Preparation of 1-D nanoparticle superstructures with tailorable thicknesses using gold-binding peptide conjugates†‡
Leekyoung
Hwang
,
Chun-Long
Chen
and
Nathaniel L.
Rosi
*
Department of Chemistry, University of Pittsburgh, 219 Parkman Avenue, Pittsburgh, PA 15260, USA. E-mail: nrosi@pitt.edu; Fax: +1-412-624-8611; Tel: +1-412-624-3987
First published on 23rd August 2010
Abstract
We describe the preparation of new 1-D gold nanoparticle superstructures with tailorable thicknesses formed using a self-assembled gold-binding peptide conjugate template and examine how the synthesis and assembly mechanism impacts the organization of the superstructures.
Ensembles of inorganic nanoparticles exhibit unique assembly-dependent physical properties which depend largely on the local order of the nanoparticles.1 To control these properties, methods must be developed to precisely control the assembly of nanoparticles.1a Short oligopeptides have emerged as promising molecules for directing nanoparticle assembly.2 However, many peptide-based assembly methods yield nanoparticle superstructures with poor local nanoparticle order.2a
We recently introduced methodology that utilizes a new class of peptide2a,3 conjugate molecules designed for directing both the synthesis andassembly of nanoparticles to yield nanoparticle superstructures with unprecedented structural complexity and excellent local nanoparticle order.4 We specifically showed that C12-PEPAu, a gold-binding peptide5 (PEPAu = AYSSGAPPMPPF) modified at its N-terminus with an aliphatic 12-carbon chain, could be used to expeditiously prepare 1-D double-helical nanoparticle assemblies in a single preparative step.4a Further, we demonstrated that various synthetic parameters could be systematically modified to carefully tailor the structure and metrics of these assemblies.4b
This unique one-step synthetic approach and the integrity and structural complexity of the nanoparticle superstructures it produces prompted us to explore how structural modifications to the peptide conjugate molecule impact the synthesis and assembly process. We reasoned that these studies would provide insight into the mechanism of nanoparticle assembly and illuminate specific factors which distinguish this methodology from other peptide-based nanostructure assembly methods. In this communication, we specifically investigate both how the identity of the organic moiety appended to the PEPAu terminus and how amino acid additions to the PEPAu sequence impact the formation of nanoparticle superstructures.
Several observations from our previous studies using C12-PEPAu serve as important precedent for the studies reported herein. In those studies, 1-D double-helical nanoparticle superstructures were produced upon addition of a gold salt to a solution of C12-PEPAu in HEPES buffer (HEPES = 4-(2-hydroxyethyl)-piperazineethanesulfonic acid). HEPES serves as the principal reducing agent for forming the gold nanoparticles.4a,5,6 We found that addition of the gold salt significantly promotes C12-PEPAu self-assembly into nanofibers. We also discovered that filtering the C12-PEPAu/HEPES solution to remove any assembled structures prior to adding the gold salt was important in order to produce gold nanoparticle double helices as product.4a This indicates that dispersed, non-assembled peptide conjugates are required at the initial stages of reaction. Collectively, these observations suggested that nanofiber self-assembly and nanoparticle synthesis are coupled into a simultaneous process. We note that this one-step process is markedly different from typical peptide-based nanoparticle assembly methods which consist of two or more independent preparative steps, including (i) peptide self-assembly into a template and (ii) either nucleation of nanoparticles or assembly of pre-synthesized nanoparticles onto the template.2 We hypothesize that a simultaneous synthesis and self-assembly process is critical for the formation of well-ordered nanoparticle superstructures.
To test this hypothesis, we aimed to prepare a peptide conjugate that, unlike C12-PEPAu, self-assembles rapidly in the absence of gold salt; we expected that such a peptide conjugate would direct nanoparticle assemblyvia a different mechanism than C12-PEPAu. We first prepared C12H9CO-PEPAu, hereafter referred to as BP-PEPAu (BP = biphenyl), reasoning that the BP moiety would promote π–π interactions and facilitate rapid self-assembly. BP-PEPAu was synthesized by coupling succinimide-activated 4-phenylbenzoic acid to the N-terminus of PEPAu (see Supporting Information for details). We studied the self-assembly of BP-PEPAu in HEPES buffer (pH = 7.3). However, neither transmission electron microscopy (TEM) nor tapping-mode atomic force microscopy (AFM) indicated formation of self-assembled BP-PEPAu structures. Our previous studies of C12-PEPAu self-assembly4a suggested that the amino acids at the N-terminus of PEPAu, in particular AYSS, played a key role in directing the assembly of nanofibers, possibly because they promote the formation of β-sheets.7 We therefore decided to modify the N-terminus of PEPAu with an additional ‘AYSS’ segment which could promote self-assembly.
