Quantitative, label-free and site-specific monitoring of molecular recognition: a multivariate resonance Raman approach†‡
Stephan
Niebling
a,
Hannes Y.
Kuchelmeister
b,
Carsten
Schmuck
b and
Sebastian
Schlücker
*a
aUniversity of Osnabrück, Department of Physics, Barbarastr. 7, 49069 Osnabrück, Germany. E-mail: sebastian.schluecker@uos.de; Fax: +49 541-96913592; Tel: +49 541-9693592
bInstitute of Organic Chemistry, University Duisburg-Essen, Universitätsstr. 7, 45117 Essen, Germany. E-mail: carsten.schmuck@uni-due.de; Fax: +49 201-1834259; Tel: +49 201-1833097
First published on 7th September 2010
Abstract
A site-specific and quantitative approach for label-free monitoring of molecular recognition is presented. Specifically, the binding site of an artificial receptor is probed selectively by UVRR spectroscopy. The ligand binding constant can be determined by non-negative matrix factorization.
The association of ligands by biological or artificial receptors is of great importance in many fields. Examples range from the screening of potential drug candidates to molecular recognition of ligands by small artificial or large biological receptors for signal transduction. The central thermodynamic quantity for binding efficiency is the association or binding constant.1 There exist numerous techniques to determine binding constants, including isothermal titration calorimetry, mass spectrometry, NMR and SPR spectroscopy. Among the most common approaches are electronic spectroscopies such as fluorescence and absorption measurements.2 Vibrational spectroscopic techniques based on infrared absorption or Raman scattering are very sensitive towards small deviations in bond lengths and strengths, making them ideal candidates for label-free monitoring of molecular recognition events in solution. In particular resonance Raman (RR) spectroscopy additionally offers selectivity and sensitivity as two important advantages. Firstly, the signal from a particular chromophoric subunit of the receptor, e.g. the binding site, can be selectively enhanced by tuning the laser excitation wavelength to the respective electronic absorption band. Due to the enhancement, this molecular part of the receptor can be selectively monitored without spectral interferences from the ligand or the rest of the receptor. Secondly, investigations at micromolar concentrations under physiologically relevant conditions in water are possible due to the RR effect, and the resulting signal increase by a few orders of magnitude (typically 3–4); this would not be feasible with electronically non-resonant Raman spectroscopy. Surprisingly, this approach has only rarely been exploited so far for monitoring molecular recognition.3,4 As one of the very few examples, Flood and coworkers have recently employed RR spectroscopy to determine the binding constant of an organic host–guest complex in solution by analyzing signal band intensities of a charge-transfer complex.4
Here, we present a label-free resonance Raman approach to determine the binding constant between an artificial peptide receptor and a tetrapeptide ligand in a multivariate manner.
Artificial peptide receptors are an important tool in bioorganic chemistry to understand the principles of molecular recognition of peptides and proteins in nature.5 The class of artificial peptide receptors developed by Schmuck and coworkers show remarkable properties: despite their small size, high binding constants to peptides even in polar solvents like water and a high sequence- and diastereoselectivity can be observed.6,7
The structure of the peptide receptor CBS-KKF is shown in Fig. 1. The most important part is a guanidiniocarbonyl pyrrole cation that binds the C-terminus of the peptide ligand. This carboxylate binding site (CBS) strongly interacts with the C-terminus of peptides by a combination of electrostatic interaction and hydrogen bonds (Fig. 1, top right).8 Sequence- and diastereoselectivity towards a given ligand is introduced by the tripeptide part (R in Fig. 1), in this case L-Lys-L-Lys-L-Phe-NH2 (KKF). In solution there is an acid/base equilibrium between the protonated and the neutral CBS (HA+ and A in Fig. 1). The ratio between the protonated (HA+) and the neutral species (A) is determined by the Henderson–Hasselbalch equation:
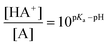 | (1) |
 | ||
| Fig. 1 The peptide receptor CBS-KKF comprises the carboxylate binding site (CBS) and a tripeptide part (R = L-Lys-L-Lys-L-Phe-NH2, KKF). In solution there is an acid/base equilibrium (2) between the protonated (HA+, top left) and the neutral CBS (A, bottom). For the sake of clarity, the solvation by water is not shown, and only one tautomer of the neutral CBS (A) is depicted. Upon addition of a peptide ligand (L−), the complexed CBS (HA+⋯L−, top right) appears as a third species (1). | ||
If a ligand (L−) is added, the ion paired complex HA+⋯L− (Fig. 1, top right) appears as a third species. In the case of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation, the association constant Kassoc and the ligand concentration determine the ratio between the complex (HA+⋯L−) and the protonated CBS (HA+):
1 complexation, the association constant Kassoc and the ligand concentration determine the ratio between the complex (HA+⋯L−) and the protonated CBS (HA+):
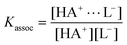 | (2) |
Since the CBS shows a strong electronic absorption at 300 nm,3 we employ UV resonance Raman (UV RR) spectroscopy with the 275 nm line from an argon ion laser to selectively monitor the cationic oxoanion binding subunit of the receptor.3,9,10 In the absence of a peptide ligand, the experimental UV RR spectra can be described as a superposition of two basis spectra: the spectrum of the protonated CBS and the spectrum of the neutral CBS (top left and bottom of Fig. 1, respectively).
