Pyrene-fused subphthalocyanine†‡
Soji
Shimizu
*,
Shota
Nakano
,
Takahisa
Hosoya
and
Nagao
Kobayashi
*
Department of Chemistry, Graduate School of Science, Tohoku University, Sendai 980-8578, Japan. E-mail: ssoji@m.tohoku.ac.jp; nagaok@m.tohoku.ac.jp; Fax: +81-22-795-7728; Tel: +81-22-795-7728
First published on 23rd August 2010
Abstract
A pyrene-fused subphthalocyanine synthesized from a reaction of 2,7-di-tert-butyl-4,5,9,10-tetracyanopyrene and tetrafluorophthalonitrile exhibits red-shifted Q-band absorption and a unique linear arrangement in the solid state caused by a π–π stacking interaction. The concave conjugation of the SubPc moiety and the planar conjugation of the pyrene moiety enhanced its co-crystallization with C60 molecules.
Subphthalocyanine (SubPc)1 comprising three isoindole units linked by aza-nitrogen atoms has attracted much attention as a functional molecule, due to the intense fluorescence, unique non-linear optical properties,2 and inherent molecular chirality when unsymmetrically substituted,3 which stem from the bowl-shaped molecular structures with an aromatic 14π-conjugation system. Its large π-conjugation curvature also provides a surface for convex–concave interaction, which was first suggested experimentally by Torres and Claessens with facile encapsulation of a C60 molecule into their SubPc cage.4 Peripheral π-expansion and contraction have been important modification methods towards novel conjugated molecules with different properties, as have been intensively investigated in the phthalocyanine chemistry.5 However, these types of modified SubPc species have been limited to only a few examples, such as subnaphthalocyanine,6subtriazaporphyrin,6a,7 and A2B and AB2 type low-symmetry subnaphthalocyanines.8 We report herein, a novel peripherally π-expanded SubPc analogue containing a pyrene unit (pyrene-fused subphthalocyanine, PySubPc) which was synthesized using 2,7-di-tert-butyl-4,5,9,10-tetracyanopyrene as a key synthetic precursor. Fusion of a pyrene unit to the periphery causes an alteration of the spectroscopic properties and leads to a unique molecular packing diagram in the solid state. Due to the combination of the planar π-conjugation system of pyrene and the curved π-conjugation system of the SubPc, convex–concave interaction with a C60 molecule was enhanced, to give a SubPc-C60 cocrystallate, the structure of which was elucidated for the first time by X-ray crystallographic analysis.
A key precursory nitrile for PySubPc synthesis, 2,7-di-tert-butyl-4,5,9,10-tetracyanopyrene (1), was synthesized by a reaction of 2,7-di-tert-butyl-4,5,9,10-tetrabromopyrene9 with CuCN in 1,3-dimethyl-2-imidazolidinone (DMI) at 220 °C. A mixed-condensation reaction of 1 and tetrafluorophthalonitrile in the presence of boron trichloride (1.0 M solution in p-xylene) in 1-chloronaphthalene at 230 °C provided a reaction mixture which included perfluorinated SubPc (2) and PySubPc (3) (Scheme 1).10 The axial chlorine ligand of 3 was replaced by a phenoxy group prior to silica gel column separation in order to prevent adsorption of 3 and its axially hydroxy substituted species formed during column separation onto silica gel. The mixture was then purified by silica gel chromatography and recycling GPC-HPLC to provide axially phenoxy substituted PySubPc (4) in 0.9% yield.
