High-nuclearity, mixed-valence Mn17, Mn18 and {Mn62}n complexes from the use of triethanolamine†
Theocharis C.
Stamatatos‡
*a,
Dolos
Foguet-Albiol
a,
Wolfgang
Wernsdorfer
b,
Khalil A.
Abboud
a and
George
Christou
*a
aDepartment of Chemistry, University of Florida, Gainesville, Florida 32611-7200, USA
bInstitut Néel, CNRS & Université J. Fourier, BP-166, Grenoble, Cedex 9, France
First published on 25th August 2010
Abstract
The use of both azide and triethanolamine, with or without the presence of carboxylate groups, has provided new Mn17, Mn18 and {Mn62}n complexes with aesthetically-pleasing cage, layered, and linked-chain-type structures; two are also new single-molecule magnets.
One of the current challenges in inorganic chemistry is the synthesis and characterization of new molecular 3d metal polynuclear clusters at moderate oxidation states.1 Reasons for this are varied, and not least among them is the aesthetic beauty that develops as the nuclearity of the clusters increases and the complexity of their molecular structures becomes apparent. From a more practical viewpoint, such large clusters can represent an alternative, ‘bottom-up’ route to nanoscale particles complementary to the traditional ‘top-down’ approach.2 An example is the discovery that molecular 3d-metal clusters can function as magnets, providing a ‘bottom-up’ approach to nanoscale magnetic materials, and such individual molecular species have come to be known as single-molecule magnets (SMMs).3 SMMs are molecules that display slow magnetization relaxation below a blocking temperature, TB, due to a large ground state spin (S) combined with a large Ising (easy-axis) magnetoanisotropy (negative zero-field splitting (zfs) parameter, D).3 In addition, SMMs are true ‘mesoscale’ particles straddling the classical/quantum interface, and thus display quantum properties such as quantum tunnelling of the magnetization vector (QTM)4 and quantum phase interference5 through the barrier. For all these reasons, SMMs have been proposed for various specialized applications, such as ultra-high density memory devices, spintronics, and quantum computing.6
Although complexes displaying SMM behaviour are known for several metals, manganese cluster chemistry continues to be the most fruitful source,7 giving a wide range of Mnx nuclearities with x having values up to 84, the latter being the largest known SMM.7c We have been developing new synthetic methods to Mn clusters of various nuclearities and structural types, and have recently been exploring the use of N3− in higher oxidation state Mn chemistry in combination with various chelating/bridging ligands.8 From a magnetic viewpoint, the N3− ion bridging in the 1,1-fashion (end-on) is one of the strongest ferromagnetic mediators in molecular magnetism, and thus it constitutes an attractive route to new high-spin molecules and SMMs.8,9
In the present work, we report Mn reactions with azides and the potentially tetradentate (N,O,O,O) triethanolamine (teaH3) chelating/bridging group, which has previously been found a useful route to high nuclearity non-azido and some lower nuclearity azido-based metal clusters.10,11 We have been targeting higher nuclearity Mn products by exploring the reactions between teaH3, NaN3 and various Mn reagents, with or without the co-presence of carboxylates. We herein report some results from this study, which has produced new mixed-valence Mn17, Mn18 and {Mn62}n molecular species with tea3−/teaH2−/N3− and tea3−/teaH2−/N3−/RCO2− ligand combinations.
