Preparing water-dispersed palladium nanoparticles via polyelectrolyte nanoreactors†
Matthew M.
Coulter
a,
Jose Amado
Dinglasan
b,
Jane B.
Goh
ab,
Sreekumari
Nair
b,
Darren J.
Anderson
*b and
Vy M.
Dong
*a
aDepartment of Chemistry, University of Toronto, 80 St. George Street, Toronto, Canada. E-mail: vdong@chem.utoronto.ca; Tel: +416 978 0364
bVive Nano Incorporated, 700 Bay Street, Suite 1100, Toronto, Canada. E-mail: danderson@vivenano.com; Fax: +416 260 8839; Tel: +416 260 8889
First published on 22nd October 2010
Abstract
A counterion-induced polyelectrolyte collapse strategy has been developed for the preparation of poly(acrylic acid) stabilized palladium nanoparticles (NP-Pd-PAA). The structure and composition of the nanoparticles were investigated via transmission electron microscopy, dynamic light scattering, powder X-ray diffraction, and X-ray photoelectron spectroscopy. NP-Pd-PAA catalyzed fully aqueous Suzuki coupling reactions at loadings as low as 0.01 mol% Pd.
Introduction
Inorganic metal nanoparticles have a variety of uses in catalysis, electronics and chemical sensing.1 As academic and commercial demands for nanoparticles grow, the development of versatile, cost-effective, and scalable nanoparticle syntheses becomes increasingly important. Many syntheses incorporate components such as polymers, micelles or surfactants, which contribute to the stability of the nanoparticles.2 Polyelectrolytes are a class of readily available polymers that contain ionizable functional groups such as carboxylic acids or amines. These polymers can undergo a counterion-induced coil-to-globule transition—a unique property that we have recently begun exploiting for nanoparticle synthesis.3,4 This method has allowed for the aqueous preparation of a wide variety of nanomaterials.We became interested in applying this strategy to synthesizing nanoparticles of catalytically important metals and testing them in synthetic applications. Herein, we disclose a novel synthesis of palladium nanoparticles via polyelectrolyte nanoreactors, which are generated by a counterion-induced coil-to-globule transition. Our strategy is an aqueous, scalable and one-pot preparation of Pd nanoparticles from inexpensive polyelectrolyte precursors. Moreover, we demonstrate the use of these Pd nanoparticles for achieving Suzuki cross-couplings under fully aqueous conditions.
Results and discussion
Synthesis and characterization of palladium nanoparticles
Our proposed Pd nanoparticle synthesis is based on the polyelectrolyte collapse strategy outlined in Fig. 1. The conformation of a polyelectrolyte in an aqueous solution is determined by the interactions of charges on the chain. Highly ionized polyelectrolyte chains assume extended, swollen-coil conformations in solutions of low ionic strength due to the repulsive interactions between their charged moieties. Upon addition of ionic species, such as inorganic metal salts, these repulsive interactions are screened, inducing a coil-to-globule transition.5,6 The globules, which contain the counterions necessary for collapse, can thus be used as nanoreactors for the preparation of metal nanoparticles.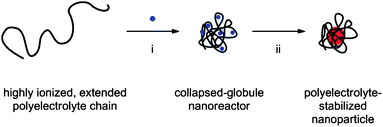 | ||
| Fig. 1 Synthesis of nanoparticles via counterion-induced polyelectrolyte collapse: (i) A solution of metal salt precursor (blue dot) is added to a solution of extended polyelectrolyte to induce a coil-to-globule transition. (ii) The collapsed nanoreactor is subjected to conditions for inorganic metal nanoparticle formation. | ||
Poly(acrylic acid) (PAA) stabilized Pd nanoparticles (NP-Pd-PAA hereafter) were synthesized according to the polyelectrolyte collapse strategy. To a partially ionized solution of PAA at pH 6.8 was added an aqueous solution of PdCl2, which had been acidified with HCl and adjusted to pH 6.4 (referred to as the PdII precursor solution; see the Experimental section for details). The transition from extended-chain to collapsed-globule was accompanied by a sharp decrease in the viscosity of the solution. UV-vis absorption spectroscopy revealed loss of the absorbance peak at ∼460 nm of the PdII precursor solution upon complexation to poly(acrylic acid). Subsequent reduction of PdII in the collapsed nanoreactor with NaBH4 generated a black solution with a featureless UV-vis spectrum containing the water-dispersed Pd nanoparticles (Fig. 2a). The nanoparticles were then exposed to UV radiation to effect cross-linking of the polymer chains. The collapse of the polyelectrolyte is clearly demonstrated by the decrease in the viscosity of the solution from 9.0 cP (PAA at pH 6.8) to 0.9 cP (NP-Pd-PAA).
