Cyclodextrin–dextran based in situ hydrogel formation: a carrier for hydrophobic drugs†
Ke
Peng
a,
Itsuro
Tomatsu
*a,
Alexander V.
Korobko
b and
Alexander
Kros
*a
aLeiden Institute of Chemistry, Leiden University, P.O. Box 9502, 2300 RA Leiden, The Netherlands. E-mail: a.kros@chem.leidenuniv.nl; Fax: + 31 71 527 4397; Tel: + 31 71 527 4234
bNano Structured Materials, TU Delft, Julianalaan 136, 2628 BL Delft, The Netherlands
First published on 23rd October 2009
Abstract
A rapid in situ hydrogel forming system composed of thiol functionalized β-cyclodextrin and maleimide functionalized dextran has been prepared and the in vitro release profile of the hydrophobic drug all-trans retinoic acid was studied.
For many years, great efforts have been made to develop hydrogels for biomedical applications because of their excellent biocompatibilities and the similarity between their highly hydrated three-dimensional polymeric networks and hydrated body tissues.1 Among these applications, hydrogel based drug delivery systems are of interest, since their physiochemical characteristics renders them suitable for targeted drug delivery, i.e. for extension of circulation time, and reduced toxicity and side effects.2 In particular, hydrogels that can be formed in situ under physiological conditions have recently received much attention as promising drug carriers.3 These gels can, in principle, be applied in vivo by direct injection with minimal surgical wounds. However, the typical hydrophilic nature of these gels prevents their use as efficient drug carriers for hydrophobic drugs because of rapid initial release of these drugs. This is due to the lack of adequate interactions between the hydrophobic drug and the hydrophilic polymer backbone of the hydrogel.4 Furthermore hydrophobic interactions among lipophilic drugs can cause formation of large drug aggregates during drug loading process, which leads to side effects or even toxicity.5 Herein, we describe a rapid in situ forming hydrogel system composed of cyclodextrin and dextran, which is able to bind and release hydrophobic drugs in a sustained manner.
β-Cyclodextrin (β-CD) is a cyclic oligosaccharide composed of seven glucopyranose units with a truncated cone shape with a hydrophobic cavity and hydrophilic surface. This particular structure allows β-CD to accommodate a wide variety of hydrophobic molecules including lipophilic drugs into its cavity to form an inclusion complex. The complex formation results in improved solubility, better stability and enhanced bioavailability of the hydrophobic drug, which makes β-CD an attractive tool in drug delivery systems.6 Moreover the chemical structure of β-CD allows it to be used as a multifunctional cross linking reagent after chemical modifications.7
Dextran is a highly hydrophilic and biocompatible natural polysaccharide consisting mainly of linear chains of α-1,6 linked glucopyranose residues, which has been widely used for therapeutic purpose.8 A number of dextran based hydrogels have been reported9 and used in biotechnological applications including drug delivery of hydrophilic drugs.10
Combining these potential materials, we designed an in situ hydrogel forming system employing maleimide functionalized dextran (Dex-mal) and thiol functionalized β-cyclodextrin (β-CDS), where Dex-mal acts as the backbone and β-CDS plays two essential roles as a cross-linker and as a host for the hydrophobic drug simultaneously (Fig. 1). Hydrogels were formed by mixing Dex-mal and β-CDS solutions via multiple Michael additions. The obtained hydrogels were characterized by rheometry and scanning electron microscopy.
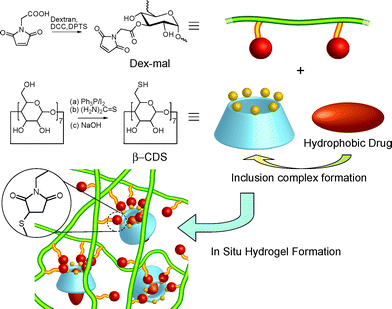 | ||
| Fig. 1 Schematic representation of the in situ hydrogel forming system in which β-CDS plays two roles: a cross-linker of dextran back bones and a host for a hydrophobic drug. Inclusion complex formation can increase the affinity of the hydrogel for hydrophobic drugs and can avoid the aggregation of drugs. | ||
To demonstrate the constant release of a hydrophobic drug from the gel over a longer period of time, all-trans retinoic acid (RA) was chosen as a model drug. RA has a strong in vivo bioactivity and plays an important role in development of animals. Major effects of RA on tissue re-growth and bone formation and patterning can easily be quantified and phenotype-based assays have been used for studying the effect of RA in vivo such as in zebrafish and chicken embryos.11 So far, RA-soaked ion exchange resin were implanted using time consuming microsurgery to study the effect of long-term local doses of RA.12 However these microscopic delivery systems lack control over the total amount, the release rate and time of RA, which makes these methods unsuitable for a more extensive analytical study on the biological functions of RA.
