Controlled rotary motion of light-driven molecular motors assembled on a gold film†
Gregory T.
Carroll
a,
Michael M.
Pollard
b,
Richard
van Delden
a and
Ben L.
Feringa
*a
aCentre for Systems Chemistry, Stratingh Institute for Chemistry and Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, Groningen, 9747 AG, The Netherlands. E-mail: B.l.feringa@rug.nl
bDepartment of Chemistry, York University, 4700 Keele St., Toronto, Ontario, Canada M3J 1P3
First published on 24th May 2010
Abstract
Using circular dichroism (CD) spectroscopy, we show that light-driven rotary molecular motors based on overcrowded alkenes can function in a self-assembled monolayer on semi-transparent gold films.
Molecular motors are ubiquitous in the nanoscale machines of nature and play a key role in nearly every important biological process.1 A current challenge is to make use of nanoscale motions in synthetic systems to develop molecular machines.2–5 The majority of studies have focused on measurements in solution, however surface-mounted motors6,7 provide nanoscale rotary systems that are interfaced with the macroscopic world and offer the most potential for developing advanced molecular machinery. Pertinent applications include probes for microviscosity,8 signal processing,9–12 tunable dielectrics,13–15 directed nano- and microscale translational16–18 and rotational19 motion and possibly the exertion of controlled nanomechanical forces20 and ultimately nanopumps and valves. Moreover, such systems are fertile ground for uncovering new paradigms in molecular motion at interfaces. One of the most widely used strategies for assembling monolayers of oriented organic molecules on solid surfaces is the adsorption of thiols on metals.21 Rotary movement at macroscopic gold surfaces has previously been investigated with STM.22–25 The motion in the systems studied involved thermally driven rotation around thiol–gold bonds or carbon–carbon single bonds. Although important aspects of rotary motion at surfaces were uncovered, a key requirement to fulfil the criteria of a rotary motor and apply the rotary motion to ultimately perform work at the molecular level is control over the direction of rotation.
Among the few synthetic systems that have demonstrated controlled unidirectional rotary motion,26–29 photoactive overcrowded alkenes are the best-studied.30 Advantages of the motor-systems based on overcrowded alkenes include the range of synthetic protocols for introducing functionality into the structure of the motor-molecule (i.e. for surface attachment) and their activation with photons, which provides a clean and traceless fuel that can be delivered with spatial control. With the long-term aim of harnessing work from the collective action of nano-motors, the simplicity of the fabrication of monolayers from organic thiols self-assembled on gold make this a very attractive approach to construct arrays of light-powered rotors. Despite many studies and advances applied to these light-driven unidirectional rotary motors,4 their assembly and rotary function on macroscopic gold films has not been reported. Although these motors were previously shown to function while grafted to the surface of gold nanoparticles dispersed in solvent,31,32 the gold particles themselves were subject to Brownian motion, thus making the application of these motor molecules more difficult.
Here we report unidirectional rotary motion of light-driven rotary molecular motors in self-assembled monolayers (SAMs)33 on gold surfaces as shown conceptually in Fig. 1. We prepared “second-generation molecular motors”34 bearing thiols for self-assembly into monolayers on a semi-transparent gold film deposited on quartz. The use of a thin (5 nm) layer of gold minimized the optical absorbance of the metal layer so as to allow the characterization of the surface-bound chiroptical motors using circular dichroism (CD) spectroscopy, and to follow the photochemical and thermal steps in the rotary cycle. We found that when the molecule was linked to the surface by shorter tethers (8 atoms), the gold interfered with the motors' excited state processes, preventing any photoisomerization of the central overcrowded alkene. However, when longer tethers (16 atoms) were used, the molecules regained the ability to undergo photochemical isomerization and unidirectional rotation.
