Total synthesis of all (−)-agelastatin alkaloids†
Mohammad
Movassaghi
*,
Dustin S.
Siegel
and
Sunkyu
Han
Massachusetts Institute of Technology, Department of Chemistry, 77 Massachusetts Avenue 18-292, Cambridge, MA 02139-4307, USA. E-mail: movassag@mit.edu
First published on 16th August 2010
Abstract
The pyrrole-imidazole family of marine alkaloids, derived from linear clathrodin-like precursors, constitutes a diverse array of structurally complex natural products. The bioactive agelastatins are members of this family that possess a tetracyclic molecular framework incorporating C4–C8 and C7–N12 bond connectivities. We provide a hypothesis for the formation of the unique agelastatin architecture that maximally exploits the intrinsic chemistry of plausible biosynthetic precursors. We report the concise enantioselective total syntheses of all known agelastatin alkaloids including the first total syntheses of agelastatins C, D, E, and F. Our gram-scale chemical synthesis of agelastatin A was inspired by our hypothesis for the biogenesis of the cyclopentane C-ring and required the development of new transformations including an imidazolone-forming annulation reaction and a carbohydroxylative trapping of imidazolones.
Introduction
The agelastatin alkaloids constitute an intriguing subset of the diverse pyrrole-imidazole family of marine alkaloids that are likely derived from linear biogenetic precursors such as clathrodin (7),1 hymenidin (8),2 and oroidin (9, Fig. 1).3,4 (−)-Agelastatins A (1) and B (2) were first isolated from the Coral Sea sponge Agelas dendromorpha by Pietra et al. in 1993 who successfully identified and chemically studied their unique tetracyclic structures.5–8 In 1998, Molinski et al. isolated (−)-agelastatins C (3) and D (4) from Cymbastela sp. native to the Indian Ocean.9 Recently, Al-Mourabit et al. reported the isolation of (−)-agelastatins E (5) and F (6) from the New Caledonian sponge A. dendromorpha.10 (−)-Agelastatin A (1) exhibits significant antitumor activity and inhibits osteopontin-mediated neoplastic transformation and metastasis in addition to slowing cancer cell proliferation by causing cells to accumulate in the G2 phase of the cell cycle.11,12 (−)-Agelastatin A (1) also exhibits toxicity towards arthropods,9 and selectively inhibits the glycogen synthase kinase-3β, which is a potential target for the treatment of Alzheimer's disease and bipolar disorder.13,14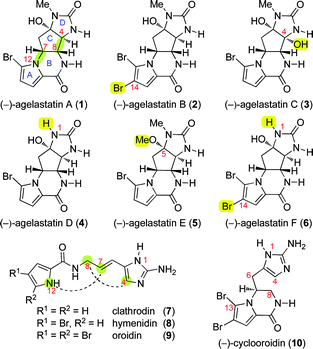 | ||
| Fig. 1 The molecular structures of all agelastatin alkaloids (1–6) and biogenetically related naturally occurring simpler pyrrole-imidazole alkaloids (7–10). | ||
These properties have prompted considerable efforts toward the total synthesis of agelastatin A (1), resulting in inventive syntheses from 10 different research groups. In 1999, Weinreb et al. completed the first total synthesis of agelastatin A (1) employing a key N-sulfinyl dienophile hetero-Diels–Alder reaction.15,16 Feldman et al. reported the first enantioselective syntheses of (−)-agelastatin A (1) and (−)-agelastatin B (2) using an alkylidenecarbene C–H insertion reaction.17,18 While Hale et al. applied aziridine opening chemistry to the synthesis of (−)-1,19,20 Davis et al. utilized ring-closing metathesis and N-sulfinyl imine based methodologies to access (−)-agelastatin A (1).21,22 Sequential palladium-catalyzed asymmetric allylic alkylation reactions were key to Trost and Dong's concise total synthesis of (+)-1.23,24 Ichikawa et al.'s sigmatropic rearrangement of an allyl cyanate25 in addition to Wardrop and Dickson’s, and Chida et al.'s respective use of the Overman rearrangement constituted additional successful approaches to 1.26,27 The interest in the field has continued with application of aziridination methodologies by Yoshimitsu et al.,28,29 and most recently by Du Bois and Wehn’s elegant solution to (−)-1.30 Interestingly, all of these syntheses have in common an early introduction of the C-ring followed by further elaboration to afford the desired tetracyclic framework. Additionally, the reported syntheses of agelastatin A (1) do not specifically evaluate existing hypotheses for biogenesis of its intriguing molecular architecture. Furthermore, there are currently no total syntheses of agelastatins C (3), D (4), E (5), or F (6). Herein, we report the first biogenetically inspired and unified approach to all (−)-agelastatins (1–6) using a concise synthetic strategy empowered by the inherent chemistry of potential biosynthetic intermediates.