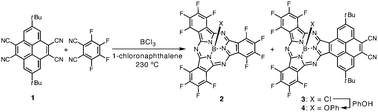 | ||
| Scheme 1 Synthesis of a pyrene-fused subphthalocyanine. | ||
The high-resolution FT-ICR mass spectrum characterized the molecule with a molecular ion peak at m/z = 941.2160 (calcd for C50H27B1F8N8O1Na1 = 941.2165 [M+ + Na]). The mass spectrum revealed that one of two phthalonitrile moieties of the starting tetracyanopyrene remains intact. The 1H NMR spectrum of 4 in CDCl3 exhibits two singlet peaks due to the pyrene unit at 10.71 and 8.85 ppm, one of which shows a fairly down-field shift due to the diatropic ring current effect of the subphthalocyanine moiety. Three peaks due to the axial phenoxy ligand are observed at 6.85 (meta), 6.72 (para), and 5.50 (ortho) ppm and tert-butyl protons are observed at 1.85 ppm. Finally, the structure was elucidated by X-ray single crystallographic analysis.§4 takes an essentially similar bowl-shaped structure to those of general SubPcs, with deviation of a boron atom by 0.63 Å from the mean plane defined by three boron-coordinating nitrogen atoms (Fig. 1).2 The axial phenoxy ligand takes a parallel arrangement and points the opposite direction relative to the pyrene moiety, which then serves for co-facial π–π stacking with the nearest neighbor in a head-to-tail manner. The opposite surface of the pyrene moiety contributes to π–π stacking with the pyrene moiety of another nearest neighbor. These π–π stacking interactions lead to a unique one-dimensional packing diagram in the solid state (Fig. 1c).
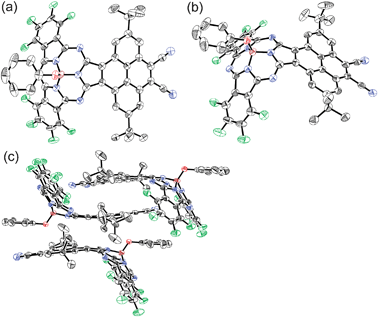 | ||
| Fig. 1 X-ray crystal structure of 4: (a) top view, (b) side view, and (c) molecular packing diagram. The thermal ellipsoids are scaled to the 50% probability level. Hydrogen atoms of 4 and solvent molecules are omitted for clarity. | ||
The absorption spectrum of 4 in CHCl3 exhibits a split Q-band absorption at 593 and 565 nm with shoulders at 547 and 515 nm (Fig. 2). In the higher energy region, a sharp absorption at 305 nm is observed along with broad bands at 290 nm. Compared to the absorption spectrum of 2 exhibiting a sharp Q-band at 573 nm, the Q-band of 4 exhibits a slight red-shift by 20 nm and becomes rather complex.
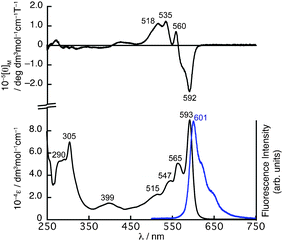 | ||
| Fig. 2 UV/vis absorption (bottom) and MCD (top) spectra of 4 in CHCl3. The fluorescence spectrum (bottom) in CHCl3 is shown as a blue line. | ||
The magnetic circular dichroism (MCD) spectrum of 4 shows negative and positive MCD signals at 592 and 560 nm in the Q-band region followed by positive MCD signals at 535 and 518 nm. The signal pattern in the Q-band region of the MCD spectrum can be assigned as Faraday B terms, which are generally observed for molecules having a symmetry axis lower than C3, and these kinds of SubPc molecules generally have non-degenerated excited states.11
TDDFT calculations at the B3LYP/6-31G(d) level predicted three transitions at 535, 485, and 469 nm having oscillator strengths greater than 0.1 in the Q-band region, which are mainly composed of transitions from the HOMO to LUMO, HOMO to LUMO + 1, and HOMO − 1 to LUMO + 2, respectively (see ESI‡). The frontier molecular orbitals related to these bands are depicted in Fig. 3. The density distribution pattern of the HOMO is similar to that of 2, having electron density mainly on the SubPc moiety though the electron density is delocalized on the pyrene moiety to some extent, while the nodal patterns of the LUMO and LUMO + 1 of 4 are exchanged relative to those of 2 due to stabilization of the LUMO + 1 of 2 upon fusion of the electron-withdrawing dicyanopyrene unit. This results in non-degeneracy of the excited states as suggested by the Faraday B terms in the Q-band region of the MCD spectrum. The HOMO − 1, LUMO + 2, and LUMO + 3 are mainly localized on the pyrene moiety, and these orbitals have the main contribution to the bands at 393 and 296 nm in the TDDFT calculations. Based on the calculations, the band at 469 nm is a kind of intramolecular charge transfer band composed of transition from the HOMO − 1 to LUMO + 1, which is implied by solvent-dependent changes in the shapes of the Q-band (see ESI‡). Although these bands cannot be easily assigned in the absorption spectrum of 4 due to the overlap of several vibronic bands, the predicted contribution of these bands indicates that the electronic structure of the pyrene unit is rather maintained in the electronic structure of 4.
