A bis-salicylaldoximato-copper(II) receptor for selective sulfate uptake†‡
Marco
Wenzel
,
Quintin W.
Knapp
and
Paul G.
Plieger
*
Institute of Fundamental Sciences, Massey University, Private Bag 11 222, Palmerston North 4442, New Zealand. E-mail: p.g.plieger@massey.ac.nz; Fax: +64 6 350 5682; Tel: +64 6 356 9099 Ext 7825
First published on 22nd October 2010
Abstract
The preferential uptake of sulfate over the industrially important anions, chloride and nitrate and structurally similar dihydrogen phosphate has been achieved in aqueous media with a dicopper salicylaldoxime complex.
Binding and extraction of oxoanions such as sulfate remains a challenging task yet is of upmost importance due to the biological, environmental and commercial relevance. Specific binding can be accomplished with highly pre-organised molecules such as indolocarbazole oligomers,1 macrocyclic or macrobicyclic ligands.2 However, complex formation is often slow, which has disadvantages in circulation industrial processes such as solvent extraction. Flexible, open chain ligands3 or metallocyclic supramolecules4 proffer a suitable alternative. The later have additional advantages; the host structure is formed in situ and benefits from additional improvements in strength and selectivity by direct metal–host binding.5 Ligands capable of existing in a zwitterionic form offer further opportunities,6 since controlled binding of both the cation and attendant anion can be triggered by a change in the pH of the solution.7
The ligand L, N,N′-dimethyl-N,N′-hexamethylenedi-(3-hydroxyiminomethyl-2-hydroxy-5-tert-butylbenzylamine), consisting of two 5-tert-butylsalicylaldoxime molecules, forms a helical twisted 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metallo-macrocycle upon Cu(II)-complexation, which can encapsulate anions (Fig. 1).8 In this work, we show that the extraction of the hydrophilic sulfate anion from aqueous solution into a water-immiscible organic phase is possible using this receptor; even in the presence of high concentrations of industrially relevant anions such as chloride and nitrate, which, based on their solvation energies should be preferentially extracted. In addition, we show unequivocally that anion shape is not a factor in this anti-Hofmeister behaviour as the structurally similar yet more hydrophobic dihydrogenphosphate anion is not captured in preference to sulfate within this system.
2 metallo-macrocycle upon Cu(II)-complexation, which can encapsulate anions (Fig. 1).8 In this work, we show that the extraction of the hydrophilic sulfate anion from aqueous solution into a water-immiscible organic phase is possible using this receptor; even in the presence of high concentrations of industrially relevant anions such as chloride and nitrate, which, based on their solvation energies should be preferentially extracted. In addition, we show unequivocally that anion shape is not a factor in this anti-Hofmeister behaviour as the structurally similar yet more hydrophobic dihydrogenphosphate anion is not captured in preference to sulfate within this system.
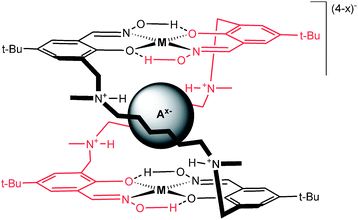 | ||
| Fig. 1 Anion encapsulated Cu2L2 metallo-macrocycle. | ||
In order to probe sulfate uptake over chloride, nitrate or phosphate, two sets of liquid–liquid extraction experiments were undertaken. The first was chosen to match the industrially relevant, Bulong circuit,9e.g. an aqueous solution containing sodium sulfate and sodium chloride was vigorously stirred for 24 h with an equal volume of chloroform containing the “copper only” complex [Cu2(L-2H)2].8 The intramolecular hydrogen bonding present around the copper centre within these pseudo-macrocyclic systems imparts improved stability to these complexes to the degree that ESMS analysis in this experiment gave a representative indication of the organic solution composition. Thus, anion uptake within the core could be conveniently analysed by ESMS with a strong isotopic peak pattern at 660 (m/z) indicative of the presence of the dicationic complex species [SO4⊂Cu2L2]2+. In a second set of experiments a large 100-fold excess of chloride, nitrate or phosphate was used with parallel results, i.e. the presence of the dicationic complex species was again observed in the ESMS in all cases with no observation of the alternative anion within the core.