The reaction of Mn(ClO4)2·6H2O, teaH3, NEt3, and NaN3 in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio in MeOH gave a dark brown solution from which was subsequently isolated [Mn18O11(OH)(OMe)(N3)12(tea)3(teaH)3(MeOH)] (1) in 65% yield.8 A very similar compound was recently reported by Murray and co-workers.11 A similar reaction, but with pivalate, between Mn(ClO4)2·6H2O, NaO2CCMe3, teaH3, NEt3, and NaN3 in a 1
3 molar ratio in MeOH gave a dark brown solution from which was subsequently isolated [Mn18O11(OH)(OMe)(N3)12(tea)3(teaH)3(MeOH)] (1) in 65% yield.8 A very similar compound was recently reported by Murray and co-workers.11 A similar reaction, but with pivalate, between Mn(ClO4)2·6H2O, NaO2CCMe3, teaH3, NEt3, and NaN3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in MeCN/DMF (2
2 ratio in MeCN/DMF (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) gave a similar dark brown solution from which was isolated [Mn17NaO10(OH)2(N3)3(O2CCMe3)13(tea)3(teaH)(DMF)] (2) in 45% yield. Interestingly, when Mn(ClO4)2·6H2O and Mn(O2CMe)2·4H2O were combined in a 1
1, v/v) gave a similar dark brown solution from which was isolated [Mn17NaO10(OH)2(N3)3(O2CCMe3)13(tea)3(teaH)(DMF)] (2) in 45% yield. Interestingly, when Mn(ClO4)2·6H2O and Mn(O2CMe)2·4H2O were combined in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio in MeCN/MeOH (5
8 ratio in MeCN/MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and treated with teaH3 and NaN3 in 1
1, v/v), and treated with teaH3 and NaN3 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio, the isolated product was now [Mn31O19(OH)(OMe)6(N3)4(O2CMe)23(tea)2(dea)2(MeOH)2]n (3) in 40% yield, where dea2− is the dianion of diethanolamine. The partial transformation of teaH3 to deaH2 in 3 is attributed to metal-assisted –(CH2)2OH bond cleavage processes12 that are sometimes seen in 3d-metal cluster chemistry. The metal oxidation states and the protonation levels of O2−, OH−, OMe−, tea3−/teaH2−, and dea2− O atoms in 1–3 were established by Mn/Na and O bond valence sum (BVS) calculations,13,14 inspection of metric parameters, and detection of MnIII Jahn–Teller (JT) elongation axes.
4 ratio, the isolated product was now [Mn31O19(OH)(OMe)6(N3)4(O2CMe)23(tea)2(dea)2(MeOH)2]n (3) in 40% yield, where dea2− is the dianion of diethanolamine. The partial transformation of teaH3 to deaH2 in 3 is attributed to metal-assisted –(CH2)2OH bond cleavage processes12 that are sometimes seen in 3d-metal cluster chemistry. The metal oxidation states and the protonation levels of O2−, OH−, OMe−, tea3−/teaH2−, and dea2− O atoms in 1–3 were established by Mn/Na and O bond valence sum (BVS) calculations,13,14 inspection of metric parameters, and detection of MnIII Jahn–Teller (JT) elongation axes.
The structure§ of 1 consists of a mixed-valent (MnII3MnIII15) Mn18 cage (Fig. 1, left) with a ‘pyramid’ or ‘cone’-like topology.14 The eight μ4-O2−, three μ3-O2−, one μ3-OH−, and one μ3-OMe− ions hold the core together, as well as chelating/bridging tea3−/teaH2− and both terminal and bridging azide groups. The core of 1 can be conveniently dissected into four layers, ABCD, of different sizes (Fig. 1, right) but all comprising fused [Mn3O] triangular units: the MnII monomeric layer A is the apex of the Mn18 ‘cone’, linked to layer B which is a MnIII3 triangle, thus giving a combined-layer AB tetrahedral topology; layer C is a large MnII2MnIII4 triangle comprising three corner-sharing MnIIMnIII2 and MnIII3 triangles connected to an additional, extrinsic MnIII atom; and layer D consists of a MnIII7 disk-like unit. Each layer is held together and linked to neighbouring layers by a combination of oxide, alkoxide, and μ3-1,1,1 or μ-1,1 (end-on) azide ligands. The three tea3− and teaH2−groups are of four types: η3:η1:η2:η2:μ5 and η1:η1:η3:η2:μ4 for the former, and η2:η1:η3:η1:μ4 and η2:η1:η2:η1:μ3 for the latter,14 reflecting the bridging flexibility of the triethanolamine group.
