Remote identification of chemicals concealed behind clothing using near infrared spectroscopy
Céline M.
Canal
,
Aamer
Saleem
,
Roger J.
Green
and
David A.
Hutchins
*
School of Engineering, University of Warwick, Coventry, CV4 7AL, UK. E-mail: D.A.Hutchins@warwick.ac.uk
First published on 12th November 2010
Abstract
Measurements are presented which demonstrate that near infrared (NIR) spectroscopy can be used to identify chemicals concealed behind clothing. This has been achieved for detector stand-off distances of 3 m. The optical properties of a range of clothing materials have been investigated, and the transmission of NIR signals found to be sufficient for spectroscopy. The study has shown that granular solid materials can be identified when hidden behind a layer of clothing. Examples of chemicals used in this study include ammonium nitrate and other ammonium salts. Details of the current measurements and results are given, together with suggestions for increasing detection sensitivity.
1. Introduction
There is an increasing need for reliable and cost-effective personal screening techniques for hidden drugs, explosives and other hazardous materials. At present, there are competing methods that can be employed for this purpose, such as backscattered X-ray systems1 currently being trialed for use in airports. Further research has been reported on combining X-ray dual-energy radiography and tomography for an accurate measure of the effective atomic number Zeff and the density of samples,2 and a measure of viscosity can be added for further improvement in identification.3 However, the use of X-rays for human screening is far from ideal because of their ionizing nature. Additionally, it is difficult to discriminate between water and liquid explosives with sufficiently low false positive alarm rate using this technique. Consequently, the concerned security agencies are keen on developing alternative systems that are safer for common usage in public places.Another approach to detecting objects concealed behind clothing is the use of Terahertz (THz) signals, conventionally defined as operating over the frequency range 0.3 THz–10 THz (or 1 mm–0.03 mm wavelength range).4 A clear image with frequencies in the 0.05–1 THz range can be obtained by exploiting differences in the electromagnetic properties of skin, explosives and metals. However, there is only limited spectral information available in this frequency range that can be used to provide information on the composition of hidden packages, although some studies have been reported.5 Further, with recent breakthroughs in THz sources and detectors, THz time-domain spectroscopy could prove useful in solving this problem. Indeed, the initial technique to generate THz radiation with a visible/NIR femtosecond pulsed lasers has now been replaced by solid-state THz semiconductor laser, also called quantum cascade laser (QCL), and the imaging is even performed in diffuse reflection.6 Whereas this technology now requires less cumbersome sources, the QCL still requires cryogenic cooling, which is often needed for the detector as well. Thus, further developments in compact, tunable THz sources and commensurate detectors have to be achieved before this technology becomes standard for security screening applications.
In the visible and NIR range of the spectrum, Raman spectroscopy has been used for the trace detection of explosives. Coupled with confocal microscopy, particles of explosives trapped between clothing fibers have been identified.7 Raman spectroscopy, in particular in a spatially offset configuration where the light is collected from a different area than the one illuminated by the laser, has also been studied for bulk detection (e.g. powders and liquid explosives hidden within plastic packaging8,9). Additionally, research has been carried out using NIR signals in the 0.8–2.5 μm wavelength range to assess the concentration of hydrogen peroxide in antiseptic solutions10 and for security applications.11 Despite this, NIR spectroscopy has not traditionally been the method of choice for such measurements because of the perceived complexity of the spectra for use in quantitative analysis, as compared to mid-IR spectra (2.5–30 μm). However, coupled with multivariate chemometric calibration techniques such as Partial Least Squares Regression (PLSR) and Principal Component Regression (PCR) as well as non-linear spectral calibration techniques including neural networks,12 NIR spectroscopy is now widespread in many fields.13 In the food industry, fruit can be classified according to sugar content, acidity and more recently internal defects using spectral information.14 Meat quality is assessed in monitoring the fat, protein and water content, but also in screening for authenticity.15 Additionally, NIR hyper-spectral imaging is used to differentiate wheat classes.16 NIR spectroscopy is also in common use in refining and petrochemical industries to control the chemical and physical properties of fuels such as the octane number.17 There are many production lines in pharmaceutical industries where NIR assists the quality control of tablets.18 Finally, considerable research is being conducted in the medical field for non-invasive testing procedures such as the diagnosis of some cancers.19 Hence, the numerous applications of NIR spectroscopy indicate that spectra in the 0.9–2.5 μm wavelength range contain useful information for chemical identification.