The agelastatins are the only isolated pyrrole-imidazole alkaloids with C4–C8 and C7–N12 connectivities (Fig. 1). Feeding studies by Kerr et al. demonstrated that histidine and ornithine (or proline) are the amino acid precursors for related pyrrole-imidazole alkaloids.31 There are currently two reported biosynthetic hypotheses for the agelastatins,5,32 and both suggest that the formation of the carbocyclic C-ring results from C8-nucleophilic trapping of a C4-electrophile in a clathrodin derivative. Furthermore, these hypotheses suggest an early stage biosynthesis of the C-ring followed by B-ring formation and attribute stereochemical control to the action of putative enzymes.
Results and discussion
Biosynthetic hypothesis and design plan for total synthesis
The fascinating molecular architecture of the agelastatins and interest in evaluating our new hypothesis for the biogenetic origins of the C-ring involving cyclization with concomitant introduction of three stereocenters motivated the studies described here. We envisioned an advanced-stage biosynthetic sequence (Scheme 1), distinct from existing hypotheses, that relies on: 1) reverse polarity in C-ring formation involving C4-nucleophilic trapping of a C8-electrophile, 2) introduction of the C-ring after the B-ring formation, and 3) substrate directed stereochemical control and use of intrinsic chemistry that is perhaps enhanced by the action of biosynthetic enzymes. Our retrosynthetic factoring of (−)-agelastatin A (1) inspired by our retrobiosynthetic analysis of 1 is illustrated in Scheme 1. Ionization of the C5-hydroxyl of 1 followed by the strategic disconnection of C4–C8 reveals N-acyliminium ion 17 and clears the carbocyclic C-ring along with three stereocenters. The mechanistic development of a transform33 linking 1 to 17 prompted consideration of a versatile precursor, pre-agelastatin A (16, Scheme 1). In the forward direction, our hypothesis asserts that pre-agelastatin A (16) may be ionized to the C8-acyliminium ion 17, allowing a 5-exo-trig cyclization followed by C5-hydroxylation to secure the C4-, C5-, and C8-stereocenters in the final stage of the biosynthesis (Scheme 1, 16 → 1). We envisioned that pre-agelastatin A (16) would result from C2-hydrolysis and C8-oxidation of the cyclooroidin analogue 14. Tricycle 14 would be formed by C4-protonation of linear precursor 11 followed by C7-trapping by the pyrrolyl-nitrogen (N12) via a 6-exo-trig cyclization.34 Notably, this pathway suggests a link between the agelastatins and the natural product cyclooroidin (10, Fig. 1),35 and is consistent with Lindel's reported acid promoted conversion of oroidin (9) to tricycle 10.36 Motivated by the potential direct conversion of pre-agelastatin A (16) to (−)-agelastatin A (1), we targeted the related structure O-methyl-pre-agelastatin A (19) and envisioned its concise synthesis from readily available D-aspartic acid derivative 22 (Scheme 1, 22 → 19).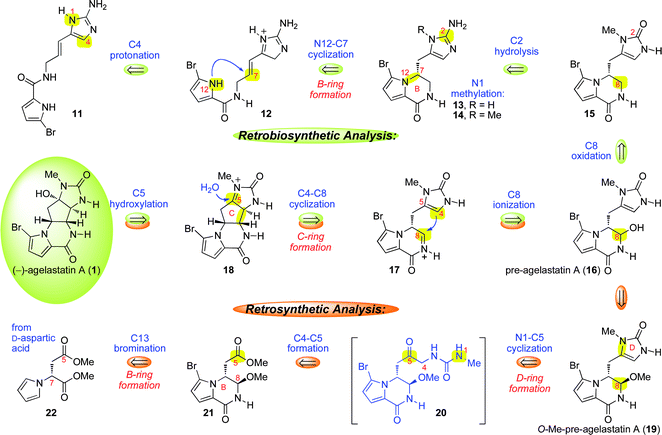 | ||
| Scheme 1 Our retrosynthetic analysis of (−)-agelastatin A (1) inspired by our biosynthetic hypothesis that involves intermediacy of pre-agelastatin A (16) in a final stage formation of the C-ring and rapid introduction of the C4-, C5-, and C8-stereochemistry (16 → 1). | ||
Total synthesis of the agelastatin alkaloids