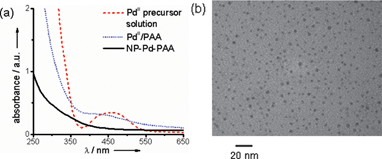 | ||
| Fig. 2 (a) UV-vis absorbance spectra of PdII precursor solution, PdII complexed to collapsed PAA, and NP-Pd-PAA after NaBH4 reduction. (b) TEM image of NP-Pd-PAA. | ||
It is well known that anions, derived from metal salt precursors and other synthetic reagents, can contribute to the stabilization of nanoparticles.7 The NP-Pd-PAA solution was found to contain approximately 6 chloride anions per Pd, which are likely significant contributors to the stability of NP-Pd-PAA in solution. A significant amount of boron, derived from the excess NaBH4 used in the nanoparticle preparation, and sodium was also incorporated into NP-Pd-PAA [see Electronic Supplementary Information† for full composition analysis].
Attempts at nanoparticle formation using the same procedure described above, but in the absence of a polyelectrolyte, led only to precipitation of the palladium. By contrast, NP-Pd-PAA is bench stable, showing no signs of aggregation or precipitation in aqueous solution over a period of more than 12 months. NP-Pd-PAA can be precipitated using 3 M NaCl in ethanol, stored, and completely re-dispersed. This synthesis has been performed reproducibly on multigram scales.
Transmission electron microscopy (TEM) analysis revealed that the Pd nanoparticles had an average size of 5.0 nm ± 1.5 nm (Fig. 2b).8 To investigate the in situ state of NP-Pd-PAA in aqueous solution, dynamic light scattering (DLS) measurements were performed.9 These confirmed that the nanoparticles were unaggregated in solution, and had a size range consistent with TEM analysis (Fig. 3).
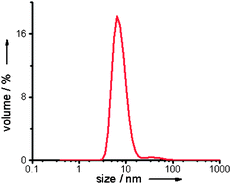 | ||
| Fig. 3 DLS measurement of NP-Pd-PAA. | ||
The nanoparticles were further analyzed by powder X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) measurements. The XRD pattern allowed indexing to the (111), (200), (220) and (311) planes of face-centered-cubic Pd (Fig. 4).10 XPS analysis revealed the presence of both Pd0 and PdII phases [see Electronic Supplementary Information† for XPS spectrum]. A partially oxidized palladium nanoparticle surface could thus serve as an additional source of stabilization.7
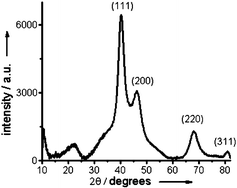 | ||
| Fig. 4 Powder X-ray diffraction (XRD) pattern of NP-Pd-PAA. | ||
Suzuki coupling reactions
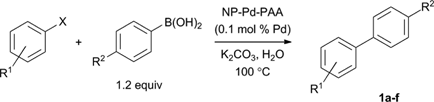
Synthetic chemists seek green coupling conditions that minimize the use of palladium, avoid phosphine ligands, and employ water as an innocuous and abundant solvent.11 In addition, emerging biology-inspired technologies, such as DNA-encoded chemical libraries, rely on aqueous transformations for chemical diversification.12 As such, we tested our NP-Pd-PAA as a catalyst for the Suzuki cross-coupling between aryl halides and arylboronic acids, in water.13 In the presence of 0.1 mol% of Pd, cross-coupling of 4′-bromoacetophenone and phenylboronic acid produced 4-acetylbiphenyl 1a in 99% isolated yield under fully aqueous conditions (Table 1, entry 1).14,15 Excellent yield of 1a could still be obtained with a reduced 0.01 mol% Pd catalyst loading (92%, entry 2).16 A lower temperature of 60 °C was also effective, but a longer reaction time was required to obtain a similar yield (98%, entry 3).|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | X | R2 | Atmb | Time | Product | Yield (%)c |
| a Reaction conditions: aryl halide (1.0 equiv), arylboronic acid (1.2 equiv), K2CO3 (1.5 equiv), NP-Pd-PAA (0.1 mol% Pd loading), 100 °C, H2O. b Atmosphere under which the reaction was performed. c Isolated yields. d 0.01 mol% Pd used. e Reaction temperature of 60 °C. f 2.0 equiv of 4-methoxyphenylboronic acid used. g Conversion based on 1H NMR analysis of crude reaction mixture. h Reaction run with 1.5 equiv of phenylboronic acid and 1 equiv tetrabutylammonium bromide. | |||||||