Maleimide functionalized dextrans were obtained by a conventional N,N′-dicyclohexylcarbodiimide mediated esterification of the hydroxyl groups of dextran with N-maleoylamino acid. N-maleoylamino acid was prepared by a synthesis procedure as previously reported.13 The obtained Dex-mal was characterized by 1H NMR; signals at δ 6.9 and 4.4 from maleimide were clearly observed in addition to the signals attributed to dextran. The conjugation of N-maleoylamino acid to dextran was further confirmed with the peaks of the glucose unit at δ 5.3 and 5.1 those showed shifts after the esterification. The degree of substitution (DS; the number of substitutes per 100 anhydroglucose units) was determined based on the integrals of signals corresponding to maleimide at δ 6.9 and the dextran glucosidic protons at δ 3.2–4.0, 5.1, and 5.3. A series of Dex-mals with different DS of 10, 17 and 26 were obtained by varying the feeding ratio and the reaction time.
All the primary hydroxyl groups of β-CD at the 6 positions were changed to thiol groups by the reported procedure.14 In order to improve the water solubility, the obtained thiolated β-CD was deprotonated by sodium hydroxide, forming the sodium salt of per-6-thio-β-cyclodextrin (β-CDS).
As shown in Fig. 2 (a), hydrogels were formed via Michael addition between maleimide and thiol groups by mixing solutions of β-CDS and Dex-mal in phosphate buffered saline (PBS). Upon the addition of β-CDS, a solution of 15 wt% Dex-mal (DS = 17) turned into a gel within 20 s. The gelation time was shown to depend on the polymer concentration and the DS. (DS = 10, gelation time = 30 s; DS = 27, gelation time = 15 s).
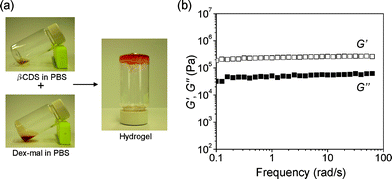 | ||
| Fig. 2 (a) Photographs of in situ hydrogel formation. Hydrogel was formed immediately after mixing solutions of β-CDS (24 mg in 400 μL PBS) and Dex-mal (DS = 17, 180 mg in 800 μL PBS). (b) Frequency dependency of storage moduli (G′) and loss moduli (G′′) of the obtained hydrogel. | ||
The obtained hydrogels were characterized with rheology measurements and scanning electron microscopy (SEM). Dependences of storage (G′) and loss (G′′) moduli on angular frequency were used to characterize the viscoelastic behavior, where G′ and G′′ reflect the elastic and viscous properties of the sample, respectively. It was confirmed that individual solutions of β-CDS and Dex-mal showed a dominant viscous property whereas the mixed solution showed a dominant elastic property. In Fig. 2 (b), G′ and G′′ were plotted as a function of angular frequency for a mixed sample of 15 wt% Dex-mal (DS = 17) and β-CDS solutions. The mixed sample exhibited the characteristic viscoelastic behavior of gel, the values of G′ varied little with angular frequency, and G′ was significantly larger than the G′′ over all measured frequencies indicating an elastic solid behavior.
The structure of the obtained hydrogels were studied with SEM on freeze-dried hydrogel samples. As shown in Fig. 3, the hydrogel had a macroporous network with a relatively regular structure indicating a highly hydrated structure and a homogeneous reaction during the gelation, respectively. In contrast only dense amorphous solids were observed for the freeze-dried individual solution of β-CDS or Dex-mal.
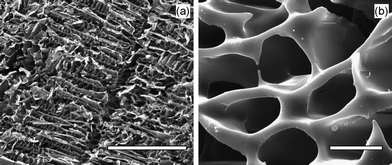 | ||
| Fig. 3 SEM images of the freeze-dried hydrogel prepared from Dex-mal (15 wt%) and β-CDS solutions; with lower and higher magnifications. Scale bars in the images (a) and (b) represent 100 and 5 μm, respectively. | ||
To examine the release characteristics of a hydrophobic drug from the hydrogel, all-trans retinoic acid (RA) carrying hydrogels were prepared. It is noteworthy that addition of RA did not alter the gelation time and also RA did not react with the hydrogel components. The released amount of RA per day from the hydrogel was determined by HPLC over a period of two weeks.15
As shown in Fig. 4, the release rate of RA became constant in a few days (20 nmol day−1), which was controlled by the solubility of RA in PBS.16 And the release was sustained: ca. 270 nmol of the loaded RA was released in two weeks. It is important to note that the release profile did not show an initial rapid release phase (burst effect), which has been commonly observed in other hydrogel systems.4,17 In this hydrogel, the β-CDS acts not only as a cross-linker but also as a host for RA,18 which increases the affinity for the hydrophobic drug, thus resulting in a constant release from the hydrogel without showing a burst effect. In addition, by forming the inclusion complex with β-CDS, aggregation of hydrophobic drugs during the drug loading process is prevented, thus evading the problems caused by a high local concentration of the drugs.
