Copper-mediated cross-coupling of 1,1-dibromo-1-alkenes with dialkyl phosphites: a convenient synthesis of 1-alkenylphosphonates†‡
Gwilherm
Evano
*,
Krishnaji
Tadiparthi
and
François
Couty
Institut Lavoisier de Versailles, UMR CNRS 8180, Université de Versailles Saint-Quentin en Yvelines, 45, avenue des Etats-Unis, 78035 Versailles Cedex, France. E-mail: evano@chimie.uvsq.fr; Fax: +33 1 39 25 44 52; Tel: +33 1 39 25 44 11
First published on 12th July 2010
Abstract
An efficient and stereoselective procedure for the preparation of E-1-alkenylphosphonates by copper-mediated cross-coupling between 1,1-dibromo-1-alkenes and dialkyl phosphites is reported. The reaction allows for formal substitution of both bromine atoms, respectively, by an hydrogen and a dialkoxyphosphoryl.
1-Alkenylphosphonates are especially useful small organic molecules.1 In addition to their extensive use in polymer science as copolymers, additives or flame-retardants,1,2 they are routinely used as building blocks for the introduction of remote phosphonate functional groups or to prepare biologically active molecules.1 Quite recently, the alkenylphosphonate scaffold itself has been used as a promising pharmacophore.3 They have also been shown over the years to be key starting materials for an impressive range of chemical transformations such as, for example, asymmetric hydrogenation,4 enantioselective organocatalytic condensation with aldehydes,5 enantioselective intramolecular Stetter reaction,6 or dipolar 1,3-cycloaddition.7
In addition to a number of traditionally employed approaches that often lack generality and selectivity,1,8 several transition-metal mediated methods for the synthesis of 1-alkenylphosphonates have recently emerged.9 They include the cross metathesis reaction with ethenylphosphonate,10 hydrophosphonylation of alkynes,11 Heck cross-coupling strategies,12 addition of zirconocene to alkynylphosphonates followed by reaction with electrophiles,13 and palladium-14 or copper-15 catalyzed coupling of dialkyl phosphites with vinyl halides, vinyl boronates16 and hypervalent alkenyl iodonium salts.17 Despite their efficiency, preparation of 1-alkenylphosphonates is far from being a trivial task and there is still a strong need for alternative methods that would allow the synthesis of alkenylphosphonates from readily available starting materials.
Drawing inspiration from our experience in copper catalysis18,19 and from recent studies of our group on the behaviour of vinyl dibromides with copper(I) salts,20,21 we thought that an attractive alternative for the preparation of 1-alkenylphosphonates 4 would rely on the cross-coupling of readily available 1,1-dibromo-1-alkenes 122 with dialkyl phosphites 2 (Scheme 1). By using an excess of the latter, it should be able to act as both a selective reducing agent23 and cross-coupling partner to give E-1-alkenylphosphonates 4.24 If successful, this procedure would be especially convenient for the preparation of these useful building blocks and would also avoid a preliminary stereoselective synthesis of the cross-coupling partner 3, which would now be generated in situ. Herein, we describe the successful development of such a one pot sequence that allows a straightforward preparation of a wide range of 1-alkenylphosphonates.
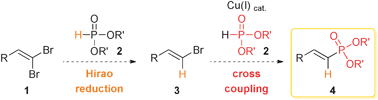 | ||
| Scheme 1 1-Alkenylphosphonates by copper-mediated cross-coupling of 1,1-dibromo-1-alkenes with dialkyl phosphites. | ||
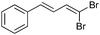
To test our hypothesis, β,β-dibromostyrene 5 was reacted with excess (2.5 equiv.) diisopropylphosphite 6 in the presence of various copper-based catalytic systems. After quite some experimentation, we eventually found that the reaction was best performed using potassium phosphate as base, copper iodide as a source of copper(I) and N,N′-dimethylethylenediamine as a ligand in toluene at 120 °C for a day (Scheme 2).25 Using these conditions, 1-alkenylphosphonate 7 could be obtained in 60% yield with useful levels of stereoselectivity (E/Z: 90/10) and with minor amounts of side products resulting from the dimerization of starting dibromide 5.20c The exclusive formation of 7 is in sharp contrast with results from the Hayes group who showed that alkynylphosphonates were predominantly formed upon reaction of alkenyl-dibromides and dialkyl phosphites with palladium acetate, dppf and propylene oxide in DMF at 80 °C.26 The present method therefore offers a complementary catalytic system that allows the selective formation of alkenylphosphonates.