The similarity in the molecular mass and the isotopic distribution of sulfate and dihydrogen phosphate prevents their discrimination via low resolution ESI-MS. However, support for the preferred uptake of sulfate over dihydrogen phosphate is given by a carefully calibrated HR-MS study. The observed signal of the +2 fragment at 665.2882 m/z (lowest-mass) and the isotopic pattern is in agreement to the theoretical model of [SO4⊂Cu2L2]2+ (C64H100N8O8Cu2SO4)2+m/z = 665.2886 (Fig. 2), with no indication of a peak associated with encapsulated dihydrogen phosphate species at ∼+0.01.
![Experimental high resolution ESI-MS isotope pattern for the dicationic fragment [SO4Cu2L2]2+ (top) and the expected theoretical isotope pattern (bottom).](/image/article/2011/CC/c0cc02230f/c0cc02230f-f2.gif) | ||
| Fig. 2 Experimental high resolution ESI-MS isotope pattern for the dicationic fragment [SO4Cu2L2]2+ (top) and the expected theoretical isotope pattern (bottom). | ||
UV-vis
titration experiments of the “copper only” complex [Cu2(L–2H)2]‡ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of isopropanol/1,2-dichloroethane were undertaken with nitrate and the halide anions to underline the observed stronger affinity towards sulfate. The calculated stability constants§ show the expected trend with smaller log K values for both nitrate and the halides as compared with the recently reported values for the tetrahedral anions sulfate and dihydrogen phosphate.8 It is worth noting that based on the complex stability data for sulfate and phosphate, a preference in binding these anions is not expected (Table 1).
1 mixture of isopropanol/1,2-dichloroethane were undertaken with nitrate and the halide anions to underline the observed stronger affinity towards sulfate. The calculated stability constants§ show the expected trend with smaller log K values for both nitrate and the halides as compared with the recently reported values for the tetrahedral anions sulfate and dihydrogen phosphate.8 It is worth noting that based on the complex stability data for sulfate and phosphate, a preference in binding these anions is not expected (Table 1).
To emphasise the preference of the receptor to bind sulfate over either chloride, nitrate or phosphate, L in methanol was mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of CuSO4 and either NaCl, NaNO3 in water or in a 1
4 ratio of CuSO4 and either NaCl, NaNO3 in water or in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio with CuSO4 and K2HPO4 in water. Crystallisation of the resulting bulk materials resulted in the isolation of the mixed anion complexes [SO4⊂Cu2L2]Cl2, [SO4⊂Cu2L2](NO3)2 and [SO4⊂Cu2L2](H2PO4)2.¶ An addition experiment utilising a mixture of three potential anions SO42−, NO3− and HPO42− also resulted in the formation of [SO4⊂Cu2L2](NO3)2 as evidenced by a space group check which matched exactly the data obtained for the above sulfate/nitrate complex. Each of the structures exhibit the 2
2 ratio with CuSO4 and K2HPO4 in water. Crystallisation of the resulting bulk materials resulted in the isolation of the mixed anion complexes [SO4⊂Cu2L2]Cl2, [SO4⊂Cu2L2](NO3)2 and [SO4⊂Cu2L2](H2PO4)2.¶ An addition experiment utilising a mixture of three potential anions SO42−, NO3− and HPO42− also resulted in the formation of [SO4⊂Cu2L2](NO3)2 as evidenced by a space group check which matched exactly the data obtained for the above sulfate/nitrate complex. Each of the structures exhibit the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 metal to ligand helical assembly common to these lengthy bis-salicylaldoxime ligands (Fig. 3). In all cases the four tertiary amines on the complex are protonated as evidenced from identification of the accompanying anions. With the exception of the phosphate containing complex, X-ray structures of the complexes confirmed the encapsulation of SO42− at the complex core in preference to the other anions (Fig. 3). There are subtle differences between the basic [SO4⊂Cu2L2]2+ dicationic core based on the Cu–Cu distances and helical twist angles of the individual complexes (Table 2). Interestingly, direct reaction of L with Cu(NO3)2·3H2O results in a NO3− encapsulated complex [NO3⊂Cu2L2](NO3)3 (Fig. S5, ESI‡) with similar Cu–Cu distances (Table 2), indicating that the Cu2L2 core with its additional intra-hydrogen bonding has limited flexibility and probably plays a part in the overall stability of the bound sulfate complex. For the phosphate complex, both data quality and the similarity in size between the dihydrogen phosphate and sulfate anions preclude direct identification of sulfate at the core of this helicate, however, additional evidence for encapsulation of SO42− over H2PO4− is given by HR-MS and elemental analysis on the bulk crystalline sample as used in the X-ray analysis. A detailed description of the structures is given in the ESI.‡