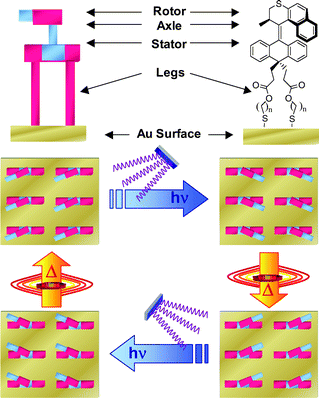 | ||
| Fig. 1 Self-assembly of light-driven molecular motors on a solid surface provides a monolayer of nanoscale motors. The four stages of the 360° rotary cycle can be addressed with light and heat.‡ | ||
The motors employed for assembly on thin gold films are based on the parent-overcrowded alkene 1 (Fig. 2), a recently designed light-driven molecular motor that undergoes unidirectional rotary motion upon irradiation with UV light.35 The stereogenic center is crucial in achieving unidirectional rotation. The methyl-substituent at the stereogenic center adopts a pseudoaxial conformation in the stable isomer, and a disfavoured pseudoequatorial conformation in the unstable isomer. The relative stabilities of the two isomers originate from differences in the degree of steric strain imposed by crowding of the methyl group with the adjacent aromatic ring in the lower-half. The configuration of the stereogenic center dictates the helicity of the chromophore, (P) or (M), in both the stable and unstable isomers. The R configuration of 1 will induce an M helicity in the stable isomer and a P helicity in the unstable isomer, whereas the S configuration of 1 induces a P helicity in the stable isomer and an M helicity in the unstable isomer. Consequently, the configuration at the stereogenic center controls the direction of rotation.
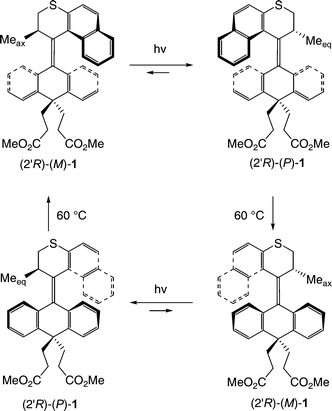 | ||
| Fig. 2 The rotary cycle of the parent motor 1 employed in surface attachment consists of four steps. A full cycle is achieved by a combination of light-driven and thermal helix inversion steps. | ||
In the first step of the rotary cycle (Fig. 2), absorption of a photon by (2′R)-(M)-1 results in an isomerization of the central overcrowded alkene to give unstable (2′R)-(P)-1. The photochemical isomerization leaves the molecule in a high-energy conformation of the opposite helicity wherein the methyl group at the stereogenic center is forced to adopt a pseudo-equatorial orientation, which results in unfavorable steric crowding between the methyl substituent and the adjacent aromatic moiety from the lower-half of the molecule. The strain imposed by steric crowding is relieved in a thermodynamically favorable helix inversion where both the methyl group and the naphthalene ring of the rotor moiety slip past the aromatic moieties of the lower-half stator, thus regenerating the stable conformation with a pseudoaxial methyl group. The large free energy gain from the thermal isomerization step provides an effectively irreversible conformational change that completes a 180° rotation of the upper-half rotor relative to the lower-half stator, regenerating (2′R)-(M)-1, and is the origin of the unidirectionality of the rotary cycle. Subsequent photo- and thermal isomerization steps complete the rotary cycle.
Parent motor 1 was selected because it has a large barrier to thermal helix inversion, making thermal isomerization at room temperature very slow and thus facilitating characterization of the separate states of the rotary cycle. Overcrowded alkene 1 was designed with two ester groups in the lower-half of the molecule to allow the incorporation of two linkers for its subsequent attachment to a surface. Both points of attachment to the surface are required to prevent uncontrolled thermal rotation of the entire system with respect to the surface, and thus transforming relative rotary motion of the two “halves” (rotor and stator) of the molecule into absolute rotary motion of the rotor relative to the stator. In order to enable self-assembly onto a gold surface, 1 was elaborated with tethers (or “legs”) that terminate with thiol moieties (Fig. 3). The diester 1 was saponified to give the corresponding diacid, which was in turn converted to the diacid chloride upon treatment with oxalyl chloride in benzene in the presence of a catalytic amount of DMF. The diacid chloride was then treated with the bromoalcohol (n = 1 or 9) to give the dibromide, which was then converted to the dithioester by displacement with potassium thioacetate in DMF. The molecules were resolved with chiral HPLC at this stage. The thioesters in the resulting enantiopure materials were then hydrolyzed under an inert atmosphere immediately prior to surface attachment by either treatment with piperidine in THF at 50 °C for 2 h or, by treatment for 20 min with MeOH that was pre-saturated with NH3 (g). Thioacetate-protected (2′R)-2 has a signal that is similar to structurally related molecules, while its enantiomer clearly displays a spectrum that is its mirror-image (Fig. 3).
