Twofold pH and temperature stimuli-responsive behaviour in block copolypeptide-decorated single wall carbon nanotubes†‡
Chaoxu
Li
a,
Jozef
Adamcik
a,
Afang
Zhang
b and
Raffaele
Mezzenga
*a
aFood & Soft Materials Science, Institute of Food, Nutrition & Health, ETH Zurich, Schmelzbergstrasse 9, 8092 Zürich, Switzerland. E-mail: raffaele.mezzenga@agrl.ethz.ch
bDepartment of Polymer Materials, Shanghai University, Chengzhong 20, 201800 Shanghai, China
First published on 28th July 2010
Abstract
Temperature- and pH-responsive dispersions of carbon nanotubes in water have been obtained by using di- and tri-block copolypeptides, in which the temperature responsive behaviour is provided by a poly(N-isopropylacrylamide) block, while the pH responsiveness is provided by two different polypeptides with an ampholytic behaviour, poly(L-glutamic acid) and poly(L-lysine). The molecular architectures of the block copolypeptides can be engineered to fine-tune the stimuli-responsive behaviour.
Due to their exceptional combination of thermal, mechanical, optical and electrical properties,1 application of carbon nanotubes (CNTs) in biological fields,2 biosensors, drug delivery and tissue engineering, are highly attractive, although their real outcome still remains to be assessed. Factors hindering their widespread use include strong tendency to segregate, poor biological compatibility and the difficulty in designing a stimuli-responsive behaviour responding to variable biological conditions, such as pH, temperature and ionic strength.
Covalent modification of CNTs has been widely attempted to disperse CNTs into various solvents, although this typically jeopardizes the CNTs' long-range π-conjugation and thus, most of CNTs' unique properties.3 Alternatively, physical adsorption of proteins on the surface of CNTs has been shown to be highly promising, due to their biological relevance, their biocompatibility, and their complex secondary and tertiary structures.4 Dordick et al. found that various natural proteins could efficiently disperse single wall carbon nanotubes (SWCNTs) in water.5 Hydrophobic interactions, π–π interactions or specific interactions triggered by the amine functionalities, have typically been exploited to tune adsorption of proteins onto CNTs. By precisely engineering the hydrophobic, hydrophilic and aromatic residues, Dieckmann et al. synthesized a short peptide which not only did solubilise CNTs but could also control their assembly.6 Additionally, Wang et al. found that amphiphilic peptides and flexible peptide sequences in solution are very efficient in binding to the nanotubes.7 Wang and Chen found that polylysine in the protonated state could disperse SWCNTs but not in the de-protonated state.8
Another interesting feature of proteins is their pH responsiveness. By changing the electric charge and conformation of bovine serum albumin, Edri et al. studied the pH dependence of the dispersion of SWCNTs in water.9 Nepal et al. carried out a comparable study using lysozyme.10 On the other hand, few attempts have been made to prepare temperature-responsive aqueous dispersions of SWCNTs, probably due to lack of suitable thermo-responsive biopolymers. Most studies have been focusing on poly(N-isopropylacrylamide) (PNiPAM).11 However, the physical interaction between SWCNTs and pure PNiPAM is not sufficient, alone, for efficient stabilization of CNTs.8
In this study, we report on the temperature- and pH- responsive dispersions of SWCNTs in water by using di- and tri-block copolypeptides (1 & 2) containing both peptidic and PNiPAM blocks, as shown in Fig. 1. Since the glutamic acid unit has the average pKa of 4.05 and the lysine unit of 10.54, the copolypeptide block are positive charged at pH<4.05 or negatively charged at pH >10.54.12 At pH = 7, insoluble aggregates form due to the complexation of the oppositely charged L-glutamic acid and L-lysine units. Along with the two-fold stimuli-responsive properties, the novelty of the present study also relies on the fact that the use of two polypeptides with ampholytic behaviour, rather than one single polypeptide as previously reported in literature, greatly widens the pH spectrum on which the stimuli–responsive properties can be achieved.