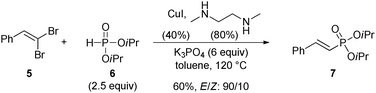 | ||
| Scheme 2 Optimized reaction conditions. | ||
Surprisingly, our initial fears that the Hirao reduction, which is typically performed in highly polar solvents (DMF, ethanol or triethylamine),23 might not be compatible with the use of a non polar solvent turned out to be unfounded, even if this reaction was found to be slower in toluene.
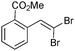
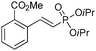
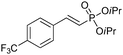
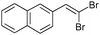
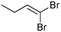
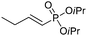
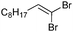
The coupling of diisopropylphosphite 6 with various gem-dibromoalkenes was next investigated using the optimized conditions: illustrative examples are shown in Table 1. The reaction was found to be rather general, and 1-alkenylphosphonates could be obtained in all cases with reasonable to good yields and with useful selectivities in favor of the E-isomer. The reaction was found to be compatible with a variety of aromatic groups (Table 1, entries 1–10), and the presence of electron withdrawing or donating groups had virtually no effect. Vinyl- and alkyl-substituted gem-dibromoalkenes were also found to be suitable reaction partners (Table 1, entries 11–15), even in the presence of a bulky substituent (Table 1, entry 14). In the case of a benzyl-substituted dibromide, migration of the double bond was, however, observed (Table 1, entry 16).
| Entry | 1,1-Dibromo-1-alkene | l-Alkenylphosphonate | E/Z | Yieldb (%) |
|---|---|---|---|---|
| a Conditions: Diisopropylphosphite (2.5 equiv.), CuI (40 mol%), N,N′-dimethylethylenediamine (80 mol%), K3PO4 (6 equiv.), toluene (0.3 mol L−1), 120 °C, 20–24 h. b Yield of pure, isolated product. c Reaction performed on a gram scale. | ||||
| 1 |

|
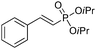
|
90/10 | 60 (60c) |
| 2 |
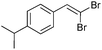
|
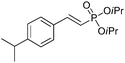
|
92/8 | 58 |
| 3 |
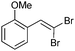
|
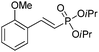
|
89/11 | 67 |
| 4 |
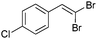
|
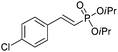
|
89/11 | 56 |
| 5 |
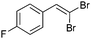
|
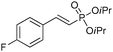
|
87/13 | 52 |
| 6 |

|
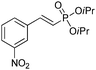
|
88/12 | 84 |
| 7 |
|
|
83/17 | 52 |
| 8 |

|
|
80/20 | 67 |
| 9 |
|
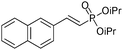
|
92/8 | 60 |
| 10 |
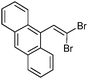
|
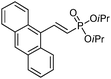
|
95/5 | 48 |
| 11 |
|
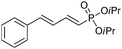
|
88/12 | 71 |
| 12 |
|
|
81/19 | 45 |
| 13 |
|
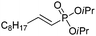
|
92/8 | 59 |
| 14 |
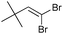
|
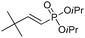
|
>95/5 | 48 |
| 15 |
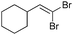
|
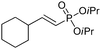
|
93/7 | 54 |
| 16 |
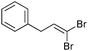
|

|
>95/5 | 62 |
The reaction is not limited to the small scale used for the coupling reactions described above (i.e., 1 mmol) since it could be conveniently performed on a gram scale with comparable yield (Table 1, entry 1).
