Electrolytically fabricated nickel microrods on screen printed graphite electrodes: Electro-catalytic oxidation of alcohols
Nadeem A.
Choudhry
and
Craig E.
Banks
*
Faculty of Science and Engineering, School of Chemistry and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester, M1 5GD, Lancs, UK. E-mail: c.banks@mmu.ac.uk; Fax: +44(0)161247116831; Tel: +44(0)1612471196
First published on 2nd November 2010
Abstract
Nickel modified graphite screen printed electrodes are explored towards the sensing of alcohols in alkaline solutions. Electrolytically formed nickel microrods with average lengths and diameters of 12 μM and 2 μM respectively are shown to be readily formed on the surfaces of graphite screen printed electrodes. This is the first example of electrolytically formed nickel nanorods which exhibit electro-catalysis towards the sensing of ethanol over the range 2.6–23 mM and glycol over the range 230–1840 μM with limits of detection of 1.4 mM and 186 μM respectively.
1. Introduction
The electrochemical oxidation of alcohols is of wide importance in high performance fuel cells which hold promise as renewable and low cost alternative fuels sources.1,2 A plethora of electro-catalytic materials have been explored with platinum based materials which are the current contenders, with research into other possible materials a highly active area in order to reduce the associated cost.3 One such potential electro-catalyst is nickel oxide which has been explored as a potential catalyst towards alcohols.1,4–7 While research is heavily focused with a view to develop fuel cell technologies, the analytical utilisation of such a system should not be overlooked and novel low cost, simple and disposable analytical tools are urgently required. For example, this might be utilised as a sensor for the monitoring of operational safety in the form of fuel cell leaks.Towards these goals, recently nickel nano-particle modified boron-doped diamond electrodes have been explored towards the sensing of ethanol and glycerol8 as well as a nickel micro-particle modified boron-doped diamond electrode for methanol sensing.9 There is considerable merit in this approach over that of a solid electrode consisting completely of nickel which includes reduced cost, ease of fabrication and a renewable surface and an improvement in the accessible linear ranges towards the target analyte due to improvements in diffusion to effectively a nickel micro/nano array. An alternative to commercially available solid electrodes such as boron-doped diamond and glassy carbon are disposable and cost effective screen printed electrodes.10–13
In this paper we explore the fabrication of electrolytically fabricated nickel microstructures using graphite screen printed graphite electrodes. It is found that nickel microrods can be readily formed at the surface of a screen printed electrode with average lengths and diameters of 12 μM and 2 μM respectively. While screen printed electrodes have been routinely used as templates for the electro-deposition of different nanoparticles compositions for a range of analytical targets,14–16 we find no literature reports of producing microrod structures in this way. Additionally we note that nickel hydroxide microrods have seldom been reported with fabrication methods involving solvothermal approaches17 and vertically aligned nickel oxide microrods on silicon substrates have also been reported via thermal heating.18 The electrolytically fabricated nickel microrods are explored as a potential electro-catalyst, in particular, towards the electro-catalytic oxidation of alcohols.
2. Experimental section
All chemicals used were of analytical grade and were used as received without any further purification from Sigma-Aldrich. All solutions were prepared with deionised water of resistively not less than 18.2 MΩ cm. All solutions were vigorously degassed with nitrogen to remove oxygen.Voltammetric measurements were carried out using a μ-Autolab III (Eco Chemie, The Netherlands) potentiostat/galvanostat and controlled by Autolab GPES software version 4.9 for Windows XP. Screen printed graphite macroelectrodes which have a 3 mm diameter working electrode were fabricated as reported previously.19 These electrodes have been characterised electrochemically in a prior paper and have heterogeneous electron transfer rate constants ∼ 1.7 × 10−3 cm s−1.12 In addition to the screen printed graphite electrodes being used as the working electrode, a large surface area platinum wire as a counter electrode and a Saturated Calomel Electrode (SCE) as the reference electrode were utilised. Connectors for the efficient connection of the screen printed electrochemical sensors were purchased from Kanichi Research Services Ltd.20
Scanning electron microscopy (SEM) images were obtained using a JEOL JSM-5600LV model.