Our convergent synthesis for the desired O-methyl-pre-agelastatin A (19) commenced with pyrrole (+)-22 (Scheme 2), accessible in one step from commercially available D-aspartic acid dimethyl ester.37 Exposure of pyrrole (+)-22 to N-bromosuccinimide (NBS) in the presence of 2,6-di-tert-butyl-4-methylpyridine (DTBMP) afforded the bromopyrrole (+)-23 in 92% yield and 99% ee. Treatment of bromopyrrole (+)-23 with chlorosulfonyl isocyanate afforded amide (+)-24 in 82% yield. Subsequently, addition of sodium borohydride followed by p-toluenesulfonic acid (TsOH) to a methanolic solution of (+)-24 generated bicycle (+)-21 as a single diastereomer in 90% yield and 99% ee on a greater than 10 g scale. The conversion of (+)-24 to bicycle (+)-21 occurs via formation and immediate C8-reduction of the imide 25, preventing an undesired C7-epimerization.38 Identical B-ring formation with the desbromopyrrole derivative of 24 resulted in significant erosion of enantiopurity. This observation was consistent with our postulate that the C7–H bond would be forced to adopt a pseudo-equatorial conformation to minimize allylic strain between the C13-bromine and C6-methylene, which suppressed undesired C7-deprotonation. Interestingly, the use of pyrrole (+)-24, possessing the C13-bromine present in all known agelastatins, provided chemical reactivity beneficial to our synthetic strategy (vide infra).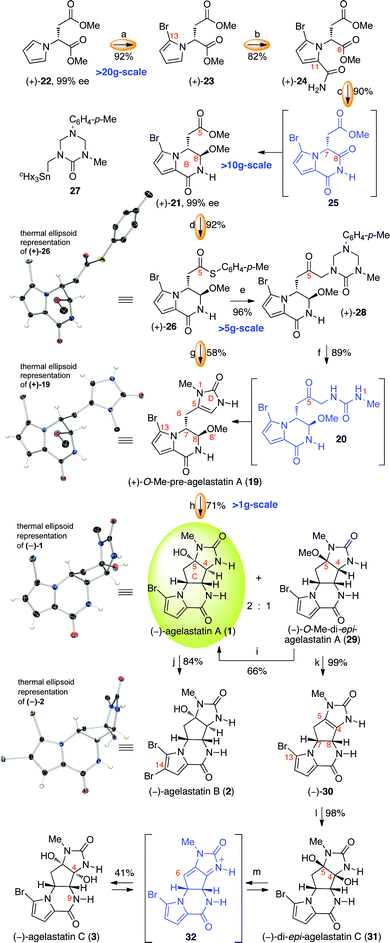 | ||
Scheme 2 Gram-scale enantioselective total synthesis of (−)-agelastatin A (1) and elaboration to (−)-agelastatin B (2) and (−)-agelastatin C (3). Reagents and conditions: (a) NBS, DTBMP, THF (92%); (b) ClSO2NCO, MeCN, 0 °C; Na(Hg), NaH2PO4 (82%); (c) NaBH4, MeOH, 0 °C; TsOH·H2O, 23 °C (90%); (d) HSC6H4-p-Me, AlMe3, CH2Cl2, 0 °C (92%); (e) 27, CuTC, THF, 50 °C (96%); (f) HCl (0.5 N), MeOH, 65 °C (89%); (g) cHx3SnCH2NH(CO)NHMe (33), CuTC, THF, 50 °C; HCl (0.5 N), MeOH, 23 °C (58%); (h) MeSO3H, H2O, 100 °C; MeOH (71% (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (−)-1 1, (−)-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (−)-29)); (i) MeSO3H, H2O, 100 °C; MeOH (66% of (−)-1, and 30% of recovered (−)-29); (j) NBS, DTBMP, THF, H2O, 0 °C (84%); (k) pyr., 115 °C (99%); (l) DMDO, acetone, H2O (98%); (m) Amberlyst 15, H2O, 100 °C, 5 d (41% of (−)-3, and 42% of recovered (−)-31); NBS = N-bromosuccinimide, DTBMP = 2,6-di-tert-butyl-4-methylpyridine, TsOH = p-toluenesulfonic acid, CuTC = copper(I)-thiophene-2-carboxylate, DMDO = dimethyldioxirane, pyr. = pyridine. (−)-29)); (i) MeSO3H, H2O, 100 °C; MeOH (66% of (−)-1, and 30% of recovered (−)-29); (j) NBS, DTBMP, THF, H2O, 0 °C (84%); (k) pyr., 115 °C (99%); (l) DMDO, acetone, H2O (98%); (m) Amberlyst 15, H2O, 100 °C, 5 d (41% of (−)-3, and 42% of recovered (−)-31); NBS = N-bromosuccinimide, DTBMP = 2,6-di-tert-butyl-4-methylpyridine, TsOH = p-toluenesulfonic acid, CuTC = copper(I)-thiophene-2-carboxylate, DMDO = dimethyldioxirane, pyr. = pyridine. | ||
We next aimed to develop a general strategy for the introduction of the imidazolone substructure present in the targeted pre-agelastatin 16.39 Initially, we focused on the direct addition of transmetallated derivatives of triazone 27 (Scheme 2, metal = Li, Mg, Cu, Ce, Zn) to the bicyclic C5-ester (+)-21. Unfortunately, these attempts were plagued by either lack of reactivity, the formation of by-products associated with metal–halogen exchange, double addition, or competing decomposition pathways. Furthermore, these metallated40–42 triazone derivatives were generally unstable at temperatures above 0 °C. Thus, the development of a new strategy for the union of a stable metallated triazone and ester (+)-21 as the prelude to introduction of the imidazolone was necessary.