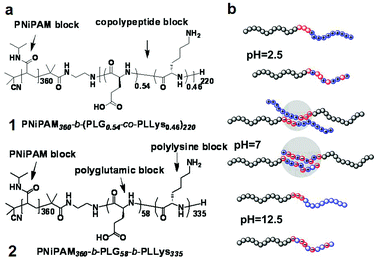 | ||
| Fig. 1 (a) Chemical structures of block copolypeptides 1 & 2 used in this study and (b) their charged states at pH 2.5, 7 and 12.5. | ||
Fig. 2a and b show the efficiency of these block copolypeptides to disperse SWCNTs in water at different pHs. After centrifugation the supernatants of the SWCNT-diblock copolymer 1 dispersion is a homogeneous, dark solution both at pH 2.5 and 12.5 while a transparent colourless solution at pH 7 is found, indicating that SWCNTs constitute a fine-enough dispersion only at pH 2.5 and 12.5, while they precipitate at pH 7. All the dispersions are stable for several months. The transitions were found to be fully reversible in the time scale of tens of minutes.
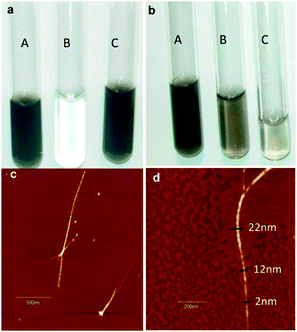 | ||
| Fig. 2 Dispersions of SWCNTs by use of diblock copolymer 1 (a) and triblock copolymer 2 (b), pH = 2.5 (A), 7(B), 12.5(C). AFM images in the dry state at low (c) and high resolution (d). | ||
Considering the nearly symmetrical compositions of L-glutamic acid and L-lysine, at neutral pH due to complexation, PNiPAM is the only water-soluble block, whose weak affinity to SWCNTs, however, is not sufficient to produce a fine dispersion. At pH 12.5, the L-glutamic acid units are negatively charged while the L-lysine units are neutral. According to Wang et al.,8 PLLys could not solubilise SWCNTs at high pH due to two reasons: (a) the inhibition of cation-π interaction between de-protonated amine groups and the π-electron orbitals of the SWCNTs for de-protonated PLLys; (b) the rigid secondary structures (α-helix of de-protonated PLLys) prevent interactions between the SWCNT walls and the polypeptide backbones. In the present case, SWCNTs are, hereby, most probably stabilized by the anionic L-glutamic acid units at pH 12.5. On the contrary, at pH 2.5, when the L-lysine units are positive charged and the L-glutamic acid units are neutral, the SWCNTs are stabilized by the protonated L-lysine units. These results can be confirmed by using the triblock copolymer 2 (Fig. 2b). At pH 2.5, the long, protonated PLLys block of 2 is sufficient to stabilize SWCNTs via the mechanisms discussed above. At pH 7 the triblock copolymer can also stabilize SWCNTs, due to the highly asymmetrical composition of L-glutamic acid and L-lysine units, in which the excess of protonated L-lysine residues is large enough to partially disperse SWCNTs. At pH 12.5, due to very short PLG block, only a very limited amount of SWCNTs can be dispersed by the de-protonated L-glutamic residues.
Fig. 2c & d show two AFM images of SWCNTs dispersions by diblock copolymer 1 at pH 2.5 in the dry state. Other AFM images of the dispersions are not shown but are identical. Because drying occurs during a time scale of 1–2 min, which is very short compared to both the kinetics of phase changes and stability of the samples, we infer that the AFM directly reflects the solution state of the block copolypeptides/CNT mixtures. The SWCNTs appear shortened and dispersed individually after sonication. Some SWCNTs are bundled together but they maintain a rod-like morphology. Fig. 2d gives a high-resolution example of SWCNT coated by the block copolymer. Interestingly, different heights can be resolved along the contour length of one single bundle. At the chain end of the bundle, the original thickness of the SWCNT can be resolved (2 nm); by moving along the contour length the thickness increases to 12 nm corresponding to the SWCNT coated by a brush of block copolymer. By further moving along the contour length, the thickness increases to 22 nm: this corresponds to twice the cross section of a block copolymer-coated SWCNT, and indeed, the bundle unwinds upon moving further along the contour length into two-single block copolymer-coated SWCNT. This demonstrates quite straightforwardly that block copolypeptides absorb at the surface of SWCNTs to disperse them in water.