In this paper, we report the use of NIR spectroscopy to identify chemicals in non-metallic packaging through barriers such as clothing, over the 0.9–2.5 μm wavelength range. This includes a study of NIR transmission characteristics of various clothing fabrics as well as the spectra of certain chemicals in granular form. It has been observed that both the microstructure of fabrics and the chemical structure of fibers have an effect on NIR transmission at these wavelengths, whereas the dyes used by textile manufacturers affect mainly transmission in the visible region. Hence, our present measurements have been restricted to the 0.9–2.5 μm range, in order to reduce absorption within clothing dyes, and this allowed a study of several spectral overtone peaks from the chemicals. The spectral features of fabrics will then be superimposed on those of the hidden chemical to be detected. However, it has been found that scattering in the clothing material due to its weave structure is the dominant feature affecting transmission intensity levels. This means that the transmitted light will not contain excessive spectral distortion due to absorption within clothing fibers.
The research was performed with the particular aim of making these measurements at a reasonable stand-off distance. Thus, our investigation was carried out with the spectrometer positioned at a distance of 3 m from the sample. We first report optical properties of various fabrics and the spectrum of requisite chemicals. Then, we demonstrate that absorption peaks of chemicals, such as ammonium nitrate, occur at the same recognizable wavelengths when they are placed behind a layer of clothing. The work also proves that the chemical being detected does not have to be placed in very close proximity of the concealing fabric to allow a sufficiently-strong signal in diffuse backscatter, and an air gap of up to 10 mm has a manageable effect on detection through most of the tested fabrics. To the best of our knowledge, this work represents the most advanced study on NIR spectroscopy through clothing for personal screening applications to date.
2. Background to NIR spectroscopy
NIR spectroscopy in the 0.9–2.5 μm wavelength range is based on molecular overtone and combination vibrations, whereas mid-infrared (MIR) absorption, in the range 2.5–25 μm, mostly comes from fundamental vibrations. Because of the low transition probabilities of molecular overtone and combination vibrations compared to fundamental vibrations, the absorption peaks decrease from the MIR to the visible region by a factor of 10–100 between each overtone. Consequently, NIR spectra are dominated by absorption from vibrating atoms that have their 1st and 2nd overtone band in the NIR range. The fundamental absorption peak of X–H (X = C, N, O) bonds usually occurs at wavelengths lower than 5 μm, leading to their 1st overtone appearing in the NIR range below 2.5 μm. In other words, molecules that have a large mechanical anharmonicity of the vibrating atoms, such as CH, OH, and NH functionalities, will demonstrate reasonable NIR absorption.20 NIR spectroscopy is therefore a good choice for the characterization of absorption due to water, hydrogen peroxide, ammonium nitrate (NH4NO3) and other substances with suitable bonds, and tables are available to assign peaks to bonding vibrations at particular wavelengths.21The quantitative evaluation of a particular chemical's concentration with NIR spectroscopy follows Beer–Lambert law:
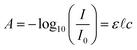 | (1) |
![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) the optical path (distance the light travels through the material), and c the concentration of the absorbing species. Accordingly, a quantitative value of absorbance requires the choice of an appropriate reference intensity level I0. In transmission spectroscopy, I0 can be the intensity of the incident light source. However, in diffuse reflectance spectroscopy, this is not appropriate and the best reference level is deemed to be the reflection from poly-tetrafluoroethylene (PTFE or Teflon™),22 as the optical properties of this material are relatively constant over the UV to NIR wavelength range. However, the use of PTFE as a standard does have its associated problems. For instance, it has been observed that physical characteristics of such a block, such as thickness and surface roughness, modify the angular properties of a diffuse reflected beam. Likewise, each chemical powder or fabric sample reflects and scatters light in different ways. The spectrum thus obtained by diffuse reflection often contains a DC offset, a linear baseline and/or a curving function as a result of scattering from the different samples and non-systematic effects. A technique known as de-trending, described below, is particularly useful for comparing the NIR spectra of granular chemicals, in situations where a constant reference sample is not always available.
the optical path (distance the light travels through the material), and c the concentration of the absorbing species. Accordingly, a quantitative value of absorbance requires the choice of an appropriate reference intensity level I0. In transmission spectroscopy, I0 can be the intensity of the incident light source. However, in diffuse reflectance spectroscopy, this is not appropriate and the best reference level is deemed to be the reflection from poly-tetrafluoroethylene (PTFE or Teflon™),22 as the optical properties of this material are relatively constant over the UV to NIR wavelength range. However, the use of PTFE as a standard does have its associated problems. For instance, it has been observed that physical characteristics of such a block, such as thickness and surface roughness, modify the angular properties of a diffuse reflected beam. Likewise, each chemical powder or fabric sample reflects and scatters light in different ways. The spectrum thus obtained by diffuse reflection often contains a DC offset, a linear baseline and/or a curving function as a result of scattering from the different samples and non-systematic effects. A technique known as de-trending, described below, is particularly useful for comparing the NIR spectra of granular chemicals, in situations where a constant reference sample is not always available.