2 metal to ligand helical assembly common to these lengthy bis-salicylaldoxime ligands (Fig. 3). In all cases the four tertiary amines on the complex are protonated as evidenced from identification of the accompanying anions. With the exception of the phosphate containing complex, X-ray structures of the complexes confirmed the encapsulation of SO42− at the complex core in preference to the other anions (Fig. 3). There are subtle differences between the basic [SO4⊂Cu2L2]2+ dicationic core based on the Cu–Cu distances and helical twist angles of the individual complexes (Table 2). Interestingly, direct reaction of L with Cu(NO3)2·3H2O results in a NO3− encapsulated complex [NO3⊂Cu2L2](NO3)3 (Fig. S5, ESI‡) with similar Cu–Cu distances (Table 2), indicating that the Cu2L2 core with its additional intra-hydrogen bonding has limited flexibility and probably plays a part in the overall stability of the bound sulfate complex. For the phosphate complex, both data quality and the similarity in size between the dihydrogen phosphate and sulfate anions preclude direct identification of sulfate at the core of this helicate, however, additional evidence for encapsulation of SO42− over H2PO4− is given by HR-MS and elemental analysis on the bulk crystalline sample as used in the X-ray analysis. A detailed description of the structures is given in the ESI.‡
![Comparison of the [SO4⊂Cu2L2]2+ dication for (a) the chloride, (b) nitrate and (c) dihydrogen phosphate mixed anion complexes emphasising the strong similarities between each.](/image/article/2011/CC/c0cc02230f/c0cc02230f-f3.gif) | ||
| Fig. 3 Comparison of the [SO4⊂Cu2L2]2+ dication for (a) the chloride, (b) nitrate and (c) dihydrogen phosphate mixed anion complexes emphasising the strong similarities between each. | ||
The stability of the sulfate core is due to a number of factors including, copper sulfate coordination and an electropositive cavity of protonated ammonium groups. However, it is the enhanced stability of the basic helicate structure brought about by intramolecular hydrogen bonding around the copper centre, coupled with an appropriate span between the copper metal centres that allows this complex to bind sulfate strongly.
Investigations of any sulfate/phosphate discrimination point to a preferred uptake of sulfate over phosphate which is noteworthy given the minor difference between the association constants (Δlog = 0.05).
Work continues in the development of systems related to [Cu2(L–2H)2] which have both a reduced strap size and more rigidity. Such minor variations appear to drastically change the anion preference.
This work was supported by grants from Massey University. For the high resolution mass spectroscopy data we thank Mrs Pat Gread and Prof. Bill Henderson from the University of Waikato. The MacDiarmid Institute for Advanced Materials and Nanotechnology and the Tertiary Education Commission of New Zealand provided funding for the Rigaku Spider optics and detector.
Notes and references
- J.-i. Kim, H. Juwarker, X. Liu, M. S. Lah and K.-S. Jeong, Chem. Commun., 2010, 46, 764–766 RSC.
- B. A. Moyer, L. H. Delmau, C. J. Fowler, A. Ruas, D. A. Bostick, J. L. Sessler, E. Katayev, G. D. Pantos, J. M. Llinares, A. Hossain, S. O. Kang and K. Bowman-James, Adv. Inorg. Chem., 2007, 59, 175–204 CAS; L. R. Eller, M. Stepien, C. J. Fowler, J. T. Lee, J. L. Sessler and B. A. Moyer, J. Am. Chem. Soc., 2007, 129, 11020–11021 CrossRef CAS; K. Bowman-James, Acc. Chem. Res., 2005, 38, 671–678 CrossRef CAS; C. A. Ilioudis and J. W. Steed, J. Supramol. Chem., 2003, 1, 165–187; K. Choi and A. D. Hamilton, Coord. Chem. Rev., 2003, 240, 101–110 CAS; V. Amendola, L. Fabbrizzi, C. Mangano, P. Pallavicini, A. Poggi and A. Taglietti, Coord. Chem. Rev., 2001, 219–221, 821–837 CrossRef CAS; P. A. Gale, Coord. Chem. Rev., 2003, 240, 191–221 CAS; P. A. Gale and R. Quesada, Coord. Chem. Rev., 2006, 250, 3219–3244 CrossRef CAS; P. Mateus, R. Delgado, P. Brandao and V. Felix, J. Org. Chem., 2009, 74, 8638–8646 CrossRef CAS.