For a binding study, 11 UV RR spectra of the receptor CBS-KKF (c = 1 mM) at pH 6.0 in the range between 0 and 10 equivalents of the ligand AE3 were acquired. No buffer was used to avoid high salt concentrations and a potential interference with ligand binding. To compensate for the lack of buffer, the pH was carefully adjusted (pH = 6.00 ± 0.01) and measured again after the acquisition of each Raman spectrum. The mean value of the absolute deviation after each Raman measurement is ΔpH = ±0.05. The spectra at 0, 1.2 and 10 equivalents are depicted in Fig. 2. Compared to our previous pH study,8,9 the spectral changes upon addition of the ligand are significantly less pronounced; minor intensity changes occur at 940 cm−1, 955 cm−1 and in the region around 1630 cm−1. The most pronounced changes are observed for the bands around 1440 cm−1 and 1470 cm−1. These bands, which show an intensity change and a slight shift to higher wavenumber values (1470 cm−1 band) upon addition of the ligand, can be mainly assigned to bending modes of the guanidinio part of the CBS. The occurrence of only small wavenumber shifts can be explained by a coordination of the binding site with water in the absence of the ligand.
 | ||
| Fig. 2 Resonance Raman binding study of 1 mM CBS-KKF in water with an excitation wavelength of 275 nm: UV RR spectra of the peptide receptor CBS-KKF (0.0 eq.) and at two different ligand (AE3) concentrations. The most sensitive bands towards complexation appear around 1440 and 1470 cm−1. | ||
In the first step, the 11 experimental UV RR spectra were used to determine the concentration of the complex species at the respective ligand concentrations. In order to extract these complex concentrations, non-negative matrix factorization (NMF) was used.11 Hereby, a matrix, which contains the experimental spectra as columns (Fig. 3, left), is factorized into the product of a matrix that contains the component spectra (Fig. 3, middle) and a matrix that contains the contributions of the components in each experimental spectrum. This multivariate approach takes into account the whole accessible spectral information. Since the pH is constant during the whole binding study, the ratio between the protonated and the neutral species is constant. NMF is not capable of resolving the respective component spectra individually as long as their ratio is constant in each experimental spectrum. Therefore, we assumed the presence of only two distinct species for the NMF analysis: the ligand-bound and the free receptor (Fig. 3). The component spectrum of the free receptor is a linear combination of the spectra of the protonated and the neutral species with a ratio of 3.5 (cf.Fig. 1). As unconstrained NMF yields non-unique solutions, we introduced additional constraints based on chemical considerations to solve the matrix equation in Fig. 3:
 | ||
| Fig. 3 The left matrix, containing the experimental spectra at different ligand concentrations from the UV RR binding study (Fig. 2), was factorized into a product of one matrix that contains the spectra of the free receptor and the complex (column vectors) and a matrix that contains the contributions of each component at different ligand concentrations (row vectors). In a second step, the contributions of the complex were used to determine the association constant Kassoc. | ||
1. The sum of the contributions in each column of the contribution matrix (3rd matrix in Fig. 3) was set to a constant value, because the molar amount of receptor (sum of free receptor and complex) is constant for each experimental spectrum (1 mM).
