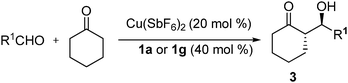Primary amine-metal Lewis acid bifunctional catalysts based on a simple bidentate ligand: direct asymmetric aldol reaction†‡
Philias
Daka
,
Zhenghu
Xu
,
Alexandru
Alexa
and
Hong
Wang
*
Department of Chemistry and Biochemistry, Miami University, Oxford, OH 45056, US. E-mail: wangh3@muohio.edu; Fax: 01 (513)529-5715; Tel: 01(513)529-2824
First published on 21st June 2010
Abstract
A novel class of primary amine-metal Lewis acid bifunctional catalysts based on a bidentate ligand was developed. These catalysts were highly efficient in catalyzing the direct asymmetric aldol reactions of ketones offering excellent stereoselectivity. The aldol reactions required a low catalyst loading (2.5 mol%), and were water compatible.
The combination of organocatalysis with transition metal catalysis has recently emerged as a promising strategy to discover new carbon-carbon and carbon-heteroatom forming reactions.1 Given the unique activation modes of organocatalysis and the well-established rich chemistry of metal catalysis, the combination of these two areas holds great potential to achieve organic transformations that are not possible through organocatalysis or metal catalysis alone. Enamine-based catalysis plays a crucial role in organocatalysis.2 In recent years, several publications combining secondary amine-based enamine catalysis with transition metal catalysis have shown unprecedented organic transformations,1,3 demonstrating the power of the combined systems. The major challenge in developing Lewis base-metal Lewis acid bifunctional catalytic systems lies in the acid–base quenching reaction (by coordination) leading to catalyst inactivation.4a The general approach to solving this problem is to use a soft metal Lewis acid along with a hard Lewis base, or vice-versa, followed by fine tuning the reaction condition until the desired product is formed as the major product.3,4 We intend to take a different approach. In our system, the Lewis base (L-amino acids) is tethered to a chelating ligand, which serves as a “trap” for the incoming metal. In this way, the base and the metal Lewis acid are brought to close proximity without interacting with each other (Fig. 1). A unique advantage of this system is that the Lewis acidity can be easily tuned by simply replacing it with a different metal, and the “softness” of the metal does not need to be considered for the purpose of preventing the acid–base interaction.
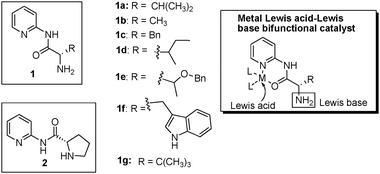 | ||
| Fig. 1 Structure of ligands and illustration of the bifunctional catalyst | ||
Despite the potentials of multi/bifunctional catalysts, the development of these systems is a formidable task due to the simultaneous requirements for the distance, rigidity, orientation and interactions of these functional groups. In previous work, we showed the first example of primary amine-metal Lewis acid bifunctional catalysts using a chelating tridentate ligand.5a Compared to a majority of those primary amine-based organocatalysts,6 the activity and diastereoselectivity of these catalysts were significantly enhanced for the asymmetric direct aldol reactions. In our continuous effort to develop enamine-metal Lewis acid multi/bifunctional catalysts, we feel that it is necessary to enrich our ligand repository in order to fully utilize this new concept, and more importantly, to open it up to wider exploitation. In this work, we wish to introduce another novel class of primary amine-metal Lewis acid bifunctional catalysts based on a simple bidentate ligand, and their successful application to the asymmetric direct aldol reactions of ketones.
Seven bidentate ligands tethered with chiral primary amines were prepared (Fig. 1). The synthesis of these ligands was achieved through coupling reactions between N-Boc protected L-α amino acids and 2-aminopyridine followed by deprotection (see details in Supporting Information). We expect that the metal will serve as a Lewis acid activating the aldol acceptor, and the primary amine will serve as a Lewis base to form an enamine intermediate with the aldol donor (Fig. 2).