Accordingly, we prepared BP-AYSS-PEPAu (C12H9CO-AYSSAYSSGAPPMPPF). In contrast to BP-PEPAu, BP-AYSS-PEPAu rapidly self-assembles (within 30 min) in HEPES buffer to yield short 1-D fibers (Scheme 1; Fig. 1a, S3a,b) with regular width (5.2 ± 0.1 nm, Fig. S4d). Further, BP-AYSS-PEPAu assembles into fibers much more rapidly than C12-PEPAu. The rapid self-assembly of BP-AYSS-PEPAu into fibers precluded dispersion of individual BP-AYSS-PEPAu conjugates. Therefore, we suspected that gold nanoparticle superstructures prepared using BP-AYSS-PEPAu would form via a two step mechanism (Scheme 1) similar to that of typical peptide-based nanoparticle assembly methods2 rather than the one step synthesis and assembly process observed for C12-PEPAu.4a
 | ||
| Scheme 1 Illustration of nanoparticle assembly process. | ||
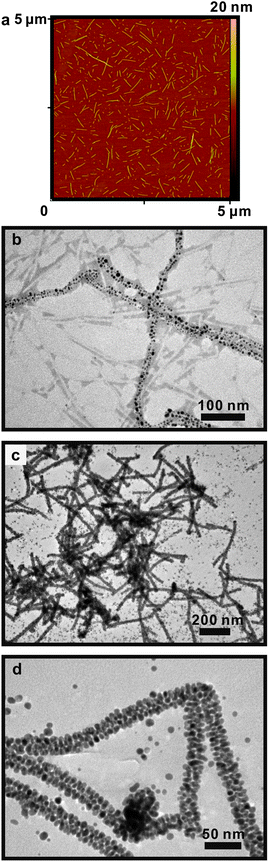 | ||
| Fig. 1 (a) AFM height image of self-assembled BP-AYSS-PEPAu fibers after 30 min incubation in HEPES buffer; (b) negative-stained TEM image of 1-D gold nanoparticle superstructures formed 30 min after adding HAuCl4 to BP-AYSS-PEPAu fibers from (a); (c) and (d) TEM images of 1-D gold nanoparticle superstructures formed 24 h after adding HAuCl4 to BP-AYSS-PEPAu fibers from (a). | ||
We proceeded to prepare 1-D nanoparticle superstructures to determine whether a two step mechanism for BP-AYSS-PEPAu would impact the structural integrity and precision of organization of the gold nanoparticles. BP-AYSS-PEPAu fibers were incubated in HEPES buffer (30 min), and then gold nanoparticle synthesis was initiated by adding a solution of chloroauric acid (HAuCl4) (see Supporting Information for details). The resulting mixture was allowed to stand at room temperature (30 min), after which a small sample was removed and characterized using TEM. Examination of multiple TEM images (Fig. 1b; Fig. S4) revealed 1-D gold nanoparticle superstructures, fibers, and free, non-assembled gold nanoparticles. The width of the superstructures (7.9 ± 0.1 nm, Fig. S4e) suggests that a single BP-AYSS-PEPAu self-assembled fiber serves as a template for their assembly. While the superstructures possess long-range order, their local nanoparticle order is poor. The structures consist of irregularly positioned nanoparticles ranging in size from ∼3 nm to ∼13 nm. The structures do not exhibit the same structural regularity, precision of nanoparticle placement, and narrow nanoparticle size distribution exhibited by the nanoparticle superstructures produced using C12-PEPAu.4 Rather, they more closely resemble structures formed using typical multi-step peptide templating methods.2a The reaction was also sampled after 24 h, and, again, the structures exhibit excellent long range order yet poor local nanoparticle order (Fig. 1c,d).
In order to probe the range of structures which can be assembled using BP-AYSS-PEPAu self-assembled fibers, we allowed BP-AYSS-PEPAu to incubate for 6 days in HEPES buffer prior to adding gold salt. TEM (Fig. S3c–f) and AFM (Fig. 2a) images reveal that BP-AYSS-PEPAu self-assembled fibers begin to align and stack together in either 2 or 3 fiber bundles with widths of 9.5 ± 0.1 nm and 15.5 ± 1.7 nm, respectively (Fig. S5). When a solution of HAuCl4 was added to this mixture of fibers, we again observed 1-D gold nanoparticle superstructures, fibers, and free, non-assembled gold nanoparticles (Fig. 2b–d; Fig. S6). A distribution of 1-D nanoparticle superstructures formed with widths of either 12.2 ± 0.2 nm or 17.6 ± 0.2 nm, suggesting that they were templated by either the 2 or 3 fiber bundles, respectively (Fig. 2c,d, Fig. S6).