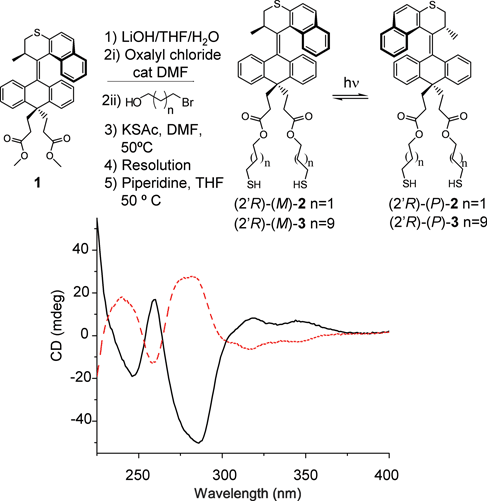 | ||
| Fig. 3 Modification of parent motor 1 with thiol-terminated linkers provides a precursor for the self-assembly of a monolayer of motors on a gold surface. The CD spectrum of (2R)-(M)-2 (black) in dodecane is shown. Irradiation produces the enantiomer (2′R)-(P)-2, which gives a CD spectrum (red) that is a pseudo-mirror image of the original due to incomplete conversion at the PSS. | ||
In order to study SAMs composed of 2 or 3 using CD spectroscopy, a semi-transparent gold film of approximately 5 nm was prepared (see ESI†).36,37 Quartz slides were cleaned with piranha§ and then treated with aminopropylmethyldiethoxysilane to generate an amine-functionalized surface that promotes adhesion of gold. The organic layer was used as the adhesive coating rather than chromium or titanium to maximize the optical transparency of the substrate. A film of approximately 5 nm of gold was deposited onto both sides of the substrate using vapor deposition. The resulting faint blue film was semi-transparent to light of both visible and UV wavelengths (see S1†) and could be handled without noticeable flaking. The absorption spectrum shows a broad band centered above 600 nm as previously reported and attributed to a localized gold surface plasmon.38 Atomic force microscopy (AFM) images show a typical island morphology (with diameters in the range of 50 nm), closely resembling previously reported films with a thickness of 5 nm (see S2†).38 The gold-coated substrates were immersed in a 10 mM solution of enantiomerically pure dithiol 2 or 3 in toluene for 15 h (Fig. 4). After removal the substrates were rinsed thoroughly and sonicated (3× 1–2 min) to remove any remaining material that was not bound to the gold surface. Diluted mixed monolayers containing enantiomerically pure dithiol 2 or 3 were prepared in an identical fashion in the presence of 10 equivalents of dodecanethiol. The CD spectra of the substrates clearly show the presence of the motor on the surface and that the coverage can be diluted by mixing with dodecanethiol (Fig. 5, S3†). The major absorption bands of the CD spectrum of the monolayer coincide with the major absorption bands observed for thioacetate-protected 3 in dodecane solution. When the enantiomer of the motor chromophore was assembled the resulting spectrum showed an approximate mirror image. CD spectra of materials in monolayers are in general rare, and are made possible here in part by the intense CD absorption of these helicene-like chromophores.35,39,40 Additionally, the IR spectrum contains a band at 1734 cm−1 corresponding to the carbonyl of the ester in the “legs” of the surface-bound motor (S4†).
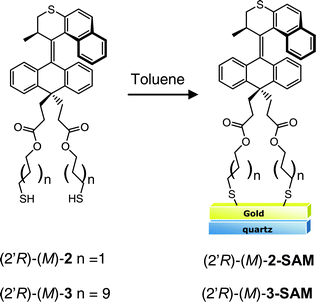 | ||
| Fig. 4 Chiral thiol-terminated molecular motors for self-assembly on gold. Note that the motor is depicted perpendicular to the surface for ease of presentation and not meant to indicate a