We envisioned that the cross-coupling of thioester derivatives43–45 with stannylurea derivatives would represent a versatile synthesis of substituted imidazolones. Thioester (+)-26 was readily prepared in 92% yield through treatment of ester (+)-21 with trimethylaluminium and 4-methylbenzenethiol in dichloromethane (Scheme 2). The structure of (+)-26 was secured via X-ray crystallographic analysis,46 revealing the pseudo-equatorial C7–H bond. After extensive experimentation, we found that the union of thioester (+)-26 with the readily available triazone 2747–49 could be achieved efficiently in the presence of stoichiometric copper(I)-thiophene-2-carboxylate (CuTC) to give the ketone (+)-28 (96%, >5 g-scale). Exposure of triazone (+)-28 to methanolic hydrogen chloride unraveled the keto-urea 20, which upon spontaneous condensative cyclization50,51 provided the desired (+)-O-methyl-pre-agelastatin A (19) in 89% yield with 99% ee (Scheme 2). The structure of (+)-19 was secured via X-ray crystallographic analysis, and its thermal ellipsoid representation illustrates that the C7-methylimidazolone and C8-methoxy group reside in a pseudo-diaxial conformation (C6–C7–C8–O8′ dihedral angle of 173°).46
With (+)-O-methyl-pre-agelastatin A (19) in hand, we proceeded to evaluate our hypothesis for C-ring biogenesis and rapid introduction of the C4-, C5-, and C8-stereocenters. Gratifyingly, heating an aqueous solution of (+)-19 with methanesulfonic acid provided (−)-agelastatin A (1, Scheme 2) as the major product along with (−)-4,5-di-epi-agelastatin A (structure not illustrated) as the minor stereoisomer (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Monitoring of this reaction by 1H NMR revealed that (−)-4,5-di-epi-agelastatin A is the kinetic product, which equilibrates to the thermodynamically favored desired product (−)-agelastatin A (1), likely via intermediacy of tetracycle (−)-30. Analysis of the rate of solvolysis of each isomer illustrated that the C5-hydroxyl of (−)-4,5-di-epi-agelastatin A ionizes significantly faster than the corresponding C5-hydroxyl of (−)-agelastatin A (1). In the event, upon complete consumption of pre-agelastatin A (16), simple exposure of the reaction mixture to methanol efficiently converted (−)-4,5-di-epi-agelastatin A to (−)-O-methyl-di-epi-agelastatin A (29), enabling facile separation of (−)-1 and (−)-29 (Scheme 2). Under preparative conditions, our putative biomimetic cyclization of (+)-19 afforded (−)-agelastatin A (1) in 49% yield (1.4 g, 99% ee) along with (−)-O-methyl-di-epi-agelastatin A (29) in 22% yield. This constitutes a total chemical synthesis of (−)-agelastatin A (1) in eight steps for the longest linear sequence from commercially available starting material with 22% overall yield. Furthermore, resubmission of (−)-29 to the above protocol afforded (−)-agelastatin A (1) in 66% yield along with recovered (−)-29 (30%) post-equilibration. The structure of (−)-1 was secured through X-ray crystallographic analysis (Scheme 2).46 It should be noted that this 5-(enol-endo)-exo-trig52 type of cyclization with an acyliminium ion is a rare and challenging reaction, as evidenced by the paucity of relevant examples in the literature.53,54 Importantly, the versatility of our new imidazolone annulation allows for the union of thioester (+)-26 and the simple stannylurea derivative 33 (cHx3SnCH2NH(CO)NHMe) to afford (+)-O-methyl-pre-agelastatin A (19) without isolation of any intermediates, providing the shortest total synthesis of (−)-agelastatin A (1, 7-steps, Scheme 2) to date.
1). Monitoring of this reaction by 1H NMR revealed that (−)-4,5-di-epi-agelastatin A is the kinetic product, which equilibrates to the thermodynamically favored desired product (−)-agelastatin A (1), likely via intermediacy of tetracycle (−)-30. Analysis of the rate of solvolysis of each isomer illustrated that the C5-hydroxyl of (−)-4,5-di-epi-agelastatin A ionizes significantly faster than the corresponding C5-hydroxyl of (−)-agelastatin A (1). In the event, upon complete consumption of pre-agelastatin A (16), simple exposure of the reaction mixture to methanol efficiently converted (−)-4,5-di-epi-agelastatin A to (−)-O-methyl-di-epi-agelastatin A (29), enabling facile separation of (−)-1 and (−)-29 (Scheme 2). Under preparative conditions, our putative biomimetic cyclization of (+)-19 afforded (−)-agelastatin A (1) in 49% yield (1.4 g, 99% ee) along with (−)-O-methyl-di-epi-agelastatin A (29) in 22% yield. This constitutes a total chemical synthesis of (−)-agelastatin A (1) in eight steps for the longest linear sequence from commercially available starting material with 22% overall yield. Furthermore, resubmission of (−)-29 to the above protocol afforded (−)-agelastatin A (1) in 66% yield along with recovered (−)-29 (30%) post-equilibration. The structure of (−)-1 was secured through X-ray crystallographic analysis (Scheme 2).46 It should be noted that this 5-(enol-endo)-exo-trig52 type of cyclization with an acyliminium ion is a rare and challenging reaction, as evidenced by the paucity of relevant examples in the literature.53,54 Importantly, the versatility of our new imidazolone annulation allows for the union of thioester (+)-26 and the simple stannylurea derivative 33 (cHx3SnCH2NH(CO)NHMe) to afford (+)-O-methyl-pre-agelastatin A (19) without isolation of any intermediates, providing the shortest total synthesis of (−)-agelastatin A (1, 7-steps, Scheme 2) to date.