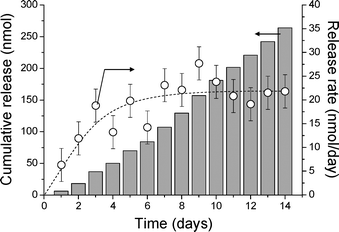 | ||
| Fig. 4 Release profile of RA from 400 μL of the cyclodextrin–dextran based hydrogel carrying 3.3 μmol RA. The cumulative release (grey bars) and released amounts per day (○) are shown. | ||
In conclusion, in situ hydrogel forming system composed of thiol functionalized β-cyclodextrin (β-CDS) and maleimide functionalized dextran (Dex-mal) was prepared and investigated the in vitro release of the hydrophobic drug all-trans retinoic acid (RA). Dex-mal with different degrees of substitution were synthesized and used for hydrogel formation. By mixing aqueous solutions of β-CDS and Dex-mal, hydrogels were formed via Michael addition within 30 s. The obtained hydrogel behaved as an elastic solid and showed to have a regular network structure. The in vitro release of RA did not show an initial burst effect making it an interesting drug carrier to study the effect of local doses of RA on development. This in situ forming hydrogel system will be further studied as an injectable drug delivery system with RA in vivo using phenotype-based assays in the near future. Furthermore, in principal it can also be applied to deliver any other hydrophobic drug that can form an inclusion complex with β-CDS such as doxorubicin,19a diltiazem19b and ketoprofen.19c
Acknowledgements
The authors acknowledge professor M. K. Richardson, Integrative Zoology Group, Institute of Biology Leiden, Leiden University, for fruitful discussion and suggestions. We are grateful to Mr Wim Jesse and Mr Ben Norder for technical assistance. The authors (A. K. and I. T.) acknowledge the support of the Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science.Notes and references
-
(a) C. Wang, R. J. Stewart and J. Kopecek, Nature, 1999, 397, 417 CrossRef CAS
; (b) W. E. Hennink and C. F. van Nostrum, Adv. Drug Delivery Rev., 2002, 54, 13 CrossRef CAS
; (c) M. P. Lutolf, J. L. Lauer-Fields, H. G. Schmoekel, A. T. Metters, F. E. Weber, G. B. Fields and J. A. Hubbell, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 5413 CrossRef CAS
; (d) N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345 CrossRef CAS
; (e) C. Hiemstra, W. Zhou, Z. Y. Zhong, M. Wouters and J. Feijen, J. Am. Chem. Soc., 2007, 129, 9918 CrossRef CAS
.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2002, 54, 3 CrossRef CAS
.
-
(a) L. Yu and J. D. Ding, Chem. Soc. Rev., 2008, 37, 1473 RSC
; (b) T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993 CrossRef CAS
.
- M. C. Chen, H. W. Tsai, C. T. Liu, S. F. Peng, W. Y. Lai, S. J. Chen, Y. Chang and H. W. Sung, Biomaterials, 2009, 30, 2102 CrossRef CAS
.
- V. P. Torchilin, Cell. Mol. Life Sci., 2004, 61, 2549 CrossRef CAS
.
-
(a) M. E. Davis and M. E. Brewster, Nat. Rev. Drug Discovery, 2004, 3, 1023 CrossRef CAS
; (b) K. Uekama, F. Hirayama and T. Irie, Chem. Rev., 1998, 98, 2045 CrossRef CAS
.
-
(a) L. Janus, G. Crini, V. El-Rezzi, M. Morcellet, A. Cambiaghi, G. Torri, A. Naggi and C. Vecchi, React. Funct. Polym., 1999, 42, 173–180 CrossRef CAS
; (b) J.-F. R. dos Santos, R. Couceiro, A. Concheiro, J.-J. Torres-Labandeira and C. Alvarez-Lorenzo, Acta Biomater., 2008, 4, 745–755 CrossRef CAS
.