- K. Wichmann, B. Antonioli, T. Soehnel, M. Wenzel, K. Gloe, K. Gloe, J. R. Price, L. F. Lindoy, A. J. Blake and M. Schroeder, Coord. Chem. Rev., 2006, 250, 2987–3003 CrossRef CAS; G. Hennrich and E. V. Anslyn, Chem.–Eur. J., 2002, 8, 2218–2224 CrossRef CAS; M. H. Filby and J. W. Steed, Coord. Chem. Rev., 2006, 250, 3200–3218 CrossRef CAS; B. Wu, J. Liang, J. Yang, C. Jia, X.-J. Yang, H. Zhang, N. Tang and C. Janiak, Chem. Commun., 2008, 1762–1764 RSC; I. Ravikumar, P. S. Lakshminarayanan, M. Arunachalam, E. Suresh and P. Ghosh, Dalton Trans., 2009, 4160–4168 RSC; F. Zhuge, B. Wu, J. Liang, J. Yang, Y. Liu, C. Jia, C. Janiak, N. Tang and X.-J. Yang, Inorg. Chem., 2009, 48, 10249–10256 CrossRef CAS; R. Custelcean and P. Remy, Cryst. Growth Des., 2009, 9, 1985–1989 CrossRef CAS; R. J. Warr, A. N. Westra, K. J. Bell, J. Chartres, R. Ellis, C. Tong, T. G. Simmance, A. Gadzhieva, A. J. Blake, P. A. Tasker and M. Schroder, Chem.–Eur. J., 2009, 15, 4836–4850 CrossRef CAS.
- A. Kumar, S.-S. Sun and A. J. Lees, Coord. Chem. Rev., 2008, 252, 922–939 CrossRef CAS; R. S. Forgan, J. E. Davidson, F. P. A. Fabbiani, S. G. Galbraith, D. K. Henderson, S. A. Moggach, S. Parsons, P. A. Tasker and F. J. White, Dalton Trans., 2010, 39, 1763–1770 RSC; J. W. Steed, Chem. Soc. Rev., 2009, 38, 506–519 RSC.
- M. Mahoney Joseph, U. Nawaratna Gayathri, M. Beatty Alicia, J. Duggan Peter and D. Smith Bradley, Inorg. Chem., 2004, 43, 5902–5907 CrossRef CAS; M. Mahoney Joseph, M. Beatty Alicia and D. Smith Bradley, Inorg. Chem., 2004, 43, 7617–7621 CrossRef CAS.
- P. A. Tasker, C. C. Tong and A. N. Westra, Coord. Chem. Rev., 2007, 251, 1868–1877 CrossRef CAS.
- S. G. Galbraith, L. F. Lindoy, P. A. Tasker and P. G. Plieger, Dalton Trans., 2006, 1134–1136 RSC.
- M. Wenzel, G. B. Jameson, L. A. Ferguson, Q. W. Knapp, R. S. Forgan, F. J. White, S. Parsons, P. A. Tasker and P. G. Plieger, Chem. Commun., 2009, 3606–3608 RSC.
- K. Soldenhoff, N. Hayward and D. Wilkins, EPD Congress 1998, Proc. Sess. Symp., held at the TMS Annual Meeting, San Antonio, United States of America, 1998, pp. 153–165 Search PubMed.
- H. Gampp, M. Maeder, C. J. Meyer and A. D. Zuberbuehler, Talanta, 1985, 32, 1133–1139 CrossRef CAS; H. Gampp, M. Maeder, C. J. Meyer and A. D. Zuberbuehler, Talanta, 1985, 32, 257–264 CAS; H. Gampp, M. Maeder, C. J. Meyer and A. D. Zuberbuehler, Talanta, 1985, 32, 95–101 CrossRef CAS; H. Gampp, M. Maeder, C. J. Meyer and A. D. Zuberbuehler, Talanta, 1986, 33, 943–951 CrossRef CAS.