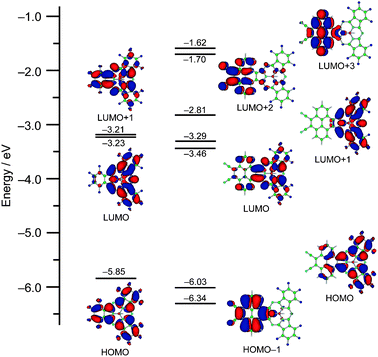 | ||
| Fig. 3 Partial MO diagram of 2 (left) and 4 (right). | ||
PySubPc 4 exhibits intense fluorescence at 601 nm with a Stokes shift of 224 cm−1 upon excitation at any wavelength at which 4 absorbs (Fig. 2). The fluorescence quantum yield of 0.0712 and the lifetime of 1.3 ns are relatively smaller and shorter than those of general SubPcs.2
The mutual π–π stacking of 4 in the solid state motivated us to investigate co-crystallization of 4 with fullerene molecules. Slow vapor diffusion of hexane into a toluene solution of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4 and C60 provided black, brick-like crystals of cocrystallates (4-C6060) suitable for X-ray crystallographic diffraction analysis (Fig. 4a).§ As is common in fullerene-containing crystal structures, the disorder of fullerene makes it difficult to determine the exact positions of the carbon atoms of fullerene, but the structural features can be adequately deduced.13 Two PySubPc molecules embrace a C60 molecule, and both the pyrene moiety and the SubPc moiety contribute to form π–π stacking with C60 with intermolecular separation of ca. 3.3 Å. The opposite surface of the pyrene moiety of 4 exhibits π–π stacking with the axial phenoxy ligand of the nearest neighbor, to form a dimer in a head-to-tail manner. These interactions thus lead to a one-dimensional zigzag array of 4 in which C60 molecules are ordered in a linear fashion along the a-axis (Fig. 4b). C60 molecules are also arranged linearly along the b-axis (Fig. 4c). The centre-to-centre separations of the C60 molecules are 16.9 Å along the a-axis and 14.8 Å along the b-axis. Although convex–concave interaction between C60 and SubPc has been highly anticipated,4b,14 to the best of our knowledge, this is the first report of the crystal structure of a cocrystallate of C60 and SubPc. The highly selective inclusion of C60 by 4 encouraged us to investigate molecular recognition in solution, but the absorption spectrum of the dissolved cocrystallates in CHCl3 merely shows a summation of the absorption spectra of 4 and C60 indicative of less significant supramolecular interactions in solution.
1 mixture of 4 and C60 provided black, brick-like crystals of cocrystallates (4-C6060) suitable for X-ray crystallographic diffraction analysis (Fig. 4a).§ As is common in fullerene-containing crystal structures, the disorder of fullerene makes it difficult to determine the exact positions of the carbon atoms of fullerene, but the structural features can be adequately deduced.13 Two PySubPc molecules embrace a C60 molecule, and both the pyrene moiety and the SubPc moiety contribute to form π–π stacking with C60 with intermolecular separation of ca. 3.3 Å. The opposite surface of the pyrene moiety of 4 exhibits π–π stacking with the axial phenoxy ligand of the nearest neighbor, to form a dimer in a head-to-tail manner. These interactions thus lead to a one-dimensional zigzag array of 4 in which C60 molecules are ordered in a linear fashion along the a-axis (Fig. 4b). C60 molecules are also arranged linearly along the b-axis (Fig. 4c). The centre-to-centre separations of the C60 molecules are 16.9 Å along the a-axis and 14.8 Å along the b-axis. Although convex–concave interaction between C60 and SubPc has been highly anticipated,4b,14 to the best of our knowledge, this is the first report of the crystal structure of a cocrystallate of C60 and SubPc. The highly selective inclusion of C60 by 4 encouraged us to investigate molecular recognition in solution, but the absorption spectrum of the dissolved cocrystallates in CHCl3 merely shows a summation of the absorption spectra of 4 and C60 indicative of less significant supramolecular interactions in solution.