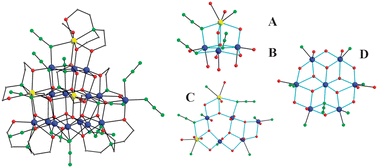 | ||
| Fig. 1 Structure of 1 (left) and the four layers of its core (right). H atoms have been omitted for clarity. Colour scheme: MnII yellow; MnIII blue; O red; N green; C grey. | ||
The structure§ of 2 (MnII4MnIII12MnIV) consists of a Mn17Na cage-like cluster (Fig. 2, top) with an irregular topology. The [Mn17Na(μ4-O)8(μ3-O)2(μ3-OH)(μ-OH)2(μ3-N3)]25+ core (Fig. 2, bottom) comprises seven [Mn4(μ4-O2−)] and one [Mn3Na(μ4-O2−)] tetrahedra fused together and linked to one adjacent [Mn2Na(μ3-OH−)] and two [Mn3(μ3-O2−)] triangles by common Mn vertices. The core can also be described as a central [Mn3Na(μ4-O2−)] tetrahedron fused to [Mn4(μ3-O2−)4] and [Mn4(μ3-O2−)3(μ3-N3)] cubanes at common atoms Mn(2) and Mn(9), respectively. All μ3-Ο2− ions in each cubane convert to a μ4 mode and bridge seven adjacent Mn atoms, three of which (Mn2,5,7) are fused to the corresponding triangular subunits. The three tea3−groups are each bridging up to six Mn atoms, acting as η3:η1:η2:η3:μ6, η2:η1:η3:η2:μ5 and η2:η1:η2:η2:μ3 ligands, the μ6 mode being seen for a first time in the coordination chemistry of this group, while the only teaH2−group is bridging in a η2:η1:η2:μ3 mode.14 Peripheral ligation is provided by ten η1:η1:μ, two η1:η2:μ and an η2:η2:μ4Me3CCO2−groups, as well as two terminal N3− ions and a terminal DMF molecule.
 | ||
| Fig. 2 Structure of complex 2 (top) and its core (bottom). Colour scheme: MnII yellow; MnIII blue; MnIV olive; Na purple; O red; N green; C grey. | ||
The structure§ of 3 (MnII11MnIII20) consists of Mn28 units comprising a central Mn14 subunit attached on each side to a Mn4 rhombus and a Mn3 triangle, with the metal atoms bridged by oxo, alkoxo, azido and acetato ions. These Mn28 units are connected by linear [MnII3(O2CMe)5(N3)] units into a 1D zig-zag chain. The structure is at first glance a polymer of Mn31 units, but the Mn3 bridge in a way that makes adjacent Mn28 inequivalent, and complex 3 is thus best described as a chain of repeating Mn62 units (Fig. 3) of formula [Mn62O38(OH)2(OMe)12(N3)8(O2CMe)46(tea)4(dea)4(MeOH)4]n. Further, the 1D chains are linked by Mn(14)–N3–Mn(18) inter-chain bridges, with the azide in an end-to-end mode, giving a 3D covalent network. The packing of the chains is provided in Fig. S11 (ESI†) which shows that (i) the linked-chains give sheets with a herring-bone pattern, and (ii) chains of adjacent linked-sheets are staggered so that when viewed along the chain axes a hexagonal close-packing is observed.
 | ||
| Fig. 3 Structure of the repeating {Mn62} unit of polymeric complex 3. Colour scheme as in Fig. 1. | ||
Solid-state dc (direct current) magnetic susceptibility (χΜ) data were collected on 1, 2, and 3·10MeCN in a 1 kG (0.1 T) field in the 5.0–300 K range. The data are plotted as χΜT vs. T in Fig. S3 (ESI†), and both 1 and 2 clearly have relatively large ground-state spin (S) values, whereas 3·10MeCN is strongly antiferromagnetically-coupled with χΜT heading for 0 cm3 K mol−1 at 0 K, indicating a diamagnetic ground state. χΜT for 1 increases from 66.53 cm3 K mol−1 at 300 K to 75.40 cm3 K mol−1 at 100.0 K, and then decreases to a plateau of ∼60.50 cm3 K mol−1 at 15.0–8.0 K, before dropping to 58.93 cm3 K mol−1 at 5.0 K; the decrease at the lowest temperatures is assigned to Zeeman effects, zero-field splitting and/or weak intermolecular interactions. For 2, χΜT steadily decreases from 44.74 cm3 K mol−1 at 300 K to a plateau of ∼35.50 cm3 K mol−1 at 40.0–20.0 K, before dropping to 33.49 cm3 K mol−1 at 5.0 K. The χΜT plateau values for 1 and 2 suggest ground-state spin values, S, of 21/2 and 17/2, respectively; the spin-only (g = 2) values are 60.38 and 40.38 cm3 K mol−1, respectively.