The scope of the reaction was next investigated with respect to the dialkyl phosphite reaction partner (Scheme 3). Other dialkyl phosphites such as diethyl- or dibutyl-phosphite were found to perform well for the reduction/cross-coupling sequence, the former being a little superior in terms of yield and selectivity. Only diphenylphosphite did not participate in the reaction since no reduced or coupled product could be detected in the crude reaction mixture.
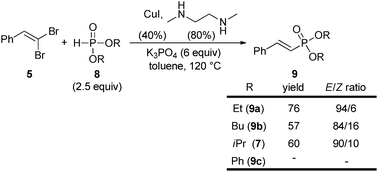 | ||
| Scheme 3 Reduction/cross-coupling using various dialkyl phosphites. | ||
We next briefly turned our attention to the more challenging synthesis of trisubstituted 1-alkenylphophonates starting from benzophenone and acetophenone-derived 1,1-dibromoalkenes 10 and 12 (Scheme 4). Again, the reaction was found to be quite efficient since the corresponding phosphonates 11 and 13 could be isolated in 58 and 78% yield. In the last case, the E-isomer27 was formed predominantly, with a selectivity similar to the one observed with disubstituted 1-alkenylphosphonates.
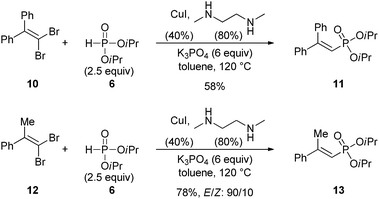 | ||
| Scheme 4 Synthesis of trisubstituted 1-alkenylphosphonates. | ||
Finally, the formation of 1-alkenylphosphonates from complex substrates was evaluated to test our procedure further (Scheme 5). Proline- and citronellal-derived dibromides 14 and 16 were converted to enantiopure 1-alkenylphosphonates 15 and 17 while (−)-menthol-derived phosphite 1928 was smoothly coupled with gem-dibromoalkene 18 to give phosphonate 20, the chirality now being introduced on the phosphonate group. Finally, double coupling performed on bis-dibromide 21 provided bis-1-alkenylphosphonate 22 in an excellent 83% yield.
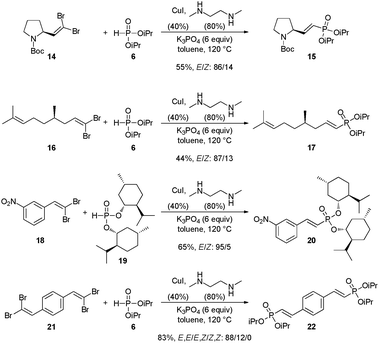 | ||
| Scheme 5 Synthesis of 1-alkenylphosphonates from complex substrates. | ||
In conclusion, an efficient stereoselective synthesis of 1-alkenylphosphonates has been described. This reaction has been shown to be general and provides a straightforward entry to 1-alkenylphosphonates that is complementary to previously reported synthetic routes. This method has the advantageous feature that it starts from readily available 1,1-dibromo-1-alkenes which act as attractive synthetic equivalents of E-alkenyl bromides. Further studies on the applications of this method, on the use of other nucleophiles and on the reaction mechanism, which is still unclear and is undoubtedly more complicated than a simple Hirao reduction/cross-coupling sequence, will be reported in due course.
The authors thank the CNRS for financial support. K. T. acknowledges the University of Versailles for a postdoctoral fellowship. We are indebted to Dr Elyane Kizilian (Lavoisier Institute) for her help with mass spectrometry analyses.