3. Results and discussion
Screen printed graphite macroelectrodes were electrolytically modified with nickel using a previously reported methodology8 from a solution containing 1 mM nickel (II) in a 0.1 M sodium acetate solution (pH 5) employing a deposition potential of −1.2 V for 300 s. Fig. 1 depicts SEM images of the modified electrode which indicates that the electrode surface has been modified with unique structures which have average lengths of 12 μm with diameters of 2 μm. It is interesting to note that using identical electrochemical parameters and solutions as reported in the literature8 that utilising boron-doped diamond electrodes results in the formation of nanoparticles which indicates that the nucleation dynamics are quite different to that observed at boron-doped diamond electrode surfaces. Given the wide range in the size of the microrods, as observed in Fig. 1, a progressive growth processes arising from continued nucleation of metallic nickel to the active sites of the screen printed surface is highly likely.21 A survey of the literature reveals that nickel microrods have been fabricated but never electrochemically as presented here.17,18 | ||
| Fig. 1 SEM images of nickel modified screen printed platforms. | ||
We next turn to exploring the nickel microrod modified screen printed graphite macroelectrodes towards the electrochemical sensing of alcohols. Fig. 2A depicts voltammetric profile of the nickel microrod modified electrodes in 1 M sodium hydroxide where an oxidation wave at +0.44 V (vs.SCE) and a reduction wave at +0.35 V (vs.SCE) are observed. EDAX analysis of the nickel microrods exhibited three peak corresponding to nickel, an oxygen peak and of course a carbon peak from the underlying electrode surface. It is well established in the literature that deposited nickel metal spontaneously forms Ni(OH)2 in alkaline solutions,8 likely surface oxide rather than complete bulk transformation and we observe an electrochemical oxidation wave at ∼+0.4 V which is due to the Ni2+/Ni3+ signal as described by eqn (1). Previous research has shown that the nickel can exist in two crystallographic forms, hydrated α-Ni(OH)2 and anhydrous β-Ni(OH)2 which produces two voltammetric oxidation peaks.8 However in our case the observation of a single voltammetric wave indicates that the nickel microrods are highly likely to be in the β-Ni(OH)2 form.8Fig. 2A also depicts the voltammetric profiles resulting from the additions of 1.3 mM ethanol using a nickel microrod modified electrode where analysis of the anodic wave, as depicted in Fig. 2B increases with additions of ethanol coupled with the position of the voltammetric peak moving to more anodic potentials with each addition. It is clear at this concentration level a linear response over the range 4 to 10 mM (Fig. 2B) is observed. The observed voltammetric profiles are due to the Ni2+/Ni3+ redox couple which is the electro-catalytic origin of the voltammetric response undergoing the following process:
| Ni(OH)2 ⇄ NiOOH + H+ + e− | (1) |
 | (2) |
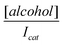 against [alcohol] allows the adsorption equilibrium constant, β, to be estimated as 356 (±3) M−1. Thus the Gibbs energy changes due to adsorption may be deduced from:
against [alcohol] allows the adsorption equilibrium constant, β, to be estimated as 356 (±3) M−1. Thus the Gibbs energy changes due to adsorption may be deduced from:| ΔG0 = −RTInβ | (3) |
 | ||
| Fig. 2 Cyclic voltammetric profiles from 1.3 mM ethanol additions into a 0.1 M sodium hydroxide obtained using a nickel microrod modified screen printed electrochemical platform. All scans recorded at 50 mVs−1vs.SCE. The analysis of the oxidation peak height (B) and reduction peak height (C) as a function of added ethanol concentrations are also shown. | ||
 | ||
| Fig. 3 Analysis of chronoamperometric response of the nickel microrod modified screen printed graphite electrode resulting from ethanol additions over the range of 0 to 26 mM. The electrode was held at a potential of +0.4 V (vs.SCE). | ||
We turn now to exploring the nickel microrod modified screen printed platforms for the sensing of glycerol. Fig. 4 depicts typical voltammetric profiles obtained from additions of glycerol into a 0.1 M NaOH solution where a linear type response is observed (Fig. 4B). To access the analytical response of the nickel microrods towards the sensing of glycerol, chronoamperometry was employed. As shown in Fig. 4C, analysis of the limiting current as a function of added glycerol concentration exhibits a linear response (I/A = 0.004 AM−1 + 1.3 × 10−6A; R2 = 0.991; N = 8) over the range of 230 μM to 1840 μM with a limit of detection (3-sigma23) found to correspond to 186 μM. Unfortunately this limit of detection is not as competitive as nickel nanoparticle modified boron-doped diamond electrodes or a nickel macroelectrode.8 Given that a very good analytical output is observed for the case of ethanol (see above) this suggests that the nickel microrods likely have a different amount of oxide on the nickel metal which might reduce the rate of reaction in the rate determining hydrogen-abstraction step in the electrochemical oxidation mechanism. While this is speculation, the unexpectedly poor analytical performance might still have analytical utility in niche applications.
