Facile immobilization of enzymes on electrospun poly(styrene-alt-maleic anhydride) nanofibres†
William J.
Cloete
a,
Craig
Adriaanse
b,
Pieter
Swart
b and
Bert
Klumperman
*a
aDepartment of Chemistry and Polymer Science, Stellenbosch University, Stellenbosch, South Africa. E-mail: bklump@sun.ac.za; Fax: +27 21 808 4967; Tel: +27 21 808 3988
bDepartment of Biochemistry, Stellenbosch University, Stellenbosch, South Africa
First published on 26th March 2011
Abstract
Facile immobilization of horseradish peroxidase (HRP) and glucose oxidase (GOX) was achieved on electrospun nanofibres made of an alternating copolymer of styrene (Sty) and maleic anhydride (MAh). The immobilized enzymes were used to perform a cascade reaction oxidizing D-glucose by GOX to yield H2O2 and employing HRP to catalyze the reaction between the generated H2O2 and Ampliflu Red to yield Resorufin. The average activity of the immobilized HRP was determined to be 21% of that of the free enzyme.
Enzymes can be immobilized on suitable polymeric materials with retention of their catalytic activity.1 Immobilization is typically achieved through a site-specific reaction between reactive moieties of the chosen material and an amino acid residue on the enzyme.2 These catalytically active, immobilized enzymes are able to catalyze degradation or neutralization reactions of certain toxins in wastewater.3 Treatment processes of the wastewater at common municipal sewage treatment plants are not efficient with regards to the removal of trace amounts of biologically active pollutants from the water.4–6 Immobilized enzyme systems are extremely sensitive for detecting these toxins as well as providing a means of bioremediation by degrading or neutralizing the pollutant species.3
Most endocrine disrupting toxins found in the wastewater are from drug residues excreted by humans, one example being estrogen, commonly found in female oral contraceptives.5 These biologically active species remain to a certain extent in the treated sewage water and are subsequently introduced into the environment with adverse effects on animal life and, via the process of bioaccumulation, ultimately on human health.4,6
Apart from providing a means of detection and remediation of toxins in the wastewater, immobilized enzymes are also essential for bioreactors in which directional synthesis can take place via cascade reactions.3 Currently, the production of polymeric surfaces for immobilization of enzymes typically entails laborious synthetic techniques for surface modification in order to ensure the availability of reactive moieties for immobilization. In this communication, a facile method for the immobilization of enzymes on nanofibrous membranes is described.
We used conventional radical copolymerization for the synthesis of the copolymer without the necessity for surface modification of the resulting polymeric membrane. Electrospinning produces sub-micron polymer fibres that form membranes with extremely high surface areas on which enzymes can be immobilized.4 The high surface areas increase the amount of enzymes that can be immobilized and cause a subsequent increase in the catalytic activity of the enzymes.1,3
We successfully immobilized two enzymes (horseradish peroxidase and glucose oxidase)7 on a membrane consisting of unmodified polymeric fibres. This was accomplished by making use of the reactive maleic anhydride units of poly(Sty-alt-MAh)copolymer.8–12 Both enzymes contain a number of accessible amine functional groups from their lysine residues. These primary amines can react in an amidation reaction with the maleic anhydride units on the polymer backbone to become covalently bonded to the polymer fibre.
Poly(Sty-alt-MAh) was chosen for this study because of several properties which make it ideal for electrospinning into sub-micron fibres from the solution.13 Specifically, it is soluble in common organic solvents and reasonably high molar masses can easily be obtained viafree radical polymerization. Conventional free radical polymerization was thus employed to make an alternating copolymer from styrene and maleic anhydride. The reaction was carried out in solution in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio under inert conditions using AIBN as an initiator at 60 °C. The polymer was characterized in a THF solution by means of size exclusion chromatography (SEC) using linear polystyrene calibration standards and a refractive index detector (Mw = 253 × 103Da,
1 molar ratio under inert conditions using AIBN as an initiator at 60 °C. The polymer was characterized in a THF solution by means of size exclusion chromatography (SEC) using linear polystyrene calibration standards and a refractive index detector (Mw = 253 × 103Da, 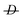 = 4.6).
= 4.6).