Notes and references
- For reviews, see: (a) T. Minami and J. Motoyoshiya, Synthesis, 1992, 333 CrossRef CAS; (b) H.-Q. Wang and Z.-J. Liu, Chin. J. Org. Chem., 2003, 23, 321 CAS; (c) V. M. Dembitsky, A. A. A. Quntar, A. Haj-Yehia and M. Srebnik, Mini-Rev. Org. Chem., 2005, 2, 91 Search PubMed; (d) A. C. Gaumont and M. Gulea, in Science of Synthesis: Houben–Weyl Methods of Molecular Transformations, ed. G. A. Molander, Thieme, Stuttgart, 2006, vol. 33, pp. 665–694 Search PubMed.
- (a) J. R. Ebdon, Recent Adv. Flame Retard. Polym. Mater., 1997, 8, 161 Search PubMed; (b) D. Price, K. Pyrah, T. R. Hull, G. Milnes, J. R. Ebdon, B. J. Hunt and P. Joseph, Polym. Degrad. Stab., 2002, 31, 1010.
- For recent examples, see: (a) A. A. A. Quntar, O. Baum, R. Reich and M. Srebnik, Arch. Pharm., 2004, 337, 76 CrossRef CAS; (b) R. Ettari, E. Nizi, M. E. Di Francesco, N. Micale, S. Grasso, M. Zappalà, R. Vičík and T. Schirmeister, ChemMedChem, 2008, 3, 1030 CrossRef CAS; (c) M. Delomenède, F. Bedos-Belval, H. Duran, C. Vindis, M. Baltas and A. Nègre-Salvayre, J. Med. Chem., 2008, 51, 3171 CrossRef CAS.
- Z.-C. Duan, X.-P. Hu, D.-Y. Wang, J.-D. Huang, S.-B. Yu, J. Deng and Z. Zheng, Adv. Synth. Catal., 2008, 350, 1979 CrossRef CAS.
- S. Sulzer-Mossé, M. Tissot and A. Alexakis, Org. Lett., 2007, 9, 3749 CrossRef CAS.
- S. C. Cullen and T. Rovis, Org. Lett., 2008, 10, 3141 CrossRef CAS.
- N. Rabasso and A. Fadel, Tetrahedron Lett., 2010, 51, 60 CrossRef CAS.
- For a Wadsworth–Horner–Emmons approach, see: (a) M.-P. Teulade, P. Savignac, E. E. Aboujaoude, S. Liétge and N. Collignon, J. Organomet. Chem., 1986, 304, 283 CrossRef CAS; (b) H. Inoue, H. Tsubouchi, Y. Nagaoka and K. Tomioka, Tetrahedron, 2002, 58, 83 CrossRef CAS.
- M. Maffei, Curr. Org. Synth., 2004, 1, 355 Search PubMed.
- A. K. Chatterjee, T. L. Choi and R. H. Grubbs, Synlett, 2001, 1034 CrossRef CAS.
- For leading references, see: (a) L. B. Han and M. Tanaka, J. Am. Chem. Soc., 1996, 118, 1571 CrossRef CAS; (b) C.-Q. Zhao, L.-B. Han, M. Goto and M. Tanaka, Angew. Chem., Int. Ed., 2001, 40, 1929 CrossRef CAS; (c) L. B. Han, C. Zhang, H. Yazawa and S. Shimada, J. Am. Chem. Soc., 2004, 126, 5080 CrossRef CAS; (d) V. P. Ananikov, L. L. Khemchyan and I. P. Beletskaya, Synlett, 2009, 2375 CrossRef CAS . For a recent review, see: L. Coudray and J.-L. Montchamp, Eur. J. Org. Chem., 2008, 3601 Search PubMed.
- Using aryldiazonium salts: (a) H. Brunner, N. Le Cousturier de Courcy and J.-P. Genêt, Synlett, 2000, 201 CAS; (b) Using arylboronic acids: G. W. Kabalka, S. K. Guchhait and A. Naravane, Tetrahedron Lett., 2004, 45, 4685 Search PubMed.