3. Experiment
The experimental arrangement is shown schematically in Fig. 1. The two main components of the instrumentation system were a source of broadband optical illumination and a diffuse reflectance light collection system attached to a spectrometer. For the light source, two 50 W halogen bulbs were collimated with lenses L1, and then focused with lenses L2 onto the sample, so as to obtain a uniform spot of illumination. L1 and L2 were uncoated spherical plano-convex lenses. Their focal lengths were 200 mm and 100 mm, while their diameters were 50.8 mm and 60 mm respectively.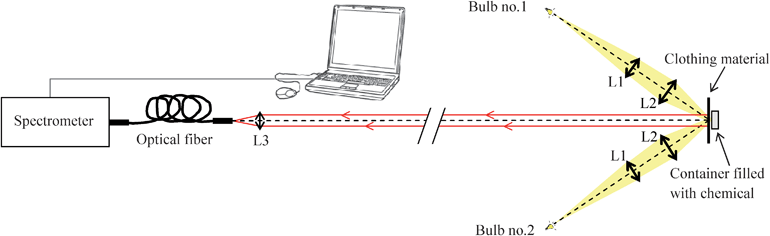 | ||
| Fig. 1 Experimental arrangement for collection of NIR spectra. | ||
The diffuse reflected light from the sample was collected via lens L3 (an uncoated spherical plano-convex lens with a 50 mm focal length and a 25.4 mm diameter) into a low OH content optical fiber, which was connected to a NIR256-2.1 spectrometer (Ocean Optics B.V). The optical fiber had a 600 μm core diameter, and its numerical aperture was 0.22. The spectrometer's sensor was an InGaAs linear array of 256 pixels, with sensitivity in the range 900 to 2100 nm. The distance between L3 and the object was around 3 m. The spectrometer, connected to a PC, was controlled via SpectraSuite™ software from Ocean Optics B.V. The integration time was set at 500 ms, and an average of four spectra was taken in each case to reduce noise.
The measurements were performed on objects generally composed of a chemical powder within a transparent container, hidden behind a layer of clothing material. In order to study different types of clothing material, different fabrics were selected, including cotton, polyester, cotton-polyester, acrylic, wool and denim in various colors. The resulting fourteen samples are shown in Fig. 2. The chemicals that were investigated included ammonium nitrate, a fertilizer used in the agricultural industry that can be found in improvised explosive devices (IEDs). Ammonium nitrate was obtained from two separate suppliers (Fisher Scientific UK and Sigma-Aldrich Ltd), in fine-grained as well as coarse-grained forms. While the fine powder contained granules with diameters in the 0.5–1 mm range, the coarse powder was composed of even granules with a 2 mm diameter to which an anti-caking agent had been added. Additional experiments were performed on other ammonium salts, such as ammonium sulfate and ammonium chloride, to determine the contribution of the ammonium ion. During the experiments, the chemicals were placed within a 50 × 50 × 10 mm3 glass container, chosen because glass has a negligible absorbance compared to the chemicals tested. For the collection of reference intensity I0, a 10 mm thick PTFE block was used. The intensity I was then recorded with the chemical instead of the PTFE reflector, and the absorbance A calculated with SpectraSuite™.
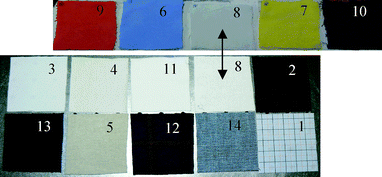 | ||
| Fig. 2 Photographs of the 14 different types of clothing, identified by sample number, which correspond to the data shown in Fig. 4. | ||
In order to enhance the chemical information in spectra, a preprocessing method was carried out using Matlab™. Thereby, spectra were first filtered with a Savitzky-Golay filter,23 which consisted of performing a local 2nd-degree polynomial regression on a series of 15 values at a time, to determine the smoothed value at each point of the spectrum. This was followed by a technique known as de-trending24 to remove linear baseline shift due to scattering effects from granular materials. This involved fitting a 1st-degree polynomial through all data points of the spectrum and then subtracting the resulting function's curve from the spectrum. Fig. 3 illustrates these successive transformations on spectra obtained from ammonium nitrate concealed behind a layer of cotton (sample no.1) or wool. It can also be noticed from Fig. 3 that the level of noise drastically increases above a wavelength of 2000 nm, due to the poor sensitivity of our spectrometer's sensor in this range. Finally, a normalization of the pre-processed spectra was often performed (as shown in Fig. 9) to allow comparison between different spectra.