Due to the strong absorption and scattering behaviour of SWCNTs, few reports have made use of light scattering as a probe technique to study the temperature responsive behaviour of PNiPAM/SWCNTs dispersion.13 Here, we have used cross correlation methods to suppress multiple scattering. As shown in Fig. 3a, by increasing the temperature close to the coil-globule transition of PNiPAM at 30–32 °C, the hydrodynamic radiuses (Rh) decreased slowly for both the pH 2.5 and 12.5 solutions dispersed by diblock copolymer 1. This is the consequence of the change of the solvent conditions for PNiPAM from good to poor in correspondence of the coil-globule transition temperature, followed by an increased aggregation among the sterically stabilized SWCNT:block copolymer systems (schemed in Fig. 4).
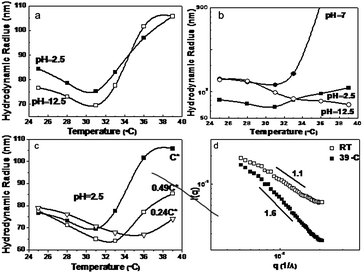 | ||
| Fig. 3 The temperature dependence of hydrodynamic radius obtained viadynamic light scattering for the solutions with block copolymer 1 (a & c) and 2 (b). Static light scattering profiles at room temperature and 39 °C for the solutions with 1 at pH 2.5 (d). | ||
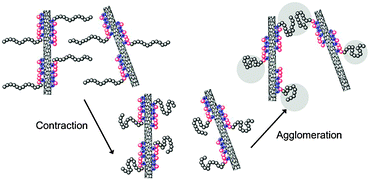 | ||
| Fig. 4 Scheme of competition between contraction and agglomeration of PNiPAM blocks upon increase of the temperature. | ||
Fig. 3b, shows the results from the same study in the case of triblock copolymer 2. The solution at pH 2.5 gave the same trend as for Fig. 3a. However, at pH 12.5, Rh decreased continuously without any sign of formation of larger aggregates above 32 °C. Considering the same block copolymer concentration and the same PNiPAM block length with diblock copolymer 1, this trend has to be closely related with much shorter charged PLG block and lower residual SWCNT concentration in the supernatant. At pH 7 above 32 °C, Rh increased rapidly and led to precipitation.
We infer that the difference in the temperature-triggered aggregation behaviour is a direct consequence of the molecular architecture of the block copolypeptides. In the case of diblock copolymer 1, because both the L-glutamic and L-lysine residues belong to the same random copolypeptide block, the entire peptidic block always participates in the SWCNTs stabilization, whereas the PNiPAM is entirely responsible for the temperature-responsive behaviour. Thus, aggregation or dispersability directly result from the quality of the solvent (water) at the different temperatures. This is consistent with the PNiPAM-driven aggregation above 32 °C. In the case of triblock copolymer 2, the specific stabilizing peptidic block changes with pH. At pH 2.5, the longest PLLys block is the one, which absorbs on the SWCNT walls. The temperature responsiveness is thus resulting from the behaviour of both the PNiPAM and PLG blocks: because the latter is very short, however, it is still the PNiPAM block, which dominates the temperature-responsive behaviour. This leads, again to the expected aggregation at higher temperatures (>32 °C). In this case, the role of PLG as a hydrophilic minority block is only that of limiting the size of the aggregates. At pH 12.5, however, the PLG block is the one absorbing on the SWCNT walls, and the temperature responsiveness depends on both the PLLys and PNiPAM blocks. In this case, since PLLys block is as large as PNiPAM and does not have an LCST, a co-brush of both hydrophilic and hydrophobic segments stabilize the SWCNTs. Thus, upon increasing temperature beyond 32 °C, the quality of the solvent does not decrease for the PLLys brush, aggregation is avoided and the Rh keeps decreasing. Finally, in the case of pH 7, both PLLys and PLG are charged. The block absorbing on the SWCNTs wall is the excess of PLLys which does not participate in the PLLys-PLG ionic complex. The temperature responsive behaviour is then driven entirely by the PNiPAM block, leading once again to observed expected behaviour.
To further investigate the role of SWCNT concentration on the aggregation behaviour, the SWCNT dispersion with diblock copolymer 1 at pH 2.5 was diluted to 0.49 and 0.24 times the original concentration keeping the same pH. As can be seen in Fig. 3c, at temperatures lower than the coil-globule transitions, only small changes are observed. At high temperatures, however, dilution reduces the average size of the clusters, which demonstrates that the final cluster size is concentration dependent.