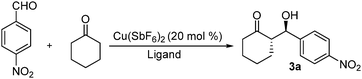
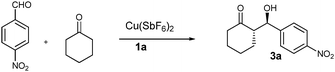
We started investigating the catalytic activities of these ligands with the aldol reaction of cyclohexanone with 4-nitrobenzaldehyde in neat conditions (Table 1). We first chose Cu(SbF6)2 as the Lewis acid, as it was the best match for our tridentate system.5 In order to understand how coordination affects the activities of these catalysts, two different ligand/metal ratios (1/1 and 2/1) (entries 1–6) were used. It turned out that 2/1 ligand/metal ratio gave much higher activity and higher stereoselectivity. The ligand/metal ratio of 2/1 was then selected to examine the rest of the ligands. While all ligands displayed very high activity in terms of yield and reaction time, 1a and lg showed exceptionally high activity completing the reaction in only three hours, much faster than the reactions catalyzed by most primary amine based organocatalysts which in general require 2 to 3 days for the completion.6 These bidentate ligand systems are also much more active than our tridentate ligand systems.5 The much higher activity may be attributed to the coexistence of the two primary amines in the symmetric 2/1 ligand/metal system. 1a and lg also provided the best diastereoselectivity (25/1, dr) and enantioselectivity (>99%, ee) (entries 2 and 10), respectively. For comparison, L-proline tethered ligand 2 was prepared. The secondary amine based ligand 2 resulted in much lower activity and stereoselectivity (entry, 11), another example of primary amines showing superior enamine catalytic activity than secondary amines.
| Entry | Ligand | T (h) | L:M | Yield (%)b | Anti/Sync | Ee (%)d |
|---|---|---|---|---|---|---|
| a The reactions were performed with 0.2 mmol of 4-nitrobenzaldehyde and 1 mL of cyclohexanone at room temperature (neat). b Combined yield. c Determined by 1H NMR. d Determined by chiral HPLC. | ||||||
| 1 | 1a | 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78 | 14/1 | 96 |
| 2 | 1a | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 25/1 | 98 |
| 3 | 1b | 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 | 10/1 | 93 |
| 4 | 1b | 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86 | 16/1 | 94 |
| 5 | 1c | 24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82 | 6/1 | 76 |
| 6 | 1c | 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 16/1 | 80 |
| 7 | 1d | 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 | 20/1 | 90 |
| 8 | 1e | 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92 | 9/1 | 90 |
| 9 | 1f | 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
64 | 7/1 | 84 |
| 10 | 1g | 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 9/1 | >99 |
| 11 | 2 | 34 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 | 3/1 | 79 |
Metal and solvent screenings were carried out next using ligand 1a in neat conditions (see Supporting Information). CuII salts offered superior activities and stereoselectivities than other metals including CoII, ZnII, YbIII, and NiII; counter anion also played a role in determining the stereoselectivity as Cu(SbF6)2 displayed much better diastereoselectivity and enantioselectivity than Cu(OTf)2. A range of solvents including DMSO, THF, CH3CN, toluene, MeOH, DCM, and DMF were examined in the presence of Cu(SbF6)2. The best results were obtained from the neat conditions (see Supporting Information).
The substrate scope of the aldol reactions of cyclohexanone was then investigated using ligand 1a and 1g under optimized conditions (Cu(SbF6)2/neat, Table 2). Very good to excellent diastereoselectivity (up to 30/1) and enantioselectivity (up to >99% ee) were achieved with all the electron-poor aromatic aldehydes (entries, 1–14) for both ligands; while 1g gave excellent enantioselectivity (>99% ee, entries 2, 4, 6, 8, 9, 10 and 12), their diastereoselectivity was lower than those of 1a. Electron-rich aromatic aldehydes also furnished with good diastereoselectivities and enantioselectivities (entries, 15–18); the aldol reaction of acetone with 4-nitrobenzaldehyde resulted in 81% ee (see Supporting Information).