Under optimal conditions, treatment of (−)-agelastatin A (1) with NBS and DTBMP in a water–tetrahydrofuran solvent mixture afforded (−)-agelastatin B (2) in 84% yield. Interestingly, X-ray crystallographic analysis of (−)-agelastatin B (2) revealed that its C-ring conformation is distinct from that of (−)-agelastatin A (1), as highlighted by the 25° and 31° difference in the C5–C4–C8–N9 and N1–C5–C4–C8 dihedral angles, respectively.46
Importantly, (−)-O-methyl-di-epi-agelastatin A (29) served as a versatile precursor for the synthesis of (−)-agelastatin C (3, Scheme 2). Heating a solution of (−)-O-methyl-di-epi-agelastatin A (29) in pyridine afforded (−)-dehydroagelastatin A (30, Scheme 2) in 99% yield.55 As anticipated, treatment of 30 with dimethyldioxirane (DMDO) gave (−)-di-epi-agelastatin C (31, 98%) via oxidation on the convex face. Significantly, heating an aqueous solution of (−)-di-epi-agelastatin C (31) with Brønsted acid slowly afforded (−)-agelastatin C (3, 41%) along with recovered 31 (42%). We propose that this equilibration occurs via the intermediate 32, consistent with our observations of deuterium incorporation at the C6-methylene when using deuterium oxide as solvent.56
Our new imidazolone annulation methodology proved most effective for accessing the desired pre-agelastatin D intermediate for the first synthesis of (−)-agelastatin D (4, Scheme 3). Under our optimized conditions, treatment of thioester (+)-26 with stannylurea 34 and CuTC followed by exposure to methanolic hydrogen chloride afforded (+)-O-methyl-pre-agelastatin D (35) in 62% yield. Despite the attenuated C4-nucleophilicity of (+)-O-methyl-pre-agelastatin D (35) compared to (+)-O-methyl-pre-agelastatin A (19) in polar-protic solvents,57 application of our key cyclization protocol as described above indeed provided the desired (−)-agelastatin D (4) in 26% yield along with (−)-O-methyl-di-epi-agelastatin D (36, 9%).58 The structure of (−)-agelastatin D (4), secured through X-ray crystallographic analysis, demonstrated a C-ring conformation similar to (−)-1.46 Despite the success of this putative biomimetic cyclization, the greater propensity of 35 to undergo competing reactions was evident by formation of the C6–C7 bond-cleavage product 38 (20%)59 and the tetracyclic by-product 40 (20%). Formation of tetracycle 40 is consistent with a competing loss of methanol to afford pyrrolopyrazinone 39, an observed intermediate, that prevents the desired C-ring formation and permits C13 to engage the imidazolone.60 The observed lower efficiency of the desired cyclization with 35 compared to 19 echoes the scarcity of natural (−)-agelastatin D (4) compared to other N-methyl agelastatin alkaloids.9,10,61
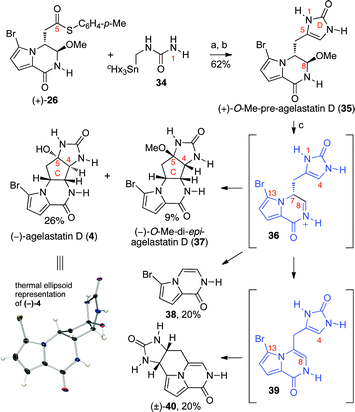 | ||
| Scheme 3 Total synthesis of (−)-agelastatin D (4). Reagents and conditions: (a) CuTC, THF, 50 °C; (b) HCl (0.5 N), MeOH, 23 °C (62% (2-steps)); (c) MeSO3H, H2O, 100 °C; HCl, MeOH (26% (−)-4, 9% (−)-37, 20% 38, 20% (±)-40); CuTC = copper(I)-thiophene-2-carboxylate. | ||
Furthermore, we have accessed the structures of the two newly isolated (−)-agelastatins E (5) and F (6) by their direct synthesis from (−)-agelastatin A (1) and D (4), respectively (Scheme 4). Heating a methanolic solution of (−)-agelastatin A (1) with Brønsted acid at 65 °C for 2 h afforded (−)-agelastatin E (5) in 96% yield (Scheme 4).7 A synthetic sample of (−)-agelastatin F (6) was generated in 86% yield by bromination of (−)-agelastatin D (4) under the optimal conditions described above for the synthesis of (−)-agelastatin B (2), thereby confirming its molecular structure.
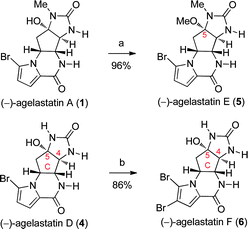 | ||
| Scheme 4 Total synthesis of (−)-agelastatin E (5) and (−)-agelastatin F (6). Reagents and conditions: (a) Amberlyst 15, MeOH, 65 °C (96%); (b) NBS, DTBMP, THF, H2O, 0 °C (86%). | ||
Our biosynthetically inspired strategy for the advanced stage C-ring formation drew on the intrinsic chemistry of our proposed pre-agelastatin intermediates for rapid generation of molecular complexity, enabling a unified approach to all known agelastatin alkaloids. Collectively, our observations hint at a plausible sequence of events for the biogenesis of the alkaloids 1–6 (Scheme 1). For example, the C13-bromopyrrole and the imidazolinone substructures (present in all agelastatins) were critical in the successful C-ring cyclization. Treatment of the desbromo derivative 41 under the optimized cyclization conditions did not afford the desired C-ring due to a more facile conversion to pyrrolopyrazinone 42 (57%, Scheme 5),62 suggesting a beneficial role for the allylic strain between the C13-bromine and C6-methylene to restrict the C7-methine in a pseudo-equatorial conformation during the key cyclization event.