| 1 | 4-Ac | Br | H | Air | 20 min | 1a | 99 |
| 2d | 4-Ac | Br | H | Air | 1.5 h | 1a | 92 |
| 3e | 4-Ac | Br | H | Air | 8 h | 1a | 98 |
| 4 | 4-Ac | Br | Cl | Air | 20 min | 1b | 96 |
| 5 | 4-Ac | Br | OMe | Air | 20 min | 1c | 99 |
| 6 | 4-OMe | Br | H | Ar | 24 h | 1d | 77 |
| 7 | 4-OMe | I | H | Ar | 4 h | 1d | 89 |
| 8 | H | I | OMe | Ar | 8 h | 1d | 73 |
| 9f | H | I | OMe | Ar | 4 h | 1d | 93 |
| 10 | 4-Me | I | H | Ar | 4 h | 1e | 65 |
| 11 | 2-Ac | Br | H | Ar | 24 h | 1f | <5g |
| 12h | 2-Ac | Br | H | Ar | 24 h | 1f | 85 |
4′-Bromoacetophenone could be coupled with other phenylboronic acids to afford 4,4′-disubstituted biaryls 1b and 1c in high yields (96 and 99%, entries 4 and 5). Coupling between electron-rich 4-bromoanisole and phenylboronic acid required a longer reaction time of 24 h and protection under argon to obtain a good yield of product 1d (77%, entry 6). This is consistent with the reduced ability of more electron-rich aryl halides to undergo oxidative addition to Pd0. By substituting 4-bromoanisole with the more reactive 4-iodoanisole, 1d could be obtained in a higher yield of 89% and in a shorter reaction time of 4 h (entry 7). Iodobenzene underwent Suzuki coupling with 4-methoxyphenylboronic acid to give 1d in 73% yield (entry 8). By increasing the number of equivalents of 4-methoxyphenylboronic acid from 1.2 to 2.0, product 1d could be obtained in an improved 93% yield (entry 9). 4-Iodotoluene underwent reaction with phenylboronic acid to give coupled product 1e in a modest 65% yield (entry 10).
Ortho-substitution was not well-tolerated, as 2′-bromoacetophenone provided only trace conversion to 1f (entry 11). A good yield of 1f could, however, be obtained via the addition of 1 equivalent of tetrabutylammonium bromide (TBAB), a well-known promoter of Pd-catalyzed cross-coupling reactions (85%, entry 12). The ability of tetrabutylammonium salts to promote cross-couplings is thought to arise from a number of effects.17,18 The exact role of TBAB in this system is as of yet unclear, but this example demonstrates that in combination with a common promoter, otherwise challenging substrates can undergo coupling in the presence of NP-Pd-PAA. No change in the catalytic activity of NP-Pd-PAA was observed over a three month period.
Experimental section
Synthesis of palladium nanoparticles
The carboxylate capped Pd nanoparticles used in this study were provided by Vive Nano, Inc. and were synthesized in aqueous solution from PdII collapsed high molecular weight poly(acrylic acid). 10 mL of PdCl2 solution was prepared by dissolving 22.5 mg PdCl2 in 10.5 mL of 50 mM HCl. This solution was then diluted to 25 mL with deionized water. The pH of this solution was then adjusted to exactly pH 6.4 with 0.5 M NaOH (as monitored by a pH meter). This solution was added dropwise to 25 mL aqueous poly(acrylic acid) solution (2 mg mL−1 in deionized water, 1.2 million MW, pH adjusted to 6.8) under vigorous stirring at a rate of 1 mL min−1. The resulting solution was brownish in color. 40 mg of solid sodium borohydride was then added to a vigorously stirred PdII/PAA solution. The resulting black solution was then exposed to four 254 nm UV lamps (Ushio G25T8) for approximately 5–6 h under continuous stirring. The nanoparticle solution was freeze-dried and reconstituted with deionized water at a concentration of 1.5 mg mL−1 (0.26 mg mL−1 Pd).Representative procedure for NP-Pd-PAA catalyzed Suzuki coupling
0.2 mmol (1.0 equiv) aryl halide, 0.24 mmol (1.2 equiv) arylboronic acid, and 0.3 mmol (1.5 equiv) potassium carbonate were added to a vial equipped with a magnetic stirbar. 920 μL of deionized water and 81 μL of a dispersion of NP-Pd-PAA (0.1 mol% palladium loading) was added via pipette. The vial was sealed with a Teflon-lined screw-cap and heated at 100 °C for the indicated period of time. The reaction mixture was then diluted with water and extracted three times with ethyl acetate. The combined organics were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated to dryness in vacuo. The desired product was isolated by preparative thin layer chromatography.Conclusions