| Entry | R1 | L | Product | Yield(%)b | Anti/sync | Ee(%)d |
|---|---|---|---|---|---|---|
| a The reactions were performed with 0.2 mmol of aldehyde at room temperature in 1 mL of cyclohexanone for 3-48 h. b Combined yield. c Determined by 1H NMR. d Determined by chiral HPLC. | ||||||
| 1 | 4-NO2C6H4 | 1a | 3a | 80 | 25/1 | 98 |
| 2 | 1g | 90 | 9/1 | >99 | ||
| 3 | 3-NO2C6H4 | 1a | 3b | 86 | 17/1 | 96 |
| 4 | 1g | 80 | 3/1 | >99 | ||
| 5 | 2-NO2C6H4 | 1a | 3c | 84 | 30/1 | 95 |
| 6 | 1g | 88 | 4/1 | >99 | ||
| 7 | 4-CNC6H4 | 1a | 3d | 83 | 16/1 | 97 |
| 8 | 1g | 85 | 3/1 | >99 | ||
| 9 | 2,6-Cl2C6H3 | 1a | 3e | 86 | 14/1 | >99 |
| 10 | 1g | 89 | 4/1 | >99 | ||
| 11 | 4-COOMeC6H4 | 1a | 3f | 94 | 12/1 | 97 |
| 12 | 1g | 90 | 3/1 | >99 | ||
| 13 | 4-BrC6H4 | 1a | 3g | 70 | 12/1 | 94 |
| 14 | 4-ClC6H4 | 1a | 3h | 84 | 7/1 | 97 |
| 15 | C6H5 | 1a | 3i | 75 | 10/1 | 83 |
| 16 | 1g | 83 | 4/1 | 89 | ||
| 17 | 2-Naphthyl | 1a | 3j | 70 | 8/1 | 92 |
| 18 | 4-MeC6H4 | 1a | 3k | 70 | 5/1 | 82 |
The effect of catalyst loading on the aldol reaction of cyclohexanone with 4-nitrobenzaldehyde using ligand 1a/Cu(SbF6)2 was examined (Table 3). Although an overall decreasing trend in activity and stereoselectivity was observed when less catalyst was used, the stereoselectivity did not show much difference with a catalyst loading between 5 to 20 mol% (∼98% ee, 16/1–25/1 dr, entries, 1–4), and the catalyst remained very active in terms of both yield and reaction time with a loading as low as 2.5 mol% (entry, 5).
| Entry | Cat. (mol%) | Time (h) | Conversion(%) | Yield(%)b | Anti/Sync | Ee(%)d |
|---|---|---|---|---|---|---|
| a The reactions were performed with 0.2 mmol of aldehyde and 1mL cyclohexanone at room temperature. b Combined yield. c Determined by 1H NMR. d Determined by chiral HPLC. | ||||||
| 1 | 20 | 3 | 100 | 80 | 25/1 | 98 |
| 2 | 15 | 4 | 100 | 84 | 16/1 | 98 |
| 3 | 10 | 6 | 100 | 80 | 16/1 | 98 |
| 4 | 5 | 10 | 100 | 85 | 16/1 | 98 |
| 5 | 2.5 | 30 | 100 | 80 | 11/1 | 96 |
| 6 | 1.0 | 144 | 95 | 67 | 9/1 | 93 |
| 7 | 0.5 | 144 | 50 | 40 | 4/1 | 89 |
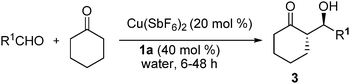
The performance of the catalytic system (1a/Cu(SbF6)2) was also examined in the presence of water using the aldol reactions of cyclohexanone with a number of aromatic aldehydes (Table 4). These aldol reactions were water tolerant (H2O/cyclohexanone, v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), however, with slight decreases in both activity and stereoselectivity. The decreasing trend became slightly more severe when more water was included in the reaction system (entries, 1–3).
1), however, with slight decreases in both activity and stereoselectivity. The decreasing trend became slightly more severe when more water was included in the reaction system (entries, 1–3).