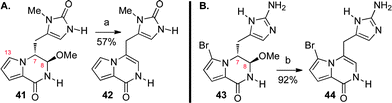 | ||
| Scheme 5 Key observations concerning our bioinspired C-ring synthesis strategy. Attempted cyclization of A) desbromo-pre-agelastatin A (41) and B) imidazole derivative 43. Reagents and conditions: (a) MeSO3H, H2O, 100 °C, 20 min (57%); (b) Dowex, H2O, 100 °C (92%). | ||
Additionally, the aminoimidazole 43 failed to undergo the desired cyclization reaction due to a more competitive pyrrolopyrazinone 44 formation (92%, Scheme 5), an observation we attribute to the greater propensity of the aminoimidazolone substructure to remain protonated and thus less nucleophilic under the reaction conditions.
Consequently, we suggest a higher probability for biosynthetic introduction of the C13-bromopyrrole and imidazolone substructures prior to C-ring formation. Moreover, our observations regarding the higher predisposition for the pre-agelastatin A derivative (+)-19, to undergo C-ring formation as compared to the desmethyl derivative (+)-35 may suggest predominant N1-methylation prior to C-ring cyclization in the biogenesis of the agelastatins. The stereochemical outcome for the key C-ring cyclization is controlled by the C7-methine to secure the desired thermodynamically favored C4-, C5-, and C8-stereocenters. Specifically, the C5-center is controlled by the C4-stereochemistry to give a cis-fused CD-ring system upon hydroxylation. It is conceivable that putative agelastatin biosynthetic enzymes have evolved to enhance the innate stereoselectivity of compounds related to those utilized in our synthesis.63 While our total syntheses of alkaloids 1–6 do not confirm our hypothesis for their biogenesis, it is gratifying to have chemical validation for our proposed mode and timing of bond and ring formations in the biosynthesis of these alkaloids.
Conclusions
We have completed the total syntheses of all known agelastatin alkaloids (1–6) through a unified strategy inspired by our hypothesis for their biogenesis. Key features of our syntheses include: 1) the concise multi-gram scale enantioselective synthesis of our proposed “pre-agelastatin” derivatives, 2) the use of the bromopyrrole substructure to suppress C7-deprotonation, 3) a versatile synthesis of imidazolone derivatives via a new [4 + 1] annulation strategy, 4) the validation of our bioinspired 5-exo-trig advanced stage C-ring formation, and 5) utilization of the intrinsic chemistry of plausible biosynthetic intermediates for rapid generation of molecular complexity. The overall efficiency of our strategy is highlighted by our 1.4 gram batch enantioselective synthesis of (−)-agelastatin A (1). With this most concise total chemical synthetic access to all natural agelastatin alkaloids and related derivatives, studies aimed at probing their chemical and biological mode of action are ongoing and will be the subject of forthcoming reports.Acknowledgements
We are grateful for financial support from NIH-NIGMS (GM074825). We thank Justin Kim for X-ray crystallographic analysis of (−)-1, (−)-2, (−)-4, (+)-19, (+)-21, and (+)-26. M. M. is an Alfred P. Sloan Research Fellow. We thank Amgen, AstraZeneca, Bristol-Myers Squibb, and DuPont for additional financial support.Notes and references
- J. J. Morales and A. D. Rodriguez, J. Nat. Prod., 1991, 54, 629–631 CrossRef CAS.
- J. Kobayashi, H. Ohizumi and Y. Hirata, Experientia, 1986, 42, 1176–1177 CAS.
- S. Forenza, L. Minale, R. Riccio and E. Fattorusso, J. Chem. Soc. D, 1971, 1129–1130 RSC.
- E. E. Garcia, L. E. Benjamin and R. I. Fryer, J. Chem. Soc., Chem. Commun., 1973, 78–79 RSC.
- M. D'Ambrosio, A. Guerriero, C. Debitus, O. Ribes, J. Pusset, S. Leroy and F. Pietra, J. Chem. Soc., Chem. Commun., 1993, 1305–1306 RSC.
- M. D'Ambrosio, A. Guerriero, G. Chiasera and F. Pietra, Helv. Chim. Acta, 1994, 77, 1895–1902 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero, M. Ripamonti, C. Debitus, J. Waikedre and F. Pietra, Helv. Chim. Acta, 1996, 79, 727–735 CAS.
- For an additional isolation report and X-ray crystallographic analysis of (−)-1, see: G. R. Pettit, S. Ducki, D. L. Herald, D. L. Doubek, J. M. Schmidt and J. Chapuis, Oncol. Res., 2005, 15, 11–20 Search PubMed.