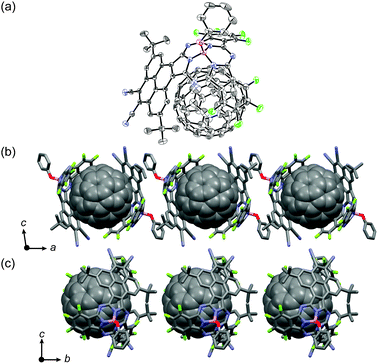 | ||
| Fig. 4 (a) Crystal structure of cocrystallate 4-C6060. The thermal ellipsoids are scaled to the 50% probability level. Hydrogen atoms of 4 are omitted for clarity. Packing diagrams along the a-axis (b) and b-axis (c). | ||
In summary, a novel periphery π-expanded PySubPc, synthesized from the reaction of 2,7-di-tert-butyl-4,5,9,10-tetracyanopyrene and tetrafluorophthalonitrile, exhibits red-shifted Q-band absorption and several shoulders due to alteration of the electronic structure by incorporation of the peripheral pyrene moiety. In addition, an enhanced π–π stacking interaction of PySubPc upon fusion of the pyrene moiety enables a unique linear arrangement of this molecule in the solid state, and the wrapping of a C60 molecule by two PySubPc molecules in the cocrystallates. The C60 molecules included in the concave spheres created by pairs of PySubPc molecules are linearly ordered. The strong interaction of periphery expanded SubPc with C60 is of interest with respect to molecular recognition in solution and supramolecular architecture in the solid state. This kind of investigation is highly promising for pyrene-bridged dimer systems which can be synthesized under similar reaction conditions. Further investigation along this direction is currently being undertaken in our laboratory.
This work was partly supported by a Grant-in-Aid for Scientific Research on Innovative Areas (No. 20108001, “pi-Space”) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and a Grant-in-Aid for Young Scientists (B) (No. 20750025) from Japan Society for the Promotion of Science (JSPS). The authors thank Prof. T. Iwamoto and Dr S. Ishida, Tohoku University for X-ray measurement, Prof. H. Uno and Dr S. Mori, Ehime University for X-ray analysis, and Dr E. Kwon, Tohoku University for helpful discussion.
Notes and references
- A. Meller and A. Ossko, Monatsh. Chem., 1972, 103, 150 CAS.
- C. G. Claessens, D. González-Rodríguez and T. Torres, Chem. Rev., 2002, 102, 835 CrossRef CAS.
- (a) C. G. Claessens and T. Torres, Tetrahedron Lett., 2000, 41, 6361 CrossRef CAS; (b) C. G. Claessens and T. Torres, Chem.–Eur. J., 2000, 6, 2168 CrossRef CAS; (c) N. Kobayashi and T. Nonomura, Tetrahedron Lett., 2002, 43, 4253 CrossRef CAS.
- (a) C. G. Claessens and T. Torres, J. Am. Chem. Soc., 2002, 124, 14522 CrossRef CAS; (b) C. G. Claessens and T. Torres, Chem. Commun., 2004, 1298 RSC.
- (a) N. Kobayashi, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 2003, vol. 15, p. 161 Search PubMed; (b) A. N. Cammidge and H. Gopee, Chem.–Eur. J., 2006, 12, 8609 CrossRef CAS; (c) Y. Fogel, M. Kastler, Z. H. Wang, D. Andrienko, G. J. Bodwell and K. Mullen, J. Am. Chem. Soc., 2007, 129, 11743 CrossRef CAS.