To determine the ground states of 1 and 2, magnetization (M) data were collected in the 0.1–1.0 T and 1.8–10.0 K ranges, and these are plotted as M/NμBvs. H/T in Fig. S4 and S5 (ESI†), respectively. The data were fit by matrix-diagonalization to a model that assumes only the ground state is populated, includes axial zero-field splitting and the Zeeman interaction, and carries out a full powder average. The best fit (solid lines in Fig. S4 and S5, ESI†) gave S = 21/2, g = 1.99(3) and D = −0.073(5) cm−1 for 1 and S = 17/2, g = 1.95(1) and D = −0.218(5) cm−1 for 2. Alternative fits with S = 19/2 or 23/2 for 1 and 15/2 or 19/2 for 2 were rejected because they gave unreasonable values of g. We conclude that 1 and 2 have S = 21/2 and 17/2 ground states, respectively. This was further confirmed by ac (alternating current) susceptibility studies.14
The S = 21/2 and 17/2 ground states and negative D values suggest that both 1 and 2 might be SMMs. At temperatures <3.0 K, frequency-dependent tails of out-of-phase (χΜ″) ac susceptibility signals for both complexes were observed.14 Such signals are an indication of the superparamagnetic-like slow relaxation of an SMM. To confirm this, magnetization vs. applied dc field data down to 0.04 K were collected on single-crystals using a micro-SQUID apparatus.15 Both complexes exhibit hysteresis loops below ∼1.0 K whose coercivities increase with increasing field sweep rate14 and decreasing temperature (Fig. 4), confirming 1 and 2 to be new Mn SMMs. Arrhenius plots were constructed from combined ac χΜ″ data and dc magnetization decay data, giving Ueff = 6.1 cm−1 = 8.8 K and τ0 = 10−7 s for 1 and Ueff = 13.2 cm−1 = 19.0 K and τ0 = 10−11 s for 2, where τ0 is the pre-exponential factor.14 Such ranges in τ0 values for high-nuclearity SMMs are becoming more common, and can be assigned to differing factors such as relaxation contributions through low-lying excited states, distribution of molecular environments, and weak intermolecular interactions.
 | ||
| Fig. 4 Magnetization (M) vs. applied dc field (H) hysteresis loops for 1·2CH2Cl2·4Et2O (top) and 2·5MeCN (bottom) at the indicated temperatures. M is normalized to its saturation value (Ms). | ||
In conclusion, azide and triethanolamine groups together, with or without the presence of carboxylate anions, have provided three Mn clusters with interesting structures, namely a multi-layer pyramid (1), a closed cage (2) and a zig-zag chain (3), two of which (1 and 2) are also new members of the SMM family. These three complexes establish the potential of this reaction system to continue giving new molecular species with unprecedented nuclearities in 3d-metal coordination chemistry.
This work was supported by the National Science Foundation (CHE-0910472).
Notes and references
- E. G. Mednikov and L. F. Dahl, Small, 2008, 4, 534 CrossRef CAS.
- G. Schmid, Nanoparticles—From Theory to Application, Wiley-VCH, Weinheim, 2005, vol. 19, p. 7 Search PubMed.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS.
- J. R. Friedman, M. P. Sarachik, J. Tejada and R. Ziolo, Phys. Rev. Lett., 1996, 76, 3830 CrossRef CAS.
- W. Wernsdorfer and R. Sessoli, Science, 1999, 284, 133 CrossRef CAS.
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179 CrossRef CAS.
- (a) E. K. Brechin, Chem. Commun., 2005, 5141 RSC; (b) R. T. W. Scott, S. Parsons, M. Murugesu, W. Wernsdorfer, G. Christou and E. K. Brechin, Angew. Chem., Int. Ed., 2005, 44, 6540 CrossRef CAS; (c) A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117 CrossRef CAS.
- Th. C. Stamatatos and G. Christou, Inorg. Chem., 2009, 48, 3308 CrossRef CAS.
- A. Escuer and G. Aromi, Eur. J. Inorg. Chem., 2006, 4721 CrossRef CAS.
- (a) M. Murugesu, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2005, 44, 892 CrossRef CAS; (b) Th. C. Stamatatos, D. Foguet-Albiol, K. M. Poole, W. Wernsdorfer, K. A. Abboud, T. A. O'Brien and G. Christou, Inorg. Chem., 2009, 48, 9831 CrossRef CAS; (c) S. K. Langley, B. Moubaraki, K. J. Berry and K. S. Murray, Dalton Trans., 2010, 4848 RSC; (d) L. M. Wittick, L. F. Jones, P. Jensen, B. Moubaraki, L. Spiccia, K. J. Berry and K. S. Murray, Dalton Trans., 2006, 1534 RSC; (e) Th. C. Stamatatos, K. M. Poole, D. Foguet-Albiol, K. A. Abboud, T. A. O'Brien and G. Christou, Inorg. Chem., 2008, 47, 6593 CrossRef CAS.