- (a) S. Ben-Valid, A. A. A. Quntar and M. Srebnik, J. Org. Chem., 2005, 70, 3554 CrossRef CAS; (b) A. A. A. Quntar, D. Rosenthal and M. Srebnik, Tetrahedron, 2006, 62, 5995 CrossRef CAS.
- (a) T. Hirao, T. Masunaga, Y. Ohshiro and T. Agawa, Tetrahedron Lett., 1980, 21, 3595 CrossRef CAS; (b) T. Hirao, T. Masunaga, Y. Ohshiro and T. Agawa, Synthesis, 1981, 56 CrossRef CAS; (c) T. Hirao, T. Masunaga, N. Yamada, Y. Ohshiro and T. Agawa, Bull. Chem. Soc. Jpn., 1982, 55, 909 CAS; (d) M. Kalek, A. Ziadi and J. Stawnski, Org. Lett., 2008, 10, 4637 CrossRef CAS.
- D. Gelman, L. Jiang and S. L. Buchwald, Org. Lett., 2003, 5, 2315 CrossRef CAS.
- G. W. Kabalka and S. K. Guchhait, Org. Lett., 2003, 5, 729 CrossRef CAS.
- S. Thielges, P. Bisseret and J. Eustache, Org. Lett., 2005, 7, 681 CrossRef CAS.
- G. Evano, M. Toumi and A. Coste, Chem. Commun., 2009, 4166 RSC.
- For reviews, see: (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (b) K. Kunz, U. Scholz and D. Ganzer, Synlett, 2003, 2428 CrossRef CAS; (c) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS; (d) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (e) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC.
- (a) A. Coste, G. Karthikeyan, F. Couty and G. Evano, Angew. Chem., Int. Ed., 2009, 48, 4381 CrossRef CAS; (b) A. Coste, F. Couty and G. Evano, Org. Lett., 2009, 11, 4454 CrossRef CAS; (c) A. Coste, F. Couty and G. Evano, Synthesis, 2010, 1500 CAS.
- For other examples on copper-catalyzed coupling reactions with vinyldibromides, see: (a) J. Yuen, Y.-Q. Fang and M. Lautens, Org. Lett., 2006, 8, 653 CrossRef CAS; (b) Z.-J. Wang, J.-G. Yang, F. Yang and W. Bao, Org. Lett., 2010, 12, 3034 CrossRef CAS.
- (a) F. Ramirez, N. B. Desai and N. McKelvie, J. Am. Chem. Soc., 1962, 84, 1745 CrossRef CAS; (b) E. J. Corey and P. L. Fuchs, Tetrahedron Lett., 1972, 13, 3769 CrossRef; (c) Y.-Q. Fang, O. Lifchits and M. Lautens, Synlett, 2008, 413 CAS.
- (a) T. Hirao, T. Masunaga, Y. Ohshiro and T. Agawa, J. Org. Chem., 1981, 46, 3745 CrossRef CAS; (b) S. Abbas, C. J. Hayes and S. Worden, Tetrahedron Lett., 2000, 41, 3215 CrossRef CAS; (c) C. Kuang, H. Senboku and M. Tokuda, Tetrahedron, 2002, 58, 1491 CrossRef CAS.
- A single example of such a transformation had been reported using palladium catalysis. See ref. 23b.
- Attempts at lowering the catalyst loading resulted in lower yields, which might be attributed to chelation of the phosphite to Cu(I).
- M. Lera and C. J. Hayes, Org. Lett., 2000, 2, 3873 CrossRef CAS.
- Configuration assigned on the basis of chemical shift and 4JC–P value for the methyl group. See: J. M. Gil and D. Y. Oh, J. Org. Chem., 1999, 64, 2950 Search PubMed.
- P. Dżygiel, P. Wieczorek, J. Å. Jonsson, M. Milewska and P. Kafarski, Tetrahedron, 1999, 55, 9923 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures, characterization, copies of 1H and 13C NMR spectra for all new compounds. See DOI: 10.1039/c0cc01617a |
| This journal is © The Royal Society of Chemistry 2011 |
