50 mg in 1 mL water).‡ The final Rietveld plot following structure solution and refinement is shown in Fig. 2.
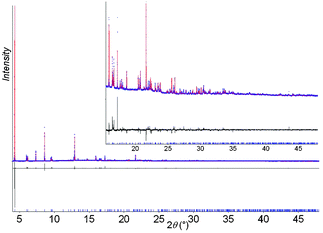 | ||
| Fig. 2 Final Rietveld plot for superhydrated MIL-53(Cr)(6.2H2O). | ||
The structure solution reveals a fully expanded form of the solid that contains close to six water molecules per unit cell (one site Ow4 is partially occupied) in an ordered arrangement (Fig. 3a). The unit cell volume of the structure in this ‘superhydrated’ state (∼1550 Å3) is larger than the dehydrated material previously reported (∼1496 Å3)11 but the symmetry (Imcm) is identical.
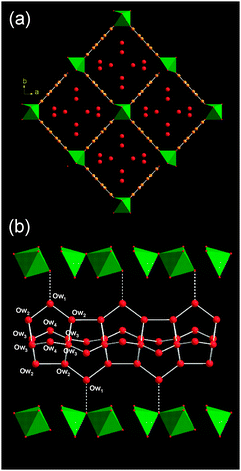 | ||
| Fig. 3 Two views of the structure of superhydrated MIL-53(Cr)(6.2H2O) (a) parallel to the lozenge-shaped channels and (b) perpendicular view of a single channel. Legend as for Fig. 2. Water–water hydrogen bonds are shown in white and the water–framework hydrogen bond as a dotted line. | ||
The water molecules in the superhydrated form of MIL-53(Cr) are linked by inter-water hydrogen bonding (with closest oxygen–oxygen distances ranging from 2.627(5) to 2.769(4) Å) to form infinite tubes running parallel to the inorganic chain (Fig. 3b). There are weaker hydrogen bonds between one of the water molecules and the framework hydroxide (3.069(8) Å). The tubular motif is constructed from four-, five- and six-rings of water molecules. A search of the Cambridge Crystal Structure Database reveals no other reported crystalline material with such a motif for hydrogen-bonded water in systems that encapsulate water. Although a variety of infinite tape (T) oligomers have been observed in organic molecular crystals,1 none reported so far has the tubular nature of the water in superhydrated MIL-53(Cr).
To study the reversibility of the hydration of MIL-53(Cr), we measured time-resolved powder X-ray diffraction data.‡Fig. 4 shows the data recorded during dehydration of a paste of the material in water over a period of 6 hours at room temperature. This confirms the ease of reversibility of the superhydration: the final product corresponds to the ‘closed pore’ hydrated material.
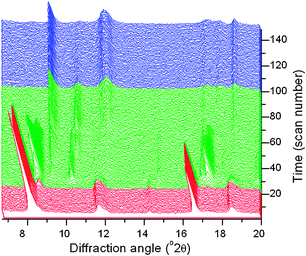 | ||
| Fig. 4 In situ powder XRD data measured during the dehydration of superhydrated MIL-53(Cr) at 30 °C under dry nitrogen flow. Three distinct regions are coloured corresponding to the superhydrate (red), intermediate hydrated forms (green) and hydrate (blue). | ||
The in situXRD data also reveal that dehydration occurs via crystalline intermediate phases. Although we are unable to isolate these partially hydrated phases, owing to their kinetic stability and the difficulty in controlling humidity to prolong their lifetime to measure high quality diffraction data, we can match their diffraction patterns to the frameworks of materials seen during the swelling of the analogous material MIL-53(Fe) with various organic solvents.13 Thus, a simulated pattern using an intermediate cell volume (∼1215 Å3) and C2/c symmetry accounts for the pattern of the most long-lived transient hydrated state (see ESI†). The reversibility of the hydration is striking whilst crystallinity is maintained: this illustrates how the restraint of the pore geometry has a strong influence on the bonding modes of the water once encapsulated. We note that as with the interaction of MIL-53(Fe) with organic guests,13 the loss of water from the superhydrated MIL-53(Cr) does not occur with a continuous evolution of unit cell volume, but rather in a stepwise fashion with only certain unit cell volumes crystallising. The similarity to MIL-53(Fe) ends here, however, since we have found it impossible to prepare a superhydrated version of the iron analogue under similar conditions, or upon heating with water to 200 °C in a sealed cell where an autogeneous pressure of ∼20 bar is generated. This illustrates how the interpretation of in situ diffraction recorded during the reactivity of flexible MOFs must be done very carefully since solvent swelling may occur readily in some, but not all, cases.