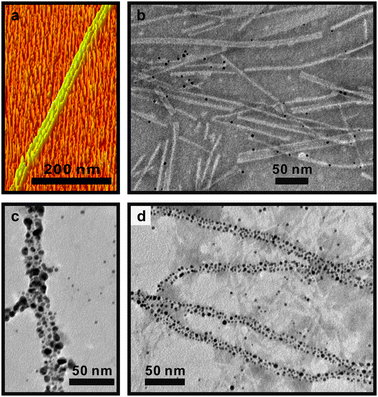 | ||
| Fig. 2 (a) 3-D AFM image of self-assembled BP-AYSS-PEPAu fibers after 6 d incubation in HEPES buffer; (b)–(d) various degrees of nanoparticle assembly and 1-D nanoparticle superstructures formed 15 min after adding HAuCl4 to BP-AYSS-PEPAu fibers which had been incubated for 6 d. | ||
In conclusion, we have shown that BP-AYSS-PEPAu first assembles into a fiber template and then nanoparticles either nucleate directly on the template or are deposited onto the template from solution to yield 1-D nanoparticle superstructures having tailorable widths of either 7.9 nm, 12.2 nm, or 17.6 nm. These structures exhibit long range order but limited local order. These results suggest that nanoparticle assembly directed by BP-AYSS-PEPAu conjugates operates by a different mechanism than C12-PEPAu. Importantly, these studies reveal the fundamental importance of a simultaneous synthesis and assembly process which is necessary for producing well-ordered nanoparticle superstructures. To produce high-quality nanoparticle superstructures using this new methodology, the peptide conjugate should not self-assemble into a template structure prior to the addition of gold salt, as this precludes a one step process in which the nanoparticles and peptide conjugates self-assemble simultaneously.
NLR acknowledges the University of Pittsburgh and and the National Science Foundation (DMR-0954380) for funding this work. The authors also thank the Peterson NFCF and the MEMS Department for provision of access to AFM and TEM, respectively.
Notes and references
- (a) N. A. Kotov and F. Stellacci, Adv. Mater., 2008, 20, 4221–4222 CrossRef CAS; (b) M. P. Pileni, J. Phys. Chem. B, 2001, 105, 3358–3371 CrossRef CAS; (c) Z. H. Nie, A. Petukhova and E. Kumacheva, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS; (d) S. Lal, S. Link and N. J. Halas, Nat. Photonics, 2007, 1, 641–648 Search PubMed; (e) W. S. Chang, L. S. Slaughter, B. P. Khanal, P. Manna, E. R. Zubarev and S. Link, Nano Lett., 2009, 9, 1152–1157 CrossRef CAS (and references therein).
- (a) C. L. Chen and N. L. Rosi, Angew. Chem., Int. Ed., 2010, 49, 1924–1942 CAS; (b) X. Y. Gao and H. Matsui, Adv. Mater., 2005, 17, 2037–2050 CrossRef CAS; (c) L. S. Li and S. I. Stupp, Angew. Chem., Int. Ed., 2005, 44, 1833–1836 CrossRef CAS; (d) N. Ostrov and E. Gazit, Angew. Chem., Int. Ed., 2010, 49, 3018–3021 CAS (and references therein).
- (a) S. R. Whaley, D. S. English, E. L. Hu, P. F. Barbara and A. M. Belcher, Nature, 2000, 405, 665–668 CrossRef CAS; (b) M. B. Dickerson, K. H. Sandhage and R. R. Naik, Chem. Rev., 2008, 108, 4935–4978 CrossRef CAS; (c) M. Sarikaya, C. Tamerler, A. K. Y. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577–585 CrossRef CAS (and references therein).
- (a) C. L. Chen, P. J. Zhang and N. L. Rosi, J. Am. Chem. Soc., 2008, 130, 13555–13557 CrossRef CAS; (b) C. L. Chen and N. L. Rosi, J. Am. Chem. Soc., 2010, 132, 6902–6903 CrossRef CAS.
- J. M. Slocik, M. O. Stone and R. R. Naik, Small, 2005, 1, 1048–1052 CrossRef CAS.
- (a) A. Habib, M. Tabata and Y. G. Wu, Bull. Chem. Soc. Jpn., 2005, 78, 262–269 CrossRef CAS; (b) J. P. Xie, J. Y. Lee and D. I. C. Wang, Chem. Mater., 2007, 19, 2823–2830 CrossRef CAS.
- D. L. Minor and P. S. Kim, Nature, 1994, 367, 660–663 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Full experimental information and additional characterization data. See DOI: 10.1039/c0cc02257h |
| This journal is © The Royal Society of Chemistry 2011 |