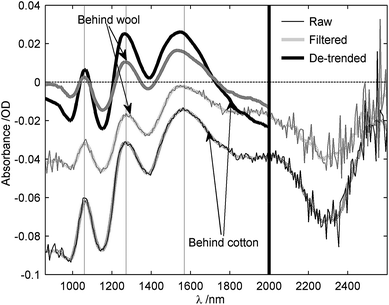 | ||
| Fig. 3 Illustration of the pre-processing steps for ammonium nitrate granules behind both cotton (sample 1) and wool. Shown are raw, filtered and de-trended spectra. | ||
4. Results
4.1 Properties of clothing fabrics in the NIR region
The optical transmission properties of various clothing materials were first measured experimentally over the 900–1300 nm range, to determine the usefulness of NIR wavelengths in this region of the spectrum. The results are shown for various clothing materials in Fig. 4. Most of the fabrics have a transmission higher than 30% within this wavelength range. It can also be seen that NIR transmission depends not only on the clothing fiber material, but also on the particular weave. This was illustrated by studying the weave pattern of three samples (cotton sample 1, cotton sample 3 and denim (a rugged cotton twill textile)) under a microscope, as shown in Fig. 5. For cotton sample 1, clear air pores between the cotton fibers were observed. On the other hand, cotton sample 3 and denim had a lower porosity due to a tighter weave and larger diameter fiber strands which serve to reduce pore density. Hence, based on the relative transmission levels observed through these samples, it was assumed that the main contribution to the NIR transmission coefficient was from photons transmitted through the pores. However, while Fig. 5d shows that the pores of polyester are small compared to the size of strands, meaning polyester has a lower porosity than that of cotton, the former was seen to have a higher transmission coefficient. This was attributed to the fact that polyester was one of the thinnest clothing materials examined, which aided in the transmission. It thus appears that material thickness as well as weaving pattern affects the scale of NIR transmission.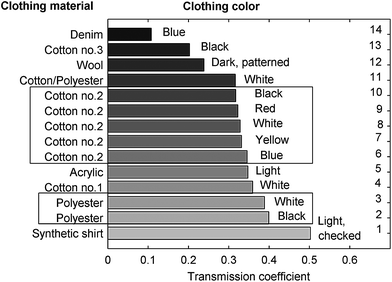 | ||
| Fig. 4 Transmission coefficient of various clothing materials. The measurement was made in through-transmission using an integrating sphere to determine the total integrated transmission intensity across the 900–1300 nm wavelength range. | ||
 | ||
| Fig. 5 Optical microscope photographs of selected fabrics, namely (a) white cotton (sample no.1), (b) black cotton (sample no. 3), (c) denim and (d) white polyester. The thickness t of each fabric is also given. | ||
Fig. 4 also shows that the dye color does not have a significant effect on optical transmission in this NIR region. To study this further, several samples of cotton (weave as in sample 2) were dyed and their spectra measured as in Fig. 6, over both the visible range (main figure) and in the NIR (inset box). These absorption spectra were measured in diffuse reflectance with the apparatus described in Fig. 1. The reference intensity I0 was taken as the intensity of light reflected by a 10 mm thick PTFE block in the absence of a sample. The clothing material was then placed in front of the PTFE reflector, and the intensity recorded. SpectraSuite™ was used to record all the intensity levels and display the corresponding absorbance. As can be seen from Fig. 6, dyes have a strong absorption in the visible wavelength range and their absorption is characteristic to the dye, and most likely based on its visible color. For instance, a red dye appears red to the human eye because it reflects rays from 600 to 750 nm. Therefore, its absorption will be in the blue-green range, as seen in Fig. 6. Another important observation is that absorbance amplitude in the NIR range is 10 times lower than in the visible range, and does not depend to any significant degree on the visible color of the sample (the four curves are plotted on top of each other in the figure). This is one reason why the NIR wavelength range is a good choice for spectroscopy of materials hidden behind clothing.