The reversible ‘superhydration’ of some nanoporous silicate zeolites has been reported previously, and can lead to the formation of infinite ‘wires’ of hydrogen-bonded water, but in those cases the effect is brought about by application of considerable applied pressure (>1 GPa).14 In the case of other hydrated MOFs, various water clusters have been observed15 and in one case the role of chains of water on the self-assembly of a MOF structure has been speculated.16 For MIL-53(Cr) the remarkable formation of complex extended tubes of water molecules upon hydration is entirely reversible under ambient conditions. Our work illustrates that there is much scope for novel host–guest chemistry in flexible MOFs, and that potentially the uptake of other small molecules may be tuned by the degree of hydration.
We thank Dr James Chisholm of the Cambridge Crystallographic Data Centre for searching the CSD for water oligomers in crystal hydrates, and Dr Andy Fitch of the ESRF for assistance with using ID31. The powder diffractometer at the University of Warwick was obtained through the Science City Advanced Materials project “Creating and Characterising Next Generation Advanced Materials” with support from Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF).
Notes and references
- L. Infantes and S. Motherwell, CrystEngComm, 2002, 4, 454 RSC.
- L. Infantes, J. Chisholm and S. Motherwell, CrystEngComm, 2003, 5, 480 RSC; M. Mascal, L. Infantes and J. Chisholm, Angew. Chem., Int. Ed., 2006, 45, 32 CrossRef.
- J. M. Ugalde, I. Alkorta and J. Elguero, Angew. Chem., Int. Ed., 2000, 39, 717 CrossRef CAS; R. Ludwig, Angew. Chem., Int. Ed., 2001, 40, 1808 CrossRef CAS; M. Henry, Cell. Mol. Biol., 2005, 51, 677 CAS.
- M. M. Teeter, Annu. Rev. Biophys. Biophys. Chem., 1991, 20, 577 CrossRef CAS.
- R. Kniep, H. G. Will, I. Boy and C. Rohr, Angew. Chem., Int. Ed. Engl., 1997, 36, 1013 CrossRef CAS; M. Henry, F. Taulelle, T. Loiseau, L. Beitone and G. Férey, Chem.–Eur. J., 2004, 10, 1366 CrossRef CAS; J. Y. Li, J. H. Yu and R. R. Xu, Phys. Chem. Chem. Phys., 2009, 11, 1291 RSC.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC; J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213 RSC.
- A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284 RSC.
- S. Kitagawa and K. Uemura, Chem. Soc. Rev., 2005, 34, 109 RSC; G. Férey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380 RSC; J. Rabone, Y. F. Yue, S. Y. Chong, K. C. Stylianou, J. Bacsa, D. Bradshaw, G. R. Darling, N. G. Berry, Y. Z. Khimyak, A. Y. Ganin, P. Wiper, J. B. Claridge and M. J. Rosseinsky, Science, 2010, 329, 1053 CrossRef CAS.
- F. Millange, C. Serre and G. Férey, Chem. Commun., 2002, 822 RSC.
- F. Millange, N. Guillou, R. I. Walton, J. Grenèche, I. Margiolaki and G. Férey, Chem. Commun., 2008, 4732 RSC; C. Volkringer, T. Loiseau, N. Guillou, G. Férey, E. Elkaim and A. Vimont, Dalton Trans., 2009, 2241 RSC.
- C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Louer and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519 CrossRef CAS.