- R. Mehvar, J. Controlled Release, 2000, 69, 1 CrossRef CAS
.
-
(a) S. J. de Jong, S. C. de Smedt, J. Demeester, C. F. van Nostrum, J. J. Kettenes-van den Bosch and W. E. Hennink, J. Controlled Release, 2001, 72, 47 CrossRef CAS
; (b) S. H. Kim, C. Y. Won and C. C. Chu, Carbohydr. Polym., 1999, 40, 183 CrossRef CAS
; (c) W. N. E. van Dijk-Wolthuis, O. Franssen, H. Talsma, M. J. van Steenbergen, J. J. Kattenes-van den Bosch and W. E. Hennink, Macromolecules, 1995, 28, 6317 CrossRef CAS
; (d) S. H. Kim, C. Y. Won and C. C. Chu, J. Biomed. Mater. Res., 1999, 46, 160 CrossRef CAS
; (e) B. G. de Geest, W. van Camp, F. E. Du Prez, S. C. de Smedt, J. Demeester and W. E. Hennink, Chem. Commun., 2008, 190 RSC
; (f) R. Jin, C. Hiemstra, Z. Y. Zhong and J. Feijen, Biomaterials, 2007, 28, 2791 CrossRef CAS
; (g) C. Hiemstra, L. J. van der Aa, Z. Y. Zhong, P. J. Dijkstra and J. Feijen, Macromolecules, 2007, 40, 1165 CrossRef CAS
; (h) S. G. Levesque and M. S. Shoichet, Bioconjugate Chem., 2007, 18, 874 CrossRef CAS
.
-
(a) L. Hovgaard and H. Brondsted, J. Controlled Release, 1995, 36, 159 CrossRef CAS
; (b) S. H. Kim and C. C. Chu, J. Biomater. Appl., 2000, 15, 23 CrossRef CAS
; (c) W. E. Hennink, S. J. de Jong, G. W. Bos, T. H. J. Veldhuis and C. F. van Nostrum, Int. J. Pharm., 2004, 277, 99 CrossRef CAS
; (d) C. Hiemstra, Z. Y. Zhong, M. J. van Steenbergen, W. E. Hennink and J. Feijen, J. Controlled Release, 2007, 122, 71 CrossRef CAS
.
-
(a) S. A. Brittijn, S. J. Duivesteijn, W. Bitter, J. D. de Bruin, D. Champagne, E. Cuppen, G. Flik, R. Janssen, I. M. de Jong, R. de Kloet, A. Kros, A. H. Meijer, A. M. van der Sar, M. J. M. Schaaf, S. Schulte-Merker, H. P. Spaink, P. P. Tak, C. M. Vandenbroucke-Grauls, M. J. Vervoordeldonk, F. F. Vonk, F. Witte and M. K. Richardson, Int. J. Dev. Biol., 2009, 53, 835–850 CrossRef CAS
; (b) C. Tickle, B. Alberts, L. Wolpert and J. Lee, Nature, 1982, 296, 564 CrossRef CAS
.
- C. Tickle, J. Lee and G. Eichele, Dev. Biol., 1985, 109, 82 CrossRef CAS
.
- D. H. Rich, P. D. Gesellchen, A. Tong, A. Cheung and C. K. Buckner, J. Med. Chem., 1975, 18, 1004 CrossRef CAS
.
- M. T. Rojas, R. Koniger, J. F. Stoddart and A. E. Kaifer, J. Am. Chem. Soc., 1995, 117, 336 CrossRef CAS
.
- G. Eichele, C. Tickle and B. M. Alberts, Anal. Biochem., 1984, 142, 542 CrossRef CAS
.
- E. Z. Szuts and F. I. Harosi, Arch. Biochem. Biophys., 1991, 287, 297 CrossRef CAS
.
- Y. L. Zhang and C. C. Chu, J. Biomed. Mater. Res., 2002, 59, 318 CrossRef CAS
.
- P. Montassiera, D. Duchêneb and M.-C. Poelmana, Int. J. Pharm., 1997, 153, 199 CrossRef
.
-
(a) E. S. Gil, J. S. Li, H. N. Xiao and T. L. Lowe, Biomacromolecules, 2009, 10, 505 CrossRef CAS
; (b) K. Uekama, T. Horikawa, Y. Horiuchi and F. Hirayama, J. Controlled Release, 1993, 25, 99 CrossRef CAS
; (c) H. S. Woldum, K. L. Larsen and F. Madsen, Drug Delivery, 2008, 15, 69 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b914166a |
| This journal is © The Royal Society of Chemistry 2010 |