2. The experimental spectra at 0 and 10 equivalents of the ligand were used as component spectra of the free and ligand-bound component, respectively (2nd matrix in Fig. 3).§
As a second step, the complex concentrations at different ligand concentrations contained in the contribution matrix (Fig. 3, bottom right) were used to determine the association constant (Kassoc) by a non-linear least-square fitting procedure with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation model (cf.eqn (2)). The degree of complexation is the ratio between the complex concentration and the initial receptor concentration:
1 complexation model (cf.eqn (2)). The degree of complexation is the ratio between the complex concentration and the initial receptor concentration:
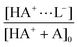 | (3) |
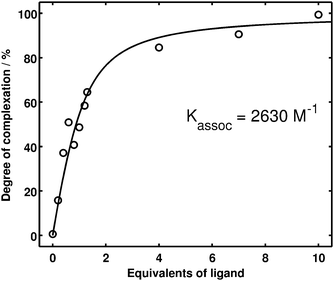 | ||
| Fig. 4 Quantitative evaluation of the UV RR binding study. The degree of complexation (circles) was determined by a non-negative matrix factorization (cf.Fig. 3) of the experimental spectra (cf.Fig. 2). The solid line is obtained from a non-linear regression, yielding an association constant of Kassoc = 2630 M−1. | ||
The information offered by this label-free methodology will help to get further insights into the pronounced sequence- and diastereoselectivity of the CBS-based receptors by comparing the binding studies performed with the same receptor but different ligands. Moreover, it can be extended to other artificial or natural receptors. The selectivity of the presented UV RR approach may also be useful for receptors with multiple binding sites in order to monitor binding only at one particular site.
Future work will aim at several aspects of resonance Raman binding studies on CBS-based receptors. First of all, new methods for data analysis should be investigated. There is still potential for improvement since we constrained the component spectra to the experimental spectra with 0 and 10 equivalents of ligand. In the case of relatively low binding constants (Kassoc < 1000 M−1), less constrained algorithms are desired, which optimize both the component spectra and the contribution matrix. This would enable the determination of concentrations without the restriction to experimentally accessible component spectra. Secondly, deep UV resonance Raman spectroscopy can be used to selectively monitor the peptide backbones of the receptor and its ligand, thus accessing further information about the interaction between these parts. Finally, it is necessary to establish spectra–structure correlations via quantum chemical calculations, in order to interpret the spectral changes observed upon molecular recognition.
Financial support from the Fonds der Chemischen Industrie (FCI doctoral fellowship for S.N.), the Studienstiftung des deutschen Volkes (doctoral fellowship for H.K.) and the German Research Foundation (DFG; Heisenberg fellowship for S.S.; SFB 630: A3 and C1 for C.S. and S.S.) is gratefully acknowledged.
Notes and references
- K. Connors, Binding constants: the measurement of molecular complex stability, John Wiley & Sons, New York, 1987 Search PubMed.
- E. V. Anslyn and D. A. Dougherty, Modern physical organic chemistry, University Science Books, 2006 Search PubMed.
- B. Küstner, C. Schmuck, P. Wich, C. Jehn, S. K. Srivastava and S. Schlücker, Phys. Chem. Chem. Phys., 2007, 9, 4598–4603 RSC.
- E. H. Witlicki, S. W. Hansen, M. Christensen, T. S. Hansen, S. D. Nygaard, J. O. Jeppesen, E. W. Wong, L. Jensen and A. H. Flood, J. Phys. Chem. A, 2009, 113, 9450–9457 CrossRef CAS.
- H.-J. Schneider, Angew. Chem., Int. Ed. Engl., 1993, 32, 848–850 CrossRef.
- C. Schmuck and M. Heil, Chem.–Eur. J., 2006, 12, 1339–1348 CrossRef CAS.
- C. Schmuck and P. Wich, Angew. Chem., Int. Ed., 2006, 45, 4277–4281 CrossRef CAS.
- D. Moiani, C. Cavallotti, A. Famulari and C. Schmuck, Chem.–Eur. J., 2008, 14, 5207–5219 CrossRef CAS.
- S. Niebling, S. K. Srivastava, C. Herrmann, P. R. Wich, C. Schmuck and S. Schlücker, Chem. Commun., 2010, 46, 2133–2135 RSC.
- S. K. Srivastava, S. Niebling, B. Küstner, P. R. Wich, C. Schmuck and S. Schlücker, Phys. Chem. Chem. Phys., 2008, 10, 6770–6775 RSC.
- D. D. Lee and H. S. Seung, Nature, 1999, 401, 788–791 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: 1. Experimental electronic absorption spectrum of the receptor CBS-KKF; 2. experimental UV resonance Raman binding study; 3. calculated contributions of free receptor and complex to the experimental spectrum of CBS-KKF and 1.2 eq. of the ligand AE3. See DOI: 10.1039/c0cc02052d |
| § For the concentrations used in this binding study this assumption is valid for association constants larger than 1000 M−1, i.e. more than 90% complexation at 10 equivalents of ligand. Typical binding constants for CBS-KKF and various tetrapeptides range from 103–104,6 so that our assumption is legitimate if the error introduced this way has to be smaller than 10%. Naturally occurring receptors show much higher binding constants (e.g. approx. 1015 M−1 for Biotin/Avidin), so that the error is expected to be much smaller. |
| This journal is © The Royal Society of Chemistry 2011 |