- G. Férey, M. Latroche, C. Serre, F. Millange, T. Loiseau and A. Percheron-Guegan, Chem. Commun., 2003, 2976 RSC; S. Bourrelly, P. L. Llewellyn, C. Serre, F. Millange, T. Loiseau and G. Férey, J. Am. Chem. Soc., 2005, 127, 13519 CrossRef CAS; C. Serre, S. Bourrelly, A. Vimont, N. A. Ramsahye, G. Maurin, P. L. Llewellyn, M. Daturi, Y. Filinchuk, O. Leynaud, P. Barnes and G. Férey, Adv. Mater., 2007, 19, 2246 CrossRef; P. L. Llewellyn, G. Maurin, T. Devic, S. Loera-Serna, N. Rosenbach, C. Serre, S. Bourrelly, P. Horcajada, Y. Filinchuk and G. Férey, J. Am. Chem. Soc., 2008, 130, 12808 CrossRef CAS; L. Alaerts, M. Maes, L. Giebeler, P. A. Jacobs, J. A. Martens, J. F. M. Denayer, C. E. A. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2008, 130, 14170 CrossRef CAS; L. Hamon, C. Serre, T. Devic, T. Loiseau, F. Millange, G. Férey and G. De Weireld, J. Am. Chem. Soc., 2009, 131, 8775 CrossRef CAS; V. Finsy, C. E. A. Kirschhock, G. Vedts, M. Maes, L. Alaerts, D. E. De Vos, G. V. Baron and J. F. M. Denayer, Chem.–Eur. J., 2009, 15, 7724 CrossRef CAS; V. Finsy, L. Ma, L. Alaerts, D. E. De Vos, G. V. Baron and J. F. M. Denayer, Microporous Mesoporous Mater., 2009, 120, 221 CrossRef CAS; M. Meilikhov, K. Yusenko and R. A. Fischer, Dalton Trans., 2009, 600 RSC; L. Alaerts, M. Maes, M. A. van der Veen, P. A. Jacobs and D. E. De Vos, Phys. Chem. Chem. Phys., 2009, 11, 2903 RSC.
- F. Millange, C. Serre, N. Guillou, G. Férey and R. I. Walton, Angew. Chem., Int. Ed., 2008, 47, 4100 CrossRef CAS.
- Y. Lee, T. Vogt, J. A. Hriljac, J. B. Parise and G. Artioli, J. Am. Chem. Soc., 2002, 124, 5466 CrossRef CAS; Y. Lee, T. Vogt, J. A. Hriljac, J. B. Parise, J. C. Hanson and S. J. Kim, Nature, 2002, 420, 485 CrossRef CAS; Y. Lee, J. A. Hriljac and T. Vogt, Phys. Chem. Miner., 2004, 31, 421 CAS; Y. Lee, C. D. Martin, J. B. Parise, J. A. Hriljac and T. Vogt, Nano Lett., 2004, 4, 619 CrossRef CAS; M. Colligan, Y. Lee, T. Vogt, A. J. Celestian, J. B. Parise, W. G. Marshall and J. A. Hriljac, J. Phys. Chem. B, 2005, 109, 18223 CrossRef CAS; Y. Lee, J. A. Hriljac, J. B. Parise and T. Vogt, Am. Mineral., 2006, 91, 247 CrossRef CAS.
- Y. Jin, Y. X. Che, S. R. Batten, P. Chen and J. M. Zheng, Eur. J. Inorg. Chem., 2007, 1925 CrossRef CAS; C. M. Jin, Z. Zhu, Z. F. Chen, Y. J. Hu and X. G. Meng, Cryst. Growth Des., 2010, 10, 2054 CrossRef CAS; R. L. Sang and L. Xu, CrystEngComm, 2010, 12, 1377 RSC.
- D. L. Reger, R. F. Semeniuc, C. Pettinari, F. Luna-Giles and M. D. Smith, Cryst. Growth Des., 2006, 6, 1068 CrossRef CAS.
- Topas V3.0: General Profile and Structure Analysis Software for Powder Diffraction Data, Bruker AXS Ltd, 2004 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Description of structure solution and assignment of in situpowder XRD. CCDC 793566–793567. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc03882b |
| ‡ MIL-53(Cr) was prepared as previously described.9 High-resolution powder X-ray diffraction data were collected on ID31 of the European Synchrotron Radiation Facility from powdered samples contained in 1 mm diameter quartz capillaries. The beamline receives X-rays from the synchrotron source (which operates with an average energy of 6 GeV and a current beam of typically 100 mA) from an undulator device. The incident X-ray wavelength was 0.79989 Å with a beam size of 2.0 mm (horizontal) × 1.0 mm (vertical) used. For the cases where the interaction of solid with water was to be studied, the liquid was injected into the capillary and the capillary then centrifuged to concentrate the solid to the end of the sample holder; this minimised the background of the liquid and prevented the loss of water from the solid during the experiment. The samples were rapidly spun during data collection to ensure good powder averaging. Extractions of the peak positions, pattern indexing and Rietveld refinements were carried out with the TOPAS program17 (see ESI†). In situpowder XRD were performed using a Bruker D8 Advance X-ray diffractometer operating with Cu Kα radiation, equipped with a VÅNTEC-1 solid-state detector and fitted with an Anton-Parr XRK900 chemical reaction chamber. The sample temperature was set at 30 °C and dry nitrogen flowed over the samples with data being recorded in scans of 2.5 minutes until structural evolution had ceased. |
| This journal is © The Royal Society of Chemistry 2011 |