- T. W. Hong, D. R. Jímenez and T. F. Molinski, J. Nat. Prod., 1998, 61, 158–161 CrossRef CAS.
- S. Tilvi, C. Moriou, M. Martin, J. Gallard, J. Sorres, K. Patel, S. Petek, C. Debitus, L. Ermolenko and A. Al-Mourabit, J. Nat. Prod., 2010, 73, 720–723 CrossRef CAS.
- C. K. Mason, S. McFarlane, P. G. Johnston, P. Crowe, P. J. Erwin, M. M. Domostoj, F. C. Campbell, S. Manaviazar, K. J. Hale and M. El-Tanani, Mol. Cancer Ther., 2008, 7, 548–558 CrossRef CAS.
- S. Kapoor, J. Cancer Res. Clin. Oncol., 2008, 134, 927–928 CrossRef CAS.
- L. Meijer, A. Thunnissen, A. W. White, M. Garnier, M. Nikolic, L. Tsai, J. Walter, K. E. Cleverley, P. C. Salinas, Y. Wu, J. Biernat, E. Mandelkow, S. Kim and G. R. Pettit, Chem. Biol., 2000, 7, 51–63 CrossRef CAS.
- K. J. Hale, M. M. Domostoj, M. El-Tanani, F. C. Campbell and C. K. Mason, in Strategies and Tactics in Organic Synthesis, ed. M. Harmata, Elsevier Academic Press, London, 2005, vol. 6, ch. 11, pp. 352–394 Search PubMed.
- G. T. Anderson, C. E. Chase, Y. Koh, D. Stien and S. M. Weinreb, J. Org. Chem., 1998, 63, 7594–7595 CrossRef CAS.
- D. Stien, G. T. Anderson, C. E. Chase, Y. Koh and S. M. Weinreb, J. Am. Chem. Soc., 1999, 121, 9574–9579 CrossRef.
- K. S. Feldman and J. C. Saunders, J. Am. Chem. Soc., 2002, 124, 9060–9061 CrossRef CAS.
- K. S. Feldman, J. C. Saunders and M. L. Wrobleski, J. Org. Chem., 2002, 67, 7096–7109 CrossRef CAS.
- K. J. Hale, M. M. Domostoj, D. A. Tocher, E. Irving and F. Scheinmann, Org. Lett., 2003, 5, 2927–2930 CrossRef CAS.
- M. M. Domostoj, E. Irving, F. Scheinmann and K. J. Hale, Org. Lett., 2004, 6, 2615–2618 CrossRef CAS.
- F. A. Davis and J. Deng, Org. Lett., 2005, 7, 621–623 CrossRef CAS.
- F. A. Davis, Y. Zhang and H. Qiu, Synth. Commun., 2009, 39, 1914–1919 CrossRef CAS.
- B. M. Trost and G. Dong, J. Am. Chem. Soc., 2006, 128, 6054–6055 CrossRef CAS.
- B. M. Trost and G. Dong, Chem.–Eur. J., 2009, 15, 6910–6919 CrossRef CAS.
- Y. Ichikawa, T. Yamaoka, K. Nakano and H. Kotsuki, Org. Lett., 2007, 9, 2989–2992 CrossRef CAS.
- P. D. Dickson and D. J. Wardrop, Org. Lett., 2009, 11, 1341–1344 CrossRef CAS.
- N. Hama, T. Matsuda, T. Sato and N. Chida, Org. Lett., 2009, 11, 2687–2690 CrossRef CAS.
- T. Yoshimitsu, T. Ino and T. Tanaka, Org. Lett., 2008, 10, 5457–5460 CrossRef CAS.
- T. Yoshimitsu, T. Ino, N. Futamura, T. Kamon and T. Tanaka, Org. Lett., 2009, 11, 3402–3405 CrossRef CAS.
- P. M. Wehn and J. Du Bois, Angew. Chem., Int. Ed., 2009, 48, 3802–3805 CrossRef CAS.
- P. Andrade, R. Willoughby, S. A. Pomponi and R. G. Kerr, Tetrahedron Lett., 1999, 40, 4775–4778 CrossRef CAS.
- A. Al Mourabit and P. Potier, Eur. J. Org. Chem., 2001, 237–243 CrossRef CAS.
- E. J. Corey and X. Cheng, The Logic of Chemical Synthesis, John Wiley & Sons, 1995, ch. 1, pp. 1–16 Search PubMed.
- Alternative timing for the N1-methylation has been considered and may occur at any point up to pre-agelastatin A (16) for our biosynthetic hypothesis for (−)-1.
- E. Fattorusso and O. Taglialatela-Scafati, Tetrahedron Lett., 2000, 41, 9917–9922 CrossRef CAS.
- C. Pöverlein, G. Breckle and T. Lindel, Org. Lett., 2006, 8, 819–821 CrossRef.
- Pyrrole (+)-22 was synthesized in one step from commercial D-aspartic acid dimethylester in 84% (99% ee) on a >15 g scale. See the ESI† for details. Also see: C. W. Jefford, F. de Villedon de Naide and K. Sienkiewicz, Tetrahedron: Asymmetry, 1996, 7, 1069–1076 Search PubMed.