Electrospinning of the polymer was done from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 2 DMF/acetone solution (15 wt%) with the collector at a distance of 15 cm and offset of 4 cm from the needle tip that was connected to a high voltage supply (25 kV, 400 microamps, 10 Watts, high output voltage supply) set at 15 kV and the polymer solution was fed at 0.01 mL min−1 by means of a feed pump (Harvard, Model 33 Twin Syringe Pump). SEM analysis was done on a series of fibres, spun from the same solution, but with varying flow rates and field strengths. The conditions described above were found to be optimal for producing visibly uniform fibres of the desired diameter. A mesh of these fibres with a diameter of ca. 500 nm (Fig. 1) was employed for the immobilization of HRP and GOX. Earlier work in our labs showed that the fiber diameter as studied by SEM has a tendency to increase upon protein immobilization. Measurements after immobilization were not performed in the present study. The fibres were immersed in 10 mL of a 4 mg mL−1 solution of HRP in PBS buffer (pH 7.4).1 Incubation of the enzyme solution with the fibres took place at ambient conditions for 1 hour in a Petri dish with the solution rotated/shaken by means of a Belly Dancer laboratory shaker. The fibres were removed and washed three times for 10 minutes at a time, with a PBS–Tween (0.01% Tween-20) solution. This buffer solution, containing the mild detergent, Tween-20, was used to get rid of any non-specific binding or adsorption of the enzyme to the polymer fibres. A substrate solution consisting of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)-ABTS and H2O2 in PBS was introduced to the fibre-immobilized enzymes and a colour change was observed relative to a control (Fig. 2).
2 DMF/acetone solution (15 wt%) with the collector at a distance of 15 cm and offset of 4 cm from the needle tip that was connected to a high voltage supply (25 kV, 400 microamps, 10 Watts, high output voltage supply) set at 15 kV and the polymer solution was fed at 0.01 mL min−1 by means of a feed pump (Harvard, Model 33 Twin Syringe Pump). SEM analysis was done on a series of fibres, spun from the same solution, but with varying flow rates and field strengths. The conditions described above were found to be optimal for producing visibly uniform fibres of the desired diameter. A mesh of these fibres with a diameter of ca. 500 nm (Fig. 1) was employed for the immobilization of HRP and GOX. Earlier work in our labs showed that the fiber diameter as studied by SEM has a tendency to increase upon protein immobilization. Measurements after immobilization were not performed in the present study. The fibres were immersed in 10 mL of a 4 mg mL−1 solution of HRP in PBS buffer (pH 7.4).1 Incubation of the enzyme solution with the fibres took place at ambient conditions for 1 hour in a Petri dish with the solution rotated/shaken by means of a Belly Dancer laboratory shaker. The fibres were removed and washed three times for 10 minutes at a time, with a PBS–Tween (0.01% Tween-20) solution. This buffer solution, containing the mild detergent, Tween-20, was used to get rid of any non-specific binding or adsorption of the enzyme to the polymer fibres. A substrate solution consisting of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)-ABTS and H2O2 in PBS was introduced to the fibre-immobilized enzymes and a colour change was observed relative to a control (Fig. 2).
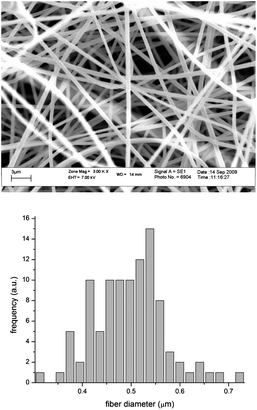 | ||
| Fig. 1 SEM image of poly(Sty-alt-MAh)copolymer fibres and the fiber diameter as determined via analSIS doco by Olympus Soft Imaging Solutions GmbH. | ||
 | ||
| Fig. 2 The colour change of substrate solution ABTS/PBS/H2O2, left is the control with no enzyme immobilized and right is the poly(Sty-alt-MAh) with covalently bonded HRP. | ||
The controls used in the study consisted of poly(Sty-alt-MAh) fibres incubated for 1 hour in the same PBS buffer solution without the enzyme as well as crosslinked fibres with no remaining anhydride moieties, incubated with and without the enzyme. The crosslinked membranes were obtained by electrospinning under the same conditions as before onto a 0.3 M ethylenediamine solution in isopropanol. The crosslinked membrane obtained was no longer soluble in acetone or DMF indicating that the electrospun fibres were fully crosslinked. An excess of amine was used in the crosslinking reaction to ensure that the fibres no longer contained any accessible reactive maleic anhydride moieties.