- P. van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, A46, 194–201.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Ligand synthesis, crystal packing, table of weak interactions of the crystal structures, crystal structure of [NO3⊂Cu2L2](NO3)3·2H2O, Job plots of the UV-Vis experiments and HR-MS spectra of [SO4⊂Cu2L2](H2PO4)2. CCDC 783210–783213. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc02230f |
§ Multi-varied analysis of the spectra (SPECFIT/32™),10 assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 anion to [Cu2L2]4+ binding model as supported by Job plots (see ESI‡). 1 anion to [Cu2L2]4+ binding model as supported by Job plots (see ESI‡). |
¶ Characterisation of complexes: [SO4⊂Cu2L2]Cl2·2.5H2O: green solid, yield 41%, MS(ESI) m/z 666 [Cu2L2SO4]2+; anal. calc. for C64H100Cu2N8O12Cl2S·2.5H2O: C 53.06, H 7.31, N 7.74%; found: C 53.02, H 7.18, N 7.65%. Green plate crystals suitable for X-ray diffraction analysis were isolated by slow diffusion of diethyl ether into an acetonitrile/ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) containing the complex over one week. Crystal data for C66H108Cl2Cu2N8O14S, M = 1467.64, monoclinic, a = 19.805(4) Å, b = 20.320(4) Å, c = 21.840(4) Å, β = 109.52(3)°, V = 8283(3) Å3, T = 100 K, P21/c (no. 14), Z = 4, 72 1) containing the complex over one week. Crystal data for C66H108Cl2Cu2N8O14S, M = 1467.64, monoclinic, a = 19.805(4) Å, b = 20.320(4) Å, c = 21.840(4) Å, β = 109.52(3)°, V = 8283(3) Å3, T = 100 K, P21/c (no. 14), Z = 4, 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 reflections measured, 14 361 reflections measured, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014 unique (Rint = 0.0821) of which 14 014 unique (Rint = 0.0821) of which 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014 were used in the calculations, R1 = 0.0650 (9908 with F > 2σ(F)). Disordered solvent regions in this complex were treated in the manner described by van der Sluis and Spek,11 as 234 e− per cell, approx. to 3.25(CH3OH) (=72 e−) per formula unit. [SO4⊂Cu2L2](NO3)2·H2O·3EtOH·MeCN: green solid, yield 88%, MS(ESI) m/z 666 [Cu2L2SO4]2+; anal. calc. for C64H100Cu2N10O18S·H2O·3EtOH·MeCN: C 52.29, H 7.50, N 9.32%, found: C 52.43, H 7.21, N 9.11%. Green block crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetonitrile/ethanol mixture (1 014 were used in the calculations, R1 = 0.0650 (9908 with F > 2σ(F)). Disordered solvent regions in this complex were treated in the manner described by van der Sluis and Spek,11 as 234 e− per cell, approx. to 3.25(CH3OH) (=72 e−) per formula unit. [SO4⊂Cu2L2](NO3)2·H2O·3EtOH·MeCN: green solid, yield 88%, MS(ESI) m/z 666 [Cu2L2SO4]2+; anal. calc. for C64H100Cu2N10O18S·H2O·3EtOH·MeCN: C 52.29, H 7.50, N 9.32%, found: C 52.43, H 7.21, N 9.11%. Green block crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetonitrile/ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over 3 days. Crystal data for C72H121Cu2N11O22S, M = 1651.94, triclinic, a = 10.464(2) Å, b = 18.668(4) Å, c = 21.678(4) Å, α = 78.77(3)°, β = 85.67(3)°, γ = 85.96(3)°, V = 4135.0(14) Å3, T = 123 K, P 1) over 3 days. Crystal data for C72H121Cu2N11O22S, M = 1651.94, triclinic, a = 10.464(2) Å, b = 18.668(4) Å, c = 21.678(4) Å, α = 78.77(3)°, β = 85.67(3)°, γ = 85.96(3)°, V = 4135.0(14) Å3, T = 123 K, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), Z = 2, 71 (no. 2), Z = 2, 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 567 reflections measured, 12 567 reflections measured, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 unique (Rint = 0.0921) of which 12 207 unique (Rint = 0.0921) of which 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 were used in the calculations, R1 = 0.0667 (8871 with F > 2σ(F)). [SO4⊂Cu2L2](H2PO4)2: yield 55%, MS(ESI) m/z 666 [Cu2L2SO4]2+, anal. calc. for C64H104Cu2N8O20P2S: C 50.35, H 6.87, N 7.34%, found: C 50.20, H 7.10, N 7.20%. Green chip crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetonitrile/methanol/ethanol mixture (1 207 were used in the calculations, R1 = 0.0667 (8871 with F > 2σ(F)). [SO4⊂Cu2L2](H2PO4)2: yield 55%, MS(ESI) m/z 666 [Cu2L2SO4]2+, anal. calc. for C64H104Cu2N8O20P2S: C 50.35, H 6.87, N 7.34%, found: C 50.20, H 7.10, N 7.20%. Green chip crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetonitrile/methanol/ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over 2 days. Crystal data C64H100Cu2N8O20P2S, M = 1522.60, orthorhombic, a = 20.2187(4) Å, b = 21.9011(4) Å, c = 38.781(3) Å, V = 17172.6(13) Å3, T = 123 K, Pbca (no. 61), Z = 8, 126 1) over 2 days. Crystal data C64H100Cu2N8O20P2S, M = 1522.60, orthorhombic, a = 20.2187(4) Å, b = 21.9011(4) Å, c = 38.781(3) Å, V = 17172.6(13) Å3, T = 123 K, Pbca (no. 61), Z = 8, 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497 reflections measured, 13 497 reflections measured, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 unique (Rint = 0.1223) of which 13 160 unique (Rint = 0.1223) of which 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 were used in the calculations, R1 = 0.0758 (7141 with F > 2σ(F)). Disordered solvent regions were treated in the manner described by van der Sluis and Spek,11 as 590 e− per cell, approximating to 1(CH3CH2OH), 2(CH3OH) and 1(H2O) (=72 e−) per formula unit. [NO3⊂Cu2L2](NO3)3·2H2O: yield 92%, MS(ESI) m/z 649 [Cu2L2NO3–H]2+; anal. calc. for C64H100Cu2N12O20·2H2O: C 50.55, H 6.89, N 11.05%, found: C 50.59, H 6.79, N 10.89%. Green chip crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetone/ethanol mixture (1 160 were used in the calculations, R1 = 0.0758 (7141 with F > 2σ(F)). Disordered solvent regions were treated in the manner described by van der Sluis and Spek,11 as 590 e− per cell, approximating to 1(CH3CH2OH), 2(CH3OH) and 1(H2O) (=72 e−) per formula unit. [NO3⊂Cu2L2](NO3)3·2H2O: yield 92%, MS(ESI) m/z 649 [Cu2L2NO3–H]2+; anal. calc. for C64H100Cu2N12O20·2H2O: C 50.55, H 6.89, N 11.05%, found: C 50.59, H 6.79, N 10.89%. Green chip crystals suitable for X-ray diffraction analysis were grown by slow diffusion of diethyl ether into an acetone/ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶ ∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) over 1 week. Crystal data for C64H100Cu2N12O20, M = 1484.64, monoclinic, a = 11.847(2) Å, b = 19.317(4) Å, c = 37.831(8) Å, β = 93.95(3)°, V = 8637(3) Å3, T = 123 K, P21/c (no. 14), Z = 4, 84 1) over 1 week. Crystal data for C64H100Cu2N12O20, M = 1484.64, monoclinic, a = 11.847(2) Å, b = 19.317(4) Å, c = 37.831(8) Å, β = 93.95(3)°, V = 8637(3) Å3, T = 123 K, P21/c (no. 14), Z = 4, 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 887 reflections measured, 13 887 reflections measured, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 unique (Rint = 0.1092) of which 13 237 unique (Rint = 0.1092) of which 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 were used in the calculations, R1 = 0.0860 (9141 with F > 2σ(F)). Disordered solvent regions were treated in the manner described by van der Sluis and Spek,11 as 250 e− per cell, approximating to 2(CH3CH2OH) and 0.75(H2O) (=61.5 e−) per formula unit. 237 were used in the calculations, R1 = 0.0860 (9141 with F > 2σ(F)). Disordered solvent regions were treated in the manner described by van der Sluis and Spek,11 as 250 e− per cell, approximating to 2(CH3CH2OH) and 0.75(H2O) (=61.5 e−) per formula unit. |
| This journal is © The Royal Society of Chemistry 2011 |