- S. K. Langley, K. J. Berry, B. Moubaraki and K. S. Murray, Dalton Trans., 2009, 973 RSC.
- S. Horikoshi, N. Watanabe, M. Mukae, H. Hidaka and N. Serpone, New J. Chem., 2001, 25, 999 RSC.
- W. Liu and H. H. Thorp, Inorg. Chem., 1993, 32, 4102 CrossRef CAS.
- See the ESI†.
- W. Wernsdorfer, Adv. Chem. Phys., 2001, 118, 99 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystallographic data (CIF format) for 1·2CH2Cl2·4Et2O, 2·5MeCN and 3·26MeCN, synthetic details for the 1–3, and various structural and magnetism figures. CCDC 700136–700138. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01701a |
| ‡ Present address: Department of Chemistry, University of Patras, Patras 26504, Greece. E-mail: thstama@chemistry.upatras.gr; Fax: +30-2610-997118; Tel.: +30-2610-996020. |
§ Crystal data for 1·2CH2Cl2·4Et2O: C56H127Cl4Mn18N42O36, Mw = 3095.72, triclinic, space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 15.3132(2) Å, b = 18.3122(11) Å, c = 21.422(2) Å, α = 68.092(2)°, β = 79.91(2)°, γ = 74.134(2)°, V = 5342.9(6) Å3, Z = 2, ρcalcd = 1.924 g cm−3, T = 173(2) K, 35 , a = 15.3132(2) Å, b = 18.3122(11) Å, c = 21.422(2) Å, α = 68.092(2)°, β = 79.91(2)°, γ = 74.134(2)°, V = 5342.9(6) Å3, Z = 2, ρcalcd = 1.924 g cm−3, T = 173(2) K, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 reflections collected, 23 186 reflections collected, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 378 unique (Rint = 0.0433), R1 [I > 2σ(I)] = 0.0577, wR2 = 0.1433 (F2, all data). CCDC 700137. Crystal data for 2·5MeCN: C102H188Mn17NaN19O51, Mw = 3453.68, orthorhombic, space groupP212121, a = 17.463(3) Å, b = 18.144(3) Å, c = 46.054(7) Å, V = 46.054(7) Å3, Z = 4, ρcalcd = 1.572 g cm−3, T = 173(2) K, 55 378 unique (Rint = 0.0433), R1 [I > 2σ(I)] = 0.0577, wR2 = 0.1433 (F2, all data). CCDC 700137. Crystal data for 2·5MeCN: C102H188Mn17NaN19O51, Mw = 3453.68, orthorhombic, space groupP212121, a = 17.463(3) Å, b = 18.144(3) Å, c = 46.054(7) Å, V = 46.054(7) Å3, Z = 4, ρcalcd = 1.572 g cm−3, T = 173(2) K, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 reflections collected, 19 542 reflections collected, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 unique (Rint = 0.1192), R1 [I > 2σ(I)] = 0.0972, wR2 = 0.2053 (F2, all data). CCDC 700136. Crystal data for 3·26MeCN: C126H215Mn31N42O84, Mw = 5365.54, monoclinic, space groupP21/c, a = 35.759(4) Å, b = 14.8904(16) Å, c = 32.943(4) Å, β = 92.562(2)°, V = 17 027 unique (Rint = 0.1192), R1 [I > 2σ(I)] = 0.0972, wR2 = 0.2053 (F2, all data). CCDC 700136. Crystal data for 3·26MeCN: C126H215Mn31N42O84, Mw = 5365.54, monoclinic, space groupP21/c, a = 35.759(4) Å, b = 14.8904(16) Å, c = 32.943(4) Å, β = 92.562(2)°, V = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524(3) Å3, Z = 4, ρcalcd = 2.034 g cm−3, T = 173(2) K, 69 524(3) Å3, Z = 4, ρcalcd = 2.034 g cm−3, T = 173(2) K, 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 613 reflections collected, 22 613 reflections collected, 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 unique (Rint = 0.1113), R1 [I > 2σ(I)] = 0.0776, wR2 = 0.1874 (F2, all data). CCDC 700138. 840 unique (Rint = 0.1113), R1 [I > 2σ(I)] = 0.0776, wR2 = 0.1874 (F2, all data). CCDC 700138. |
| This journal is © The Royal Society of Chemistry 2011 |