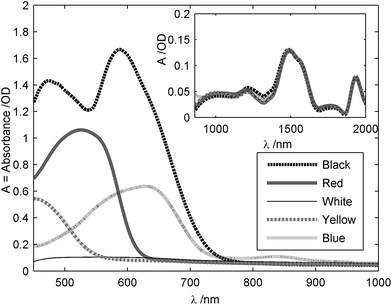 | ||
| Fig. 6 Absorption spectra of cotton sample 2 in both the visible range (main figure) and in the NIR (inset box). The measurements were made in diffuse reflection spectroscopy and the reference was a 10 mm thick PTFE block. Data were filtered with a Savitzky-Golay filter, then 1st-degree de-trended. | ||
The NIR absorption spectra of various clothing materials were then measured using the same procedure. As can be seen from the results shown in Fig. 7, the spectral features fall within two classifications: natural and synthetic fibers. For cotton, absorption peaks occur at 1210, 1488 and 1935 nm, which is commensurate with the results documented in the literature (1216, 1490 and 1930 nm25). Further, cotton and sucrose have a similar spectrum due to the cellulose, which is also a polysaccharide and the main constituent of cotton. For polyester, absorption peaks occur at 1135, 1389 and 1665 nm, with the relevant literature reporting similar peaks at 1128, 1368–1412 and 1660 nm.25 The small differences between these wavelengths are due to the fact that the sampling resolution of the spectrometer was 6.9 nm. The amplitude of the spectra is around 0.1 optical density (O.D). Absorption tends to increase at longer wavelengths, and thus a stronger influence of the cloth on the spectrum of chemicals would be expected at wavelengths greater than 1400 nm or so. One of the fabric samples, labeled in the figure as check shirt, has an unknown composition, but it can be deduced from its spectrum that the sample is composed of a synthetic fabric because it contains the polyester peak at 1665 nm.
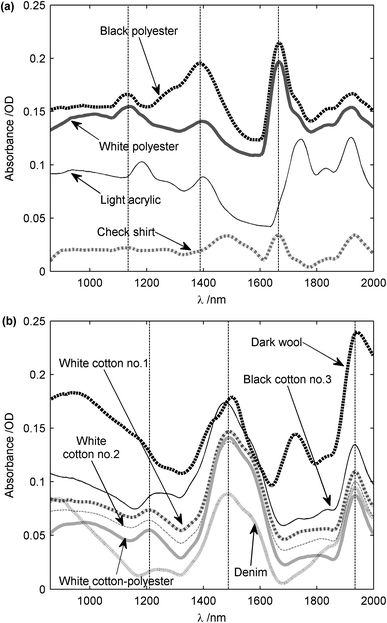 | ||
| Fig. 7 NIR spectra of fabrics. (a) Synthetic clothes including polyester and acrylic. (b) Natural clothes including cotton, cotton-polyester, wool and denim. The measurements were made in diffuse reflection spectroscopy and the reference was a 10 mm thick PTFE block. Data were filtered with a Savitzky-Golay filter, then 1st-degree de-trended. Artificial DC offsets have been added to move the spectra apart for visual clarity. | ||
The results from the above demonstrated that NIR spectra can be recorded at stand-off distances of 3 m and that the absorbance of different types of clothing is relatively low over the range 0.9–2 μm. Thus, the detection of certain chemicals behind such barriers might be possible due to the significant transmission of clothing in the NIR. The next set of measurements was thus designed to investigate this for common chemicals in granular form.
4.2 Ammonium salts in granular form placed behind clothing material
To demonstrate that NIR spectroscopy is a viable technique for detecting chemicals concealed behind clothing, granular ammonium nitrate and other ammonium salts were investigated. As explained previously, only molecules with high anharmonicity would be expected to be strong absorbers in the NIR range. Hence, ammonium salts would be expected to have similar spectral content due to N–H bonds in the NH4+ ion. Fig. 8a shows results for the chemicals only, with no clothing material present, using PTFE as the reference reflector to record I0. It can be seen that ammonium nitrate from both commercial suppliers had the same three absorption peaks at 1059, 1272 and 1568 nm, as indicated by dashed vertical lines. The fine-grained and coarse-grained forms of ammonium nitrate might have had an influence on the baseline shift (removed in Fig. 8a), but the physical form does not seem to affect the spectral content. The 1568 nm band corresponds to the 1st overtone stretch of N–H bonds, whereas the 1059 nm band corresponds to the 2nd overtone stretch. The 1272 nm band is attributed to a combination of N–H stretching and N–H bending, as this peak is also related to a 2012 nm peak, well-documented in the literature.26 It was decided to keep NIR spectra within the 860–2000 nm range, because the sensitivity of the spectrometer fell sharply at wavelengths above 2000 nm. In this way, the overall signal-to-noise ratio (SNR) was increased. The measurements indicated that ammonium sulfate had exactly the same absorption peaks as ammonium nitrate, while those of ammonium chloride were shifted by about 30 nm towards higher wavelengths. This shift could be due to differences in electro-negativity between the nitrate and chlorine ions.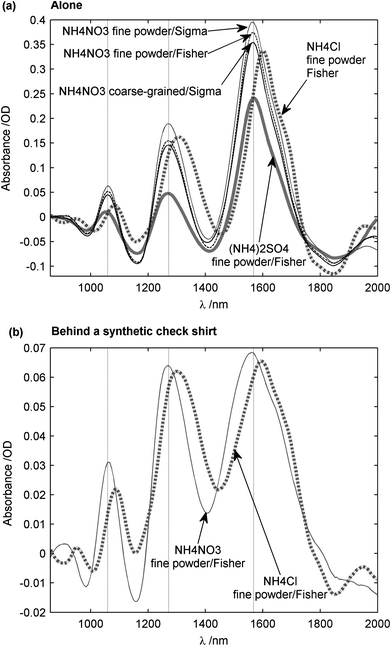 | ||