- Treatment of (+)-24 with methanol-d4 for 2 h resulted in the formation of imide 25 in 99% yield with 99% deuterium incorporation at C7 and complete loss of optical activity.
- S. I. Zav'yalov, G. I. Ezhova, N. E. Kravchenko, L. B. Kulikova, O. V. Dorofeeva, E. E. Rumyantseva and A. G. Zavozin, Pharm. Chem. J., 2004, 38, 256–260 Search PubMed.
- T. Hassel and D. Seebach, Helv. Chim. Acta, 1978, 61, 2237–2240 CrossRef CAS.
- P. Beak, W. J. Zajdel and D. B. Reitz, Chem. Rev., 1984, 84, 471–523 CrossRef CAS.
- W. H. Pearson, A. C. Lindbeck and J. W. Kampf, J. Am. Chem. Soc., 1993, 115, 2622–2636 CrossRef CAS.
- R. Wittenberg, J. Srogl, M. Egi and L. S. Liebeskind, Org. Lett., 2003, 5, 3033–3035 CrossRef CAS.
- For a review, see: T. Fukuyama and H. Tokuyama, Aldrichimica Acta, 2004, 37, 87–96 Search PubMed.
- For a recent review, see: H. Prokopcová and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 2276–2286 Search PubMed.
- Structural parameters for (−)-1, (−)-2, (−)-4, (+)-19, (+)-21, and (+)-26 are freely available from the Cambridge Crystallographic Data Center under CCDC 775302, 775303, 775304, 775306, 775305, and 775307, respectively.
- Stannyltriazone 27 was prepared in two steps from commercial material on a >10 g scale via lithiation of the corresponding triazone followed by trapping with tricyclohexylstannyl chloride.
- S. Knapp, J. J. Hale, M. Bastos and F. S. Gibson, Tetrahedron Lett., 1990, 31, 2109–2112 CrossRef CAS.
- S. D. Knight, L. E. Overman and G. Pairaudeau, J. Am. Chem. Soc., 1993, 115, 9293–9294 CrossRef CAS.
- W. Marckwald, Ber. Dtsch. Chem. Ges., 1892, 25, 2354–2373 CrossRef.
- R. Duschinsky and L. A. Dolan, J. Am. Chem. Soc., 1946, 68, 2350–2355 CrossRef.
- J. E. Baldwin and M. J. Lusch, Tetrahedron, 1982, 38, 2939–2947 CrossRef CAS.
- J. C. Gramain and R. Remuson, Heterocycles, 1989, 29, 1263–1273 CrossRef CAS.
- H. Heaney and M. O. Taha, Tetrahedron Lett., 1998, 39, 3341–3344 CrossRef CAS.
- For a previously described semi-synthesis of (−)-30 from (−)-5, see ref. 7. Notably, we observed that (−)-29 undergoes dehydration at a faster rater than (−)-5.
- Treatment of either (−)-di-epi-agelastatin C (31) or (−)-agelastatin C (3) with MeSO3H (10 equiv) in D2O at 100 °C for 3 d, afforded a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equilibrium mixture of (−)-di-epi-agelastatin C (31) and (−)-agelastatin C (3) with quantitative deuterium incorporation at the C6-methylene as indicated by 1H NMR analysis. See ESI† for details.
1 equilibrium mixture of (−)-di-epi-agelastatin C (31) and (−)-agelastatin C (3) with quantitative deuterium incorporation at the C6-methylene as indicated by 1H NMR analysis. See ESI† for details. - Direct comparison of the rates of deuterium incorporation between (+)-O-methyl-pre-agelastatin A (19) and (+)-O-methyl-pre-agelastatin D (35) revealed that (+)-19 incorporates deuterium at the C4-position 10 times faster than (+)-35.
- Resubmission of (−)-O-methyl-di-epi-agelastatin D (36) to MeSO3H in H2O at reflux for 25 h afforded (−)-agelastatin D (4) in 68% yield.
- C6–C7 bond fragmentation, requiring C5–C6 π-bond formation, is likely facilitated by diminished allylic strain imposed by the N1–H intermediate 36 compared to N1–Me derivative 17.
- 1H NMR monitoring of this reaction revealed the formation and slow consumption of 39. At lower temperature (60 °C), 39 was recovered from the reaction mixture; its resubmission to the cyclization conditions afforded 40 in 24% yield.
- Neither the optical rotation nor the 13C NMR spectrum of agelastatin D was obtained in the original isolation report as it was a minor component.
- The C8-hydroxy derivative of 41 accounted for approximately 20% of the mass balance after 20 min. Prolonged exposure of 41, the C8-hydroxy derivative of 41, or 42 to the reaction conditions did not afford the desired cyclization.
- The opposite C7-stereochemistry of (−)-cyclooroidin (10) compared to that of the agelastatins entreats the possibility that downstream biosynthetic enzymes may preferentially bind and consume derivatives of ent-cyclooroidin for the synthesis of the agelastatins.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectral data. CCDC 775302–775307. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00351d |
| This journal is © The Royal Society of Chemistry 2010 |