The substrate solution introduced to the fibres on which the enzyme was immobilized turned from light green to dark blue. This indicated the presence of covalently bonded HRP on the poly(Sty-alt-MAh) fibres since HRP catalyzes the reaction between ABTS and H2O2, in a PBS buffer solution, turning ABTS (light green) into ABTS˙+ (dark blue). The expected colour change was not observed for the fibres without incubation with the HRP or for the crosslinked fibres after incubation with and without the HRP.
In order to see if a simple cascade reaction could be done with immobilized enzymes, two further experiments were conducted. In the first experiment, HRP (4 mg mL−1) and GOX (10 mg mL−1) were immobilized together on a membrane while dissolved in PBS buffer solution. Incubation and wash steps were repeated as previously described. A substrate solution made up of Ampliflu Red/D-glucose in PBS buffer was introduced and the subsequent colour change observed (Fig. 3).1 The proposed mechanism for this colour change was that GOX oxidized D-glucose leading to the evolution of H2O2. Then, the immobilized HRP catalyzed the reaction between evolved H2O2 and Ampliflu Red to yield Resorufin. To test whether this reaction cascade had indeed occurred, in the second experiment, HRP and GOX were immobilized on two separate membranes. We first introduced Ampliflu Red substrate solution onto the membrane with the GOX to produce H2O2. The resulting solution was then transferred to the membrane on which the HRP was immobilized. The same colour change was observed as in the previous experiment, indicating that the HRP successfully catalyzed the reaction between the evolved H2O2 and Ampliflu Red to yield Resorufin.
 | ||
| Fig. 3 The colour change of substrate solution going from Ampliflu Red to Resorufin. On the left is the control with no immobilized enzyme and on the right the immobilized membrane with glucose oxidase and horseradish peroxidase. | ||
To confirm the immobilization of HRP with the retention of enzymatic activity, the immobilization was repeated. Instead of adding the substrate after immobilization of HRP onto the fibres, the fibres were incubated in a buffered solution of primary and secondary antibodies followed by testing with an appropriate substrate solution. The HRP was first incubated as previously discussed with subsequent wash steps. Then the fibres were incubated in casein buffer (10 mM Tris, pH 7.6, 0.15 M NaCl, 0.5% casein, 0.02% thiomersal) for 20 minutes in order to block all nonspecific sites where the antibodies may bind. Anti-HRPantibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 in casein buffer) were incubated along with the fibres at 37 °C for 2 hours. After washing with 0.1% Tween-20 solution as before, a secondary antibody (Sigma, anti-rabbit IgC, catalogue number: A3687), conjugated to alkaline phosphatase (1
000 in casein buffer) were incubated along with the fibres at 37 °C for 2 hours. After washing with 0.1% Tween-20 solution as before, a secondary antibody (Sigma, anti-rabbit IgC, catalogue number: A3687), conjugated to alkaline phosphatase (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000), was added to the fibres and incubated at 37 °C for 2 hours. This was followed by four wash cycles, whereafter 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt/nitro blue tetrazolium (BCIP-T/NBT) substrate was added at room temperature. A positive result was obtained with the substrate solution changing from light yellow to dark purple (Fig. 4), indicating that the enzyme was successfully immobilized with retention of its catalytic activity. The controls used in this experiment consisted of the same controls used for the previous experiments and the introduction of the BCIP-T/NBT substrate solution yielded a negative result for the crosslinked membranes as well as the fibres on which no enzymes were immobilized.
000), was added to the fibres and incubated at 37 °C for 2 hours. This was followed by four wash cycles, whereafter 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt/nitro blue tetrazolium (BCIP-T/NBT) substrate was added at room temperature. A positive result was obtained with the substrate solution changing from light yellow to dark purple (Fig. 4), indicating that the enzyme was successfully immobilized with retention of its catalytic activity. The controls used in this experiment consisted of the same controls used for the previous experiments and the introduction of the BCIP-T/NBT substrate solution yielded a negative result for the crosslinked membranes as well as the fibres on which no enzymes were immobilized.