| Fig. 8 NIR spectrum of ammonium salts. (a) Spectra of ammonium nitrate, ammonium sulfate, and ammonium chloride, the reference being a 10 mm thick PTFE block. No clothing was present. (b) Spectra of ammonium nitrate and ammonium chloride positioned behind a synthetic check shirt, where now the reference was a synthetic check shirt sample. The measurements were made in diffuse reflection spectroscopy. Spectra were filtered with a Savitzky-Golay filter, then 1st-degree de-trended. | ||
The data in Fig. 8b is for ammonium nitrate and chloride when positioned behind a checked cloth sample. The reference intensity I0, in this case, was recorded from the clothing material, before insertion of the chemical granules. Chemical detection was therefore enhanced, as most of the spectral content from the cloth sample was removed. Note that the PTFE block was not used in this set of measurements in order to allow closer approximation to a real-life application. Fig. 8b demonstrates that, even with clothing present, the expected NIR absorption peaks of ammonium nitrate and ammonium chloride remained intact and the 30 nm shift observed for ammonium chloride as mentioned above was still present. It can also be seen that the amplitude of the spectra in Fig. 8b was about 0.08 O.D (i.e. comparable to the spectra of clothing, or approximately 6 times lower than the spectra of the chemical alone).
A further study on ammonium nitrate concealed behind various clothing materials is summarized in Fig. 9. The amplitude of the recorded spectrum in each case depended on the NIR transmission of each clothing material. Hence, all spectra were normalized to an amplitude range of 0 to 1, in order to ease comparisons. Although the absorption peaks of ammonium nitrate (marked by dashed vertical lines) are seen to be well-preserved behind synthetic clothing materials, observed spectra became less distinct in the case of natural clothing materials. However, even for the worst case of denim, which has a low NIR transmission due to a tightly-woven textile structure, the three expected peaks were still present, with very little shift in the wavelength in each case. This increased attenuation of the signal transmitted through the clothing sample is thought to be due to the increased scattering by the fibers resulting from a lack of air pores.
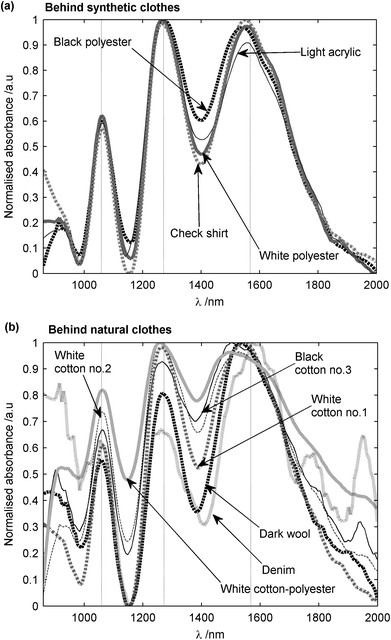 | ||
| Fig. 9 NIR spectrum of coarse-grained ammonium nitrate granules (provided by Sigma-Aldrich Ltd) (a) behind synthetic clothes and (b) behind natural clothes. The measurements were made in diffuse reflection spectroscopy and reference was the clothing material. Spectra were filtered with a Savitzky-Golay filter, then 1st-degree de-trended and normalized. | ||
The effect of any air gap between the clothing material and the chemical container was also investigated by gradually increasing this gap from 0 to 20 mm. The results are reported in Fig. 10. Although the amplitude was seen to decrease exponentially with increasing gap behind most fabrics, it was still possible to measure spectra with amplitudes greater than 0.01 O.D, with the largest air gap between the cloth and the chemical container. However, the curve for denim appeared almost flat as the amplitude of the signal reached the level of noise after a few millimeters (marked by a horizontal dashed line). This meant that the modulation of denim's spectra by ammonium nitrate was in the same range as that due to the natural variation in denim's spectrum (e.g. due to surface flatness and temperature). However, as previously mentioned, denim presents the worst case scenario. Improvements in the quality of illumination of samples would increase the SNR, especially for fabrics with thick and tight weave structures.