The structure of the dispersions can be further elucidated by studying the characteristic exponent of the scattered light intensity: I(q) ∼ q−D, where I(q) is the scattered intensity, q the scattering vector and D the fractal dimension. Fig. 3d gives the plots of I(q) ∼ q for the SWCNT solution with diblock copolymer 1 (pH 2.5) at RT and 39 °C. The linear nature of the curves indicates fractal-like morphologies. The slopes were obtained via linear regression of log[I(q)] ∼ log(q). In Fig. 3d, the slope of −1.1 at RT indicates a rod-like structure with some degrees of flexibility at the 2π/q length scales, e.g. 600 nm. This occurs is in the range of the 102 nm persistence length previously reported in literature;14 and is consistent with small angle neutron scattering from single-wall carbon nanotube suspensions providing evidence for isolated rigid rods and rod networks.15 However an increasing slope of −1.6 was observed at 39 °C, upon aggregation, which suggests either an increased flexibility at this length scales, or a self-avoiding random walk of SWCNT segments. (e.g. 1.66 is the typical fractal exponent for a self-avoiding random walk). Consistently, an agglomeration of SWCNTs into a branched, random geometrical structures in a Mikado-like clusters can be expected (as shown in Fig. 4).
AZ wishes to thank Jingguo Li and Xiuqiang Zhang for the synthesis of block copolypeptides. The Swiss Science national Foundation is kindly acknowledged for financial support.
Notes and references
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Science, 2002, 297, 787 CrossRef CAS.
- Y. Lin, S. Taylor, H. Li, K. A. Fernando, L. Qu, W. Wang, L. Gu, B. Zhou and Y. P. Sun, J. Mater. Chem., 2004, 14, 527 RSC.
- S. Banerjee, T. Hemraj-Benny and S. S. Wong, Adv. Mater., 2005, 17, 17 CrossRef CAS.
- V. Nicolosi, H. Cathcart, A. R. Dalton, D. Aherne, G. R. Dieckmann and J. N. Coleman, Biomacromolecules, 2008, 9, 598 CrossRef CAS; F. Balavoine, P. Schultz, C. Richard, V. Mallouh, T. W. Ebbesen and C. Mioskowski, Angew. Chem., Int. Ed., 1999, 38, 1912 CrossRef CAS; S. S. Karajanagi, A. A. Vertegel, R. S. Kane and J. S. Dordick, Langmuir, 2004, 20, 11594 CrossRef CAS.
- S. S. Karajanagi, H. Yang, P. Asuri, E. Sellitto, J. S. Dordick and R. S. Kane, Langmuir, 2006, 22, 1392 CrossRef CAS.
- G. R. Dieckmann, A. B. Dalton, P. A. Johnson, J. Razal, J. Chen, G. M. Giordano, E. Munoz, I. H. Musselman, R. H. Baughman and R. K. Draper, J. Am. Chem. Soc., 2003, 125, 1770 CrossRef CAS.
- S. Wang, E. S. Humphreys, S.-Y. Chung, D. F. Delduco, S. R. Lustig, H. Wang, K. N. Parker, N. W. Rizzo, S. Subramoney, Y.-M. Chiang and A. Jagota, Nat. Mater., 2003, 2, 196 CrossRef CAS.
- D. Wang and L. Chen, Nano Lett., 2007, 7, 1480 CrossRef CAS.
- E. Edri and O. Regev, Langmuir, 2009, 25, 10459 CrossRef CAS.
- D. Nepal and K. Geckeler, Small, 2006, 2, 406 CrossRef CAS.
- H. Kong, W. Li, C. Gao, D. Yan, Y. Jin, D. R. M. Walton and H. W. Kroto, Macromolecules, 2004, 37, 6683 CrossRef CAS.
- C. Li, J. Li, X. Zhang, A. Zhang and R. Mezzenga, Macromol. Rapid Commun., 2010, 31, 265 CrossRef.
- J. Y. Lee, J. S. Kim, K. H. An, K. Lee, D. Y. Kim, D. J. Bae and Y. H. Lee, J. Nanosci. Nanotechnol., 2005, 5, 1045 CrossRef CAS.
- M. Sano, A. Kamino, J. Okamura and S. Shinkai, Science, 2001, 293, 1299 CrossRef CAS.
- W. Zhou, M. F. Islam, H. Wang, D. L. Ho, A. G. Yodh, K. I. Winey and J. E. Fischer, Chem. Phys. Lett., 2004, 384, 185 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0cc01606c |
| This journal is © The Royal Society of Chemistry 2011 |