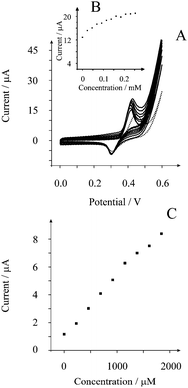 | ||
| Fig. 4 Cyclic voltammetric profiles (A) recorded showing 230 μM glycerol additions into a 1M sodium hydroxide solution using the nickel microrod modified screen printed graphite electrode. All scans recorded at 50 mVs−1vs.SCE. Part B depicts the analysis of the oxidation peak height as a function of glycerol concentrations. Part C is the analysis of chronoamperometric responses using the nickel microrod modified screen printed graphite electrode. The electrode was held at a potential of +0.4 V (vs.SCE). | ||
4. Conclusions
The first electrochemical fabrication of nickel microrods is reported which have been shown to be electroanalytical useful for the sensing of ethanol which perform analytically similar to current state-of-the-art using nickel nanoparticle modified electrodes and to a lesser extent the microrods can be utilised for glycerol sensing. The simple and low cost fabrication process of the nickel microrods suggest their use in the electroanalytical quantification of ethanol and glycol, for example in the monitoring for potential fuel cell leaks. The potential use of the nickel microrods in alcohol fuel cells as a electro-catalyst should also not be overlooked and are also being explored in other sensing applications.References
- A. E. Antolini, J. Power Sources, 2007, 170, 1 CrossRef CAS.
- E. Antolini and E. R. Gonzalez, J. Power Sources, 2010, 195, 3431 CrossRef CAS.
- N. W. Maxakato, C. J. Arendse and K. I. Ozoemena, Electrochem. Commun., 2009, 11, 534 CrossRef CAS.
- M. Fleischmann, K. Korinek and D. Pletcher, J. Electroanal. Chem., 1971, 31, 39 CrossRef CAS.
- A. Amajad, D. Pletcher and C. Smith, J. Electrochem. Soc., 1977, 124, 203 CrossRef.
- C.-C. Pang, M.-H. Chen, T.-Y. Lin and T.-C. Chou, Electroanalysis, 2000, 13, 499.
- Y. G. Lee and T.-C. Chou, Electroanalysis, 2003, 15, 1589 CrossRef CAS.
- N. R. Stradiotto, K. E. Toghill, L. Xiao, A. Moshar and R. G. Compton, Electroanalysis, 2009, 21, 2627 CrossRef CAS.
- K. E. Toghill, L. Xiao, N. R. Stradiotto and R. G. Compton, Electroanalysis, 2010, 22, 491 CrossRef CAS.
- N. A. Choudry, D. K. Kampouris, R. O. Kadara and C. E. Banks, Electrochem. Commun., 2010, 12, 6 CrossRef CAS.
- J. P. Hart and S. A. Wring, Electroanalysis, 1994, 6, 617 CAS.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Sens. Actuators, B, 2009, 138, 556 CrossRef.
- K. C. Honeychurch, J. P. Hart and D. C. Cowell, Electroanalysis, 2000, 12, 171 CrossRef CAS.
- S. Sanllorent-Mendez, O. Dominguez-Renedo and M. J. Arcos-Martinez, Electroanalysis, 2008, 21, 635.
- G. D. Liu, Y. Y. Lin, H. Wu and Y. Lin, Environ. Sci. Technol., 2007, 41, 8129 CrossRef CAS.
- M. E. B. Calvo, O. D. Renedo and A. J. A. Martinez, Talanta, 2007, 74, 59 CrossRef CAS.
- F. F. Tao, J. P. Lu, L. M. Lang and Z. Xu, Chinese J. Inorg. Chem., 2009, 25, 296 CAS.
- Z. J. Zhang, Y. Zhao and M. M. Zhu, Appl. Phys. Lett., 2006, 88, 033101 CrossRef.
- P. M. Hallam, D. K. Kampouris, R. O. Kadara and C. E. Banks, Analyst, 2010, 135, 1947 RSC.
- Kanichi Research Services Ltd, available online at: http://kanichi-research.com/ (accessed on 12 July 2010).
- M. E. Hyde and R. G. Compton, J. Electroanal. Chem., 2003, 549, 1 CrossRef CAS.
- A. Kowal, S. N. Port and R. J. Nichols, Catal. Today, 1997, 38, 483 CrossRef CAS.
- C. M. A. Brett and A. M. O. Brett, Electroanalysis, 1998, pg 81, Oxford Science Publications Search PubMed.
- H. Ju and D. Leech, J. Electroanal. Chem., 2000, 484, 150 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