- (a) J. Rauschnabel and M. Hanack, Tetrahedron Lett., 1995, 36, 1629 CrossRef CAS; (b) S. Nonell, N. Rubio, B. del Rey and T. Torres, J. Chem. Soc., Perkin Trans. 2, 2000, 1091 RSC.
- (a) J. R. Stork, J. J. Brewer, T. Fukuda, J. P. Fitzgerald, G. T. Yee, A. Y. Nazarenko, N. Kobayashi and W. S. Durfee, Inorg. Chem., 2006, 45, 6148 CrossRef CAS; (b) N. Kobayashi, T. Ishizaki, K. Ishii and H. Konami, J. Am. Chem. Soc., 1999, 121, 9096 CrossRef CAS.
- J. R. Stork, R. J. Potucek, W. S. Durfee and B. C. Noll, Tetrahedron Lett., 1999, 40, 8055 CrossRef CAS.
- Y. Miura and E. Yamano, J. Org. Chem., 1995, 60, 1070 CrossRef CAS.
- Pyrene-bridged dimers were also formed under the same reaction conditions and characterized preliminarily by MALDI-TOF mass spectrum of the reaction mixture.
- T. Fukuda, J. R. Stork, R. J. Potucek, M. M. Olmstead, B. C. Noll, N. Kobayashi and W. S. Durfee, Angew. Chem., Int. Ed., 2002, 41, 2565 CrossRef CAS.
- Quantum yield was determined relative to Rhodamine 6G (ΦF = 0.94 in ethanol) M. Fischer and J. Georges, Chem. Phys. Lett., 1996, 260, 115 Search PubMed.
- P. D. W. Boyd and C. A. Reed, Acc. Chem. Res., 2005, 38, 235 CrossRef CAS.
- C. G. Claessens, D. González-Rodríguez, R. S. Iglesias and T. Torres, C. R. Chim., 2006, 9, 1094 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Synthesis, 1H NMR spectrum of 4, and TDDFT calculation. CCDC 780572, 780573. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01877e |
§ Crystallographic data for 4: C52H29B1N8O1F8Cl6, MW = 1157.34, monoclinic, space groupC2/c (no. 15), a = 22.946(4), b = 24.128(4), c = 19.003(3) Å, β = 99.940(2)°, V = 10363(3) Å3, Z = 8, ρcalcd = 1.484 g cm−3, T = −173(2) °C, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 288 measured reflections, 9133 unique reflections (Rint = 0.0480), R = 0.0990 (I > 2σ(I)), Rw = 0.3166 (all data), GOF = 1.033, CCDC 780572. Crystallographic data for 4-C6060: C80H27B1N8O1F8, MW = 1278.91, monoclinic, space groupP2/n (no. 13), a = 16.8540(18), b = 14.8124(16), c = 24.597(3) Å, β = 99.270(1)°, V = 6060.5(11) Å3, Z = 4, ρcalcd = 1.402 g cm−3, T = −173(2) °C, 66 288 measured reflections, 9133 unique reflections (Rint = 0.0480), R = 0.0990 (I > 2σ(I)), Rw = 0.3166 (all data), GOF = 1.033, CCDC 780572. Crystallographic data for 4-C6060: C80H27B1N8O1F8, MW = 1278.91, monoclinic, space groupP2/n (no. 13), a = 16.8540(18), b = 14.8124(16), c = 24.597(3) Å, β = 99.270(1)°, V = 6060.5(11) Å3, Z = 4, ρcalcd = 1.402 g cm−3, T = −173(2) °C, 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 471 measured reflections, 13 471 measured reflections, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851 unique reflections (Rint = 0.0262), R = 0.0833 (I > 2σ(I)), Rw = 0.2548 (all data), GOF = 1.069, CCDC 780573. 851 unique reflections (Rint = 0.0262), R = 0.0833 (I > 2σ(I)), Rw = 0.2548 (all data), GOF = 1.069, CCDC 780573. |
| This journal is © The Royal Society of Chemistry 2011 |