In summary, we have prepared palladium nanoparticles via a novel polyelectrolyte collapse strategy and demonstrated their application in low-loading and fully aqueous Suzuki couplings. The polyelectrolyte collapse strategy provides a simple, scalable and aqueous method for nanoparticle synthesis, and it is thus promising that it is applicable to the preparation of catalytically active materials. The Suzuki coupling was targeted for initial studies because of its industrial and academic importance.13 Although applied to the synthesis of poly(acrylic acid) stabilized palladium nanoparticles, counterion-induced collapse is transferable to the synthesis of a number of polyelectrolyte/metal nanocomposites.3a Subsequent studies will focus on investigating the catalytic properties of other polyelectrolyte/metal systems, the delineation of mechanistic details, and immobilization of the nanoparticles on solid supports.Acknowledgements
VMD would like to thank the University of Toronto, the National Science and Engineering Council of Canada (NSERC), Canada Foundation for Innovation, and Ontario Research Fund for funding. VMD is grateful to be an Alfred P. Sloan Fellow. Vive Nano would like to thank Sustainable Development Technology Canada and Ontario's Ministry of Research of Innovation for supporting our work on nanocatalysts.Notes and references
- (a) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS; (b) T. H. J. van Osch, J. Perelaer, A. W. M. de Laat and U. S. Schubert, Adv. Mater. (Weinheim, Ger.), 2008, 20, 343 CrossRef CAS; (c) Z. Wang and L. Ma, Coord. Chem. Rev., 2009, 253, 1607 CrossRef CAS.
- (a) J. D. Aiken III and R. G. Finke, J. Mol. Catal. A: Chem., 1999, 145, 1 CrossRef; (b) B. L. Cushing, V. L. Kolesnichenko and C. J. O'Connor, Chem. Rev., 2004, 104, 3893 CrossRef CAS; (c) J. P. Wilcoxon and B. L. Abrams, Chem. Soc. Rev., 2006, 35, 1162 RSC; (d) L. Durán Pachón and G. Rothenberg, Appl. Organomet. Chem., 2008, 22, 288 CrossRef , and references therein.
- (a) US Pat., 7 645 318, 2010; US Patent Application, Publication No. US 2009/0148703, Application No. 12/116,869; (b) One of our groups reported the effects of annealing on poly(acrylic acid) capped CdTe nanoparticles, see: S. A. Rutledge, A. A. Farah, J. Dinglasan, D. J. Anderson, A. Das, J. Goh, C. Goh and A. S. Helmy, J. Phys. Chem. C, 2009, 113, 20208 Search PubMed.
- For some examples of transition-metal nanoparticle preparations that employ polyelectrolytes, see: (a) Y. Yonezawa, T. Sato, M. Ohno and H. Hada, J. Chem. Soc., Faraday Trans. 1, 1987, 83, 1559 RSC; (b) A. Henglein, B. G. Ershov and M. Malow, J. Phys. Chem., 1995, 99, 14129 CrossRef CAS; (c) A. B. R. Mayer and J. E. Mark, J. Macromol. Sci., Part A: Pure Appl. Chem., 1997, 11, 2151 Search PubMed; (d) A. B. R. Mayer, J. E. Mark and S. H. Hausner, Angew. Makromol. Chem., 1998, 259, 45 CrossRef CAS; (e) M. M. Demir, M. A. Gulgun, Y. Z. Menceloglu, B. Erman, S. S. Abramchuk, E. E. Makhaeva, A. R. Khokhlov, V. G. Matveeva and M. G. Sulman, Macromolecules, 2004, 37, 1787 CrossRef CAS; (f) L. Gröschel, R. Haidar, A. Beyer, K.-H. Reichert and R. Schomäcker, Catal. Lett., 2004, 95, 67 CrossRef; (g) M. Ballauff, Prog. Polym. Sci., 2007, 32, 1135 CrossRef CAS; (h) Z. G. Estephan, L. Alawieh and L. I. Halaoui, J. Phys. Chem. C, 2007, 111, 8060 CrossRef CAS; (i) S. Bhattacharjee and M. L. Bruening, Langmuir, 2008, 24, 2916 CrossRef CAS; (j) E. Veletanlic and M. C. Goh, J. Phys. Chem. C, 2009, 113, 18020 CrossRef CAS.