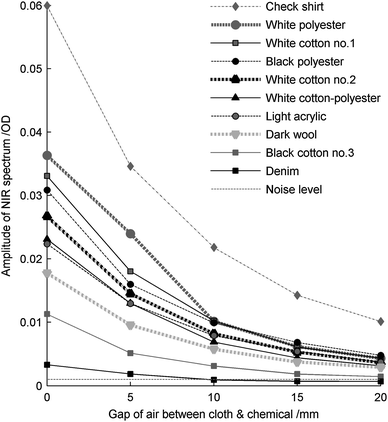 | ||
| Fig. 10 Amplitude of spectra of coarse-grained ammonium nitrate (provided by Sigma-Aldrich Ltd) behind various cloths for various air gaps between the sample and the clothing material. The peak-to-peak amplitude is plotted between the 1169 nm minimum and the 1279 nm maximum. It was obtained from Savitzky-Golay filtered and 1st-degree de-trended spectra. The spectra were measured in diffuse reflection spectroscopy with the clothing material as a reference. | ||
5. Discussion and conclusions
It has been shown that NIR spectroscopy is a promising method for identification of chemicals in non-metallic containers concealed behind layers of clothing, especially if used in conjunction with appropriate chemometric analysis. In particular, we have demonstrated that this technique could be used as a remote detection method, at stand-off detection distances of 3 m. Granular solids have a significant coefficient of diffuse reflection, which allowed the detection of recognizable NIR spectra of chemicals, such as ammonium nitrate hidden behind a set of clothing materials. Further experiments with other solid chemicals, like nitrate salts and saccharose (sucrose), have also indicated that ammonium salts have specific well-marked peaks of absorption. The present article is intended to focus on the detection of solid chemicals. However, that technique is currently being investigated for use with liquid chemicals, such as ethanol and hydrogen peroxide, and our recent results will be reported in due course. In a real-life application, such as personal screening at airports, the measurements would be more difficult, and enhancements to the technique would have to be considered. First of all, the sensitivity of the system can be improved by reducing the spectral range and choosing a more suitable detector. Detectors with greater sensivity over the range 0.9–1.5 μm and more pixels are commercially available, and sometimes even less expensive. This limitation of the spectral range would enable the study of at least two absorption peaks and would lead to a higher resolution (1.2 nm instead of 6.9 nm). This could also lead to a reduction in the total output power of the light source, an important consideration for eye safety reasons. The output power of our current light source with conventional halogen bulbs was estimated to be 5 mW nm−1, which represents 3 W to cover a range of 600 nm. In order to perform the measurements with even lower intensities, and in various environments where the background noise due to ambiant lighting may not be controllable, the light source could be modulated in order to use a lock-in amplifier after the detector array. This could significantly improve the SNR of the measurement and additionally provide suppression of background-related signals. Furthermore, the method used to identify spectra may need to be reconsidered in a practical case, as pure spectra of textile materials will not be known for reference purposes. In order to overcome this issue, the subject could be scanned and various reference spectra could be recorded at different locations (that technique could also be used for hyperspectral imaging), or new algorithms could be implemented for signal processing based on a library of spectra of textile materials. Additionally, multivariate calibration techniques (such as PCR, PLSR, neural networks) could be investigated to determine the presence and the concentration of a certain chemical, even in the form of mixtures and in the presence of interferents, such as plastic packaging in which the chemicals could be held in. The spectrum of such containers have been measured and a strong peak of absorption occurred around 1.7 μm with smaller additional peaks at 1.15 and 1.4 μm. While a transparent plastic container will not introduce too many distortions to chemical spectra, due to the small thickness of the plastic material, it is obvious that spectral features of highly diffusely scattering containers will dominate the chemical spectrum after reflection (in the same way as the clothing material). Methods already developed for various industrial applications are available and could be implemented to deal with this problem. These and other enhancements are actively being investigated by the authors.Acknowledgements
This work was funded by the Innovative Research Call in Explosives and Weapons Detection, a programme sponsored by a number of UK government departments and agencies. Thanks are due to Simon Leigh and Robert Southgate, from the University of Warwick, for helpful technical discussions, and assistance with experimental data.References
- S. Singh and M. Singh, Signal Process., 2003, 83, 31–55 CrossRef.