 | ||
| Fig. 4 The colour change of substrate solution BCIP-T/NBT, left is the control with no enzyme and antibody immobilized and right is the fibres after incubation with HRP, primary and secondary antibodies. | ||
In order to assess the activity of the immobilized enzymes, a dedicated experiment was conducted. In this experiment, the HRP was immobilized from 10 mL of a diluted solution in PBS (1.0 μg mL−1) onto a 1 cm2membrane of poly(Sty-alt-MAh)nanofibres and washed four times with 0.01% Tween-20 solution in PBS. The concentration of active HRP was determined via an assay with ABTS and H2O2 solution in a citratebuffer at pH 5.0. The substrate solution was composed of 6 mg ABTS and 6 μL H2O2 (30%) in 12 mL of citratebuffer. The concentration in the various samples was measured by UV absorbance at 405 nm and calibrated against a calibration curve of known enzyme concentrations. From the difference in the HRP concentration in the solution before and after the immobilization, it was calculated that 1.96 μg HRP was bound to the fiber surface. The activity of the immobilized enzymes was equivalent to 0.42 μg HRP, i.e. 21% activity relative to the free enzyme. This can mean that either HRP loses some of its activity upon immobilization or that the active site of a fraction of the enzyme is inaccessible to the substrate. The retention of enzymatic activity compares favorably with earlier reported values on enzymes immobilized on poly(ethylene-g-(STY-co-MAh)).11
Conclusions
This study qualitatively showed that using the reactive maleic anhydride moieties on electrospun poly(Sty-alt-MAh) fibres provides a facile method to immobilize enzymes such as HRP and GOX. Two methods confirmed the covalent attachment of HRP to the electrospun fibres. First, wash steps with Tween-20 solution were unable to remove the enzyme from the membrane. Second, poly(Sty-alt-MAh) fibres that were treated with ethylenediamine and consequently had no reactive MAh moieties were unable to immobilize HRP. The binding efficacy of the HRP and the retention of its catalytic activity were successfully verified by means of incubation with primary and secondary anti-HRPantibodies. The reported immobilization method is highly recommended as a viable alternative for enzyme immobilization on high surface area polymeric materials. No modification of the surface or functionalization thereof is necessary prior to immobilization of the enzymes. This facile method of immobilization with retention of catalytic activity makes it ideal as a detection method for toxins and possible bio-remediation. It also provides an opportunity for kinetic studies of directional biochemical reactions by spectroscopic means, since cascade reactions can easily be conducted after enzyme immobilization. Further studies on recyclability and possible enzyme leaching of the membranes are underway in our labs.Notes and references
- T. C. Logan, D. S. Clark, T. B. Stachowiak, F. Svec and J. M. J. Frechet, Anal. Chem., 2007, 79, 6592–6598 CrossRef CAS.
- M. A. Gauthier and H.-A. Klok, Chem. Commun., 2008, 2591–2611 RSC.
- M. Ignatova, O. Stoilova, N. Manolova, D. G. Mita, N. Diano, C. Nicolucci and I. Rashkov, Eur. Polym. J., 2009, 45, 2494–2504 CrossRef CAS.
- B. Kasprzyk-Hordern, R. M. Dinsdale and A. J. Guwy, Water Res., 2009, 43, 363–380 CrossRef CAS.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell, C. Ort, H. Singer, U. von Gunten and H. Siegrist, Environ. Sci. Technol., 2009, 43, 7862–7869 CrossRef CAS.
- M. Auriol, Y. Filali-Meknassi, R. D. Tyagi, C. D. Adams and R. Y. Surampalli, Process Biochem., 2006, 41, 525–539 CrossRef CAS.
- T. Wilhelm and G. Wittstock, Angew. Chem., Int. Ed., 2003, 42, 2248–2250 CrossRef CAS.
- S. Mannino, O. Brenna, S. Buratti and M. S. Cosio, Electroanalysis, 1997, 9, 1337–1340 CAS.
- L. Goldstein, in Methods in Enzymology, ed. L. L. Gertrude and E. Perlmann, Academic Press, 1970, vol. 19, pp. 935–962 Search PubMed.
- C. G. Beddows, M. H. Gil and J. T. Guthrie, Polym. Bull. (Berlin), 1985, 14, 199–206 Search PubMed.
- C. G. Beddows, H. G. Gil and J. T. Guthrie, Biotechnol. Bioeng., 1985, 27, 579–584 CrossRef CAS.
- M.-Y. Lee, A. Srinivasan, B. Ku and J. S. Dordick, Biotechnol. Bioeng., 2003, 83, 20–28 CrossRef CAS.
- C. Tang, S. Ye and H. Liu, Polymer, 2007, 48, 4482–4491 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1py00069a |
| This journal is © The Royal Society of Chemistry 2011 |