| Entry | R1 | H2O/Cyclohexanoneb | Product | Yield(%)c | Anti/Synd | Ee(%)e |
|---|---|---|---|---|---|---|
| a The reactions were performed with 0.2 mmol of aldehyde. b Volume ratio, total volume: 1 mL. c Combined yield. d Determined by 1H NMR. e Determined by chiral HPLC. | ||||||
| 1 | 4-NO2C6H4 | 1/1 | 3a | 62 | 11/1 | 96 |
| 2 | 4-NO2C6H4 | 2/1 | 3a | 65 | 10/1 | 95 |
| 3 | 4-NO2C6H4 | 3/1 | 3a | 66 | 9/1 | 93 |
| 4 | 4-CNC6H4 | 2/1 | 3d | 65 | 6/1 | 93 |
| 5 | 4-ClC6H4 | 2/1 | 3h | 57 | 7/1 | 92 |
| 6 | 4-BrC6H4 | 2/1 | 3g | 53 | 6/1 | 89 |
| 7 | 4-CO2MeC6H4 | 2/1 | 3f | 70 | 5/1 | 94 |
| 8 | 2-Naphyl | 2/1 | 3j | 45 | 8/1 | 90 |
The cooperative nature of this catalytic system was revealed by the fact that Cu(SbF6)2 or the ligand (1a) alone could not catalyze the aldol reaction of cyclohexanone with 4-nitrobenzaldehyde. All the aldol reactions of cyclohexanone predominately generated the anti aldol products with (2S, 1′R) configuration. The mechanism is likely to follow that the metal coordinates with the chelating ligand to form a rigid chiral structure and acts as a Lewis acid to activate the aldehyde; the primary amine reacts with cyclohexanone to form an enamine attacking the activated aldehyde from the re-face to generate the products (Fig. 2).
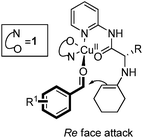 | ||
| Fig. 2 Proposed transition state | ||
In summary, we have developed a new class of primary amine-metal Lewis acid cooperative bifunctional catalysts using simple bidentate ligands. These bifunctional catalysts displayed exceptionally high activity in the direct asymmetric aldol reactions of ketones with both electron-rich and electron-poor aldehydes. The enantioselectivities obtained from these reactions are comparable with those catalyzed by the best organocatalysts, and are much higher than those of the tridentate ligand system. It is notable that these catalysts possess both advantages of metal Lewis acid and organocatalysts, as requiring low catalyst loading and being water tolerant. The in depth understanding of the coordination chemistry of these bifunctional catalysts is currently under investigation. The application of these enamine-metal Lewis acid bifunctional catalysts to activate ketone acceptors through a strong Lewis acid in aldol reactions as well as in inverse-electron-demand hetero-Diels–Alder reactions are under way, and will be reported in due course.
We thank Professor Mike Novak for useful discussions. Financial support was provided by Miami University.
Notes and references
- (a) Z. H. Shao and H. B. Zhang, Chem. Soc. Rev., 2009, 38, 2745 RSC; (b) A. Duschek and S. F. Kirsch, Angew. Chem., Int. Ed., 2008, 47, 5703 CrossRef CAS.
- (a) B. List, R. A. Lerner and C. F. Barbas, III, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS; (b) W. Notz and B. List, J. Am. Chem. Soc., 2000, 122, 7386 CrossRef CAS; (c) K. S. Sakthivel, W. Notz, T. Bui and C. F. Barbas, III, J. Am. Chem. Soc., 2001, 123, 5260 CrossRef CAS . For reviews, see; (d) P. Melchiorre, M. Marigo, A. Carlone and G. Bartoli, Angew. Chem., Int. Ed., 2008, 47, 6138 CrossRef CAS; (e) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS; (f) A. Erkkilä, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef.
- (a) I. Ibrahem and A. Cordova, Angew. Chem., Int. Ed., 2006, 45, 1952 CrossRef CAS; (b) F. Bihelovic, R. Matovic, B. Vulovic and R. N. Saicic, Org. Lett., 2007, 9, 5063 CrossRef CAS; (c) Q. Ding and J. Wu, Org. Lett., 2007, 9, 4959 CrossRef CAS; (d) J. T. Binder, B. Crone, T. T. Haug, H. Menz and S. F. Kirsch, Org. Lett., 2008, 10, 1025 CrossRef CAS; (e) T. Yang, A. Ferrali, L. Campbell and D. J. Dixon, Chem. Commun., 2008, 2923 RSC; (f) O. Abillard and B. Breit, Adv. Synth. Catal., 2007, 349, 1891 CrossRef CAS; (g) S. Chercheja and P. Eilbracht, Adv. Synth. Catal., 2007, 349, 1897 CrossRef CAS.
- (a) D. H. Paull, C. J. Abraham, M. T. Scerba, E. Alden-Danforth and T. Lectka, Acc. Chem. Res., 2008, 41, 655 CrossRef CAS; (b) J. Kofoed, T. Darbre and J. L. Reymond, Chem. Commun., 2006, 1482 RSC; (c) R. Fernandez-Lopez, J. Kofoed, M. Machuqueiro and T. Darbre, Eur. J. Org. Chem., 2005, 5268 CrossRef CAS; (d) J. Kofoed, J. L. Reymond and T. Darbre, Org. Biomol. Chem., 2005, 3, 1850 RSC.