- N. Volk, D. Vollmer, M. Schmidt, W. Oppermann and K. Huber, Adv. Polym. Sci., 2004, 166, 29 CAS.
- (a) T. Kitano, A. Taguchi, I. Noda and M. Nagasawa, Macromolecules, 1980, 13, 57 CrossRef CAS; (b) K. Huber, J. Phys. Chem., 1993, 97, 9825 CrossRef CAS; (c) V. O. Aseyev, J. Juhanoja, H. Tenhu and S. I. Klenin, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 3337 CrossRef CAS; (d) Y. Roiter, W. Jaeger and S. Minko, Polymer, 2006, 47, 2493 CrossRef CAS.
- L. S. Ott and R. G. Finke, Coord. Chem. Rev., 2007, 251, 1075 CrossRef CAS.
- The TEM image was obtained after phase-transfer of the nanoparticles into toluene. See Electronic Supplementary Information† for details and a larger TEM image.
- C. Ornelas, J. Ruiz, C. Belin and D. Astruc, J. Am. Chem. Soc., 2009, 131, 590 CrossRef CAS.
- International Center for Diffraction Data, PDF-2 Ref. No. 88–2335.
- (a) J. G. de Vries and A. H. M. de Vries, Eur. J. Org. Chem., 2003, 799 CrossRef CAS; (b) D. Astruc, Inorg. Chem., 2007, 46, 1884 CrossRef CAS; (c) S. Sawoo, D. Srimani, P. Dutta, R. Lahiri and A. Sarkar, Tetrahedron, 2009, 65, 4367 CrossRef CAS.
- M. A. Cark, R. A. Acharya, C. C. Arico-Muendel, S. L. Belyanskaya, D. R. Benjamin, N. R. Carlson, P. A. Centrella, C. H. Chiu, S. P. Creaser, J. W. Cuozzo, C. P. Davie, Y. Ding, G. J. Franklin, K. D. Franzen, M. L. Gefter, S. P. Hale, N. J. V. Hansen, D. I. Israel, J. Jiang, M. J. Kavarana, M. S. Kelley, C. S. Kollmann, F. Li, K. Lind, S. Mataruse, P. F. Medeiros, J. A. Messer, P. Myers, H. O'Keefe, M. C. Oliff, C. E. Rise, A. L. Satz, S. R. Skinner, J. L. Svendsen, L. Tang, K. van Vloten, R. W. Wagner, G. Yao, B. Zhao and B. A. Morgan, Nat. Chem. Biol., 2009, 5, 772 CrossRef.
- For reviews on Suzuki coupling, see: (a) A. Suzuki, J. Organomet. Chem., 1999, 576, 147 CrossRef CAS; (b) F.-X. Felpin, T. Ayad and S. Mitra, Eur. J. Org. Chem., 2006, 2679 CrossRef CAS.
- For Suzuki coupling reactions catalyzed by other polyelectrolyte stabilized Pd nanoparticle systems, see: (a) S. Proch, Y. Mei, J. M. Rivera Villanueva, Y. Lu, A. Karpov, M. Ballauff and R. Kempe, Adv. Synth. Catal., 2008, 350, 493 CrossRef CAS; (b) C. Ornelas, A. K. Diallo, J. Ruiz and D. Astruc, Adv. Synth. Catal., 2009, 351, 2147 CrossRef CAS.
- For examples of Pd nanoparticle catalyzed coupling reactions, including Suzuki reactions, see ref. 11 (b).
- de Vries applies the term “homeopathic dose” to ligandless Suzuki reactions employing catalyst loadings in this range, see ref. 11 (a).
- For discussions of the roles of quaternary ammonium salts in Pd-catalyzed cross-coupling reactions, see: (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS; (b) K. Kohler, W. Kleist and S. S. Prockl, Inorg. Chem., 2007, 46, 1876 CrossRef.
- For an example of tetrabutylammonium bromide and haloarene promoted change in PVP-stabilized Pd nanoparticle size and shape, see: A. Gniewek, A. M. Trzeciak, J. J. Ziółkowski, L. Kępiński, J. Wrzyszcz and W. Tylus, J. Catal., 2005, 229, 332 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details and characterization data. See DOI: 10.1039/c0sc00314j/ |
| This journal is © The Royal Society of Chemistry 2010 |