- M. Iovea, M. Neagu, O. G. Duliu and G. Mateiasi, Proceedings of DIR 2007-International Symposium on Digital industrial Radiology and Computer Tomography, Lyon, 2007 Search PubMed.
- EU Pat., EP2064535, 2009.
- A. G. Davies, A. D. Burnett, W. Fan, E. H. Linfield and J. E. Cunningham, Mater. Today, 2008, 11, 18–26 CrossRef CAS.
- M. C. Kemp, P. F. Taday, B. E. Cole, J. A. Cluff, A. J. Fitzgerald and W. R. Tribe, Proc. SPIE, 2003, 5070, 44–52, DOI:10.1117/12.500491.
- P. Dean, M. U. Shaukat, S. P. Khanna, S. Chakraborty, M. Lachab, A. Burnett, G. Davies and E. H. Linfield, Opt. Express, 2008, 16, 5997–6007 CrossRef CAS.
- E. M. A. Ali, H. G. M. Edwards and I. J. Scowen, J. Raman Spectrosc., 2009, 40, 2009–2014 CrossRef CAS.
- C. Eliasson, N. A. Macleod and P. Matousek, Vib. Spectrosc., 2008, 48, 8–11 CrossRef CAS.
- C. Eliasson, N. A. Macleod and P. Matousek, Anal. Chem., 2007, 79, 8185–8189 CrossRef CAS.
- Y.-A. Woo, H.-R. Lim, H.-J. Kim and H. Chung, J. Pharm. Biomed. Anal., 2003, 33, 1049–1057 CrossRef CAS.
- H. Itozaki and Y. Yamauchi, Proc. SPIE, 2009, 7298, 72983X, DOI:10.1117/12.819247.
- V. Centner, J. Verdu-Andres, B. Walczak, D. Jouan-Rimbaud, F. Despagne, L. Pasti, D.-L. Massart and O. E. D. Noord, Appl. Spectrosc., 2000, 54, 608–623 CrossRef CAS.
- P. Flinn, NIR news, 2005, 16, 4–8.
- H. Lin and Y. Ying, Sens. Instrum. Food Qual. Saf., 2009, 3, 130–141 Search PubMed.
- N. Prieto, R. Roehe, P. Lavín, G. Batten and S. Andrés, Meat Sci., 2009, 83, 175–186 CrossRef CAS.
- S. Mahesh, A. Manickavasagan, D. S. Jayas, J. Paliwal and N. D. G. White, Biosyst. Eng., 2008, 101, 50–57 CrossRef.
- S. J. Foulk and B. E. DeSimas, Fresenius J. Anal. Chem., 1992, 342, 292–294 CAS.
- M. Blanco, M. Alcalá, J. M. González and E. Torras, J. Pharm. Sci., 2006, 95, 2137–2144 CrossRef CAS.
- V. Kondepati, T. Oszinda, H. Heise, K. Luig, R. Mueller, O. Schroeder, M. Keese and J. Backhaus, Anal. Bioanal. Chem., 2007, 387, 1633–1641 CrossRef CAS.
- L. Bokobza, in Near-infrared spectroscopy: principles, instruments, applications, ed. H. W. Siesler, Y. Ozaki, S. Kawata and H. M. Heise, Wiley-VCH, Weinheim, 1st edn, 2002, ch. 2, pp. 11–41 Search PubMed.
- I. Murray, in The 11th International Conference on Near Infrared Spectroscopy (NIR2003), Cordoba, 2003 Search PubMed.
- A. Springsteen, Anal. Chim. Acta, 1999, 380, 379–390 CrossRef CAS.
- A. Savitzky and M. J. E. Golay, Anal. Chem., 1964, 36, 1627–1639 CrossRef CAS.
- R. J. Barnes, M. S. Dhanoa and S. J. Lister, Appl. Spectrosc., 1989, 43, 772–777 CAS.
- S. Ghosh and J. Rodgers, in Handbook of near-infrared analysis, ed. D. A. Burns and E. W. Ciurczak, CRC Press, Taylor & Francis Group, Boca Raton, 3rd edn, 2008, ch. 25, pp. 485–519 Search PubMed.
- P. Patnaik, in Dean's analytical chemistry handbook, McGraw-Hill, 2nd edn, 2004, ch. 7, p. 7.1 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