- (a) Z. Xu, P. Daka and H. Wang, Chem. Commun., 2009, 6825 RSC; (b) Z. Xu, P. Daka, I. Budik, H. Wang, F. Q. Bai and H. X. Zhang, Eur. J. Org. Chem., 2009, 4581 CrossRef CAS.
- For reviews on primary amine catalysis, see: (a) L.-W. Xu and Y. Lu, Org. Biomol. Chem., 2008, 6, 2047 RSC; (b) L.-W. Xu, J. Luo and Y. Lu, Chem. Commun., 2009, 1807 RSC; (c) F. Z. Peng and Z. H. Shao, J. Mol. Catal. A: Chem., 2008, 285, 1 CrossRef CAS; for recent examples, see: (d) M. Amedjkouh, Tetrahedron: Asymmetry, 2005, 16, 1411 CrossRef CAS; (e) A. Córdova, W.-B. Zou, I. Ibrahem, E. Reyes, M. Engqvist and W.-W. Liao, Chem. Commun., 2005, 3586 RSC; (f) Z. Jiang, Z. Liang, X. Xu and Y. Lu, Chem. Commun., 2006, 2801 RSC; (g) X. Wu, Z. Jiang, H.-M. Shen and Y. Lu, Adv. Synth. Catal., 2007, 349, 812 CrossRef CAS; (h) S. Luo, H. Xu, J. Li, L. Zhang and J.-P. Cheng, J. Am. Chem. Soc., 2007, 129, 3074 CrossRef CAS; (i) X.-Y. Xu, Y.-Z. Wang and L.-Z. Gong, Org. Lett., 2007, 9, 4247 CrossRef CAS; (j) K. Nakayama and K. Maruoka, J. Am. Chem. Soc., 2008, 130, 17666 CrossRef CAS; (k) B. L. Zheng, Q. Z. Liu, C. S. Guo, X. L. Wang and L. He, Org. Biomol. Chem., 2007, 5, 2913 RSC; (l) R. K. Zeidan and M. E. Davis, J. Catal., 2007, 247, 379 CrossRef CAS; (m) L. Li, L. W. Xu, Y. D. Ju and G. Q. Lai, Synth. Commun., 2009, 39, 764 CrossRef CAS; (n) S. Z. Luo, X. X. Zheng and J. P. Cheng, Chem. Commun., 2008, 5719 RSC; (o) F. Z. Peng, Z. H. Shao, X. W. Pu and H. B. Zhang, Adv. Synth. Catal., 2008, 350, 2199 CrossRef CAS; (p) J. Y. Li, S. Z. Luo and J. P. Cheng, J. Org. Chem., 2009, 74, 1747 CrossRef CAS; (q) S. Z. Luo, H. Xu, L. Zhang, J. Y. Li and J. P. Cheng, Org. Lett., 2008, 10, 653 CrossRef CAS; (r) X. Ma, C. S. Da, L. Yi, Y. N. Jia, Q. P. Guo, L. P. Che, F. C. Wu, J. R. Wang and W. P. Li, Tetrahedron: Asymmetry, 2009, 20, 1419 CrossRef CAS; (s) J. Zhou, V. Wakchaure, P. Kraft and B. List, Angew. Chem., Int. Ed., 2008, 47, 7656 CrossRef CAS; (t) A. Córdova, W. B. Zou, P. Dziedzic, I. Ibrahem, E. Reyes and Y. M. Xu, Chem.–Eur. J., 2006, 12, 5383 CrossRef CAS; (u) S. S. V. Ramasastry, H. Zhang, F. Tanaka and C. Barbas, J. Am. Chem. Soc., 2007, 129, 288 CrossRef CAS; (v) B. L. Zheng, Q. Z. Liu, C. S. Guo, X. L. Wang and L. He, Org. Biomol. Chem., 2007, 5, 2913 RSC.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data for all new compounds, and HPLC data. See DOI: 10.1039/c0cc00917b |
| This journal is © The Royal Society of Chemistry 2011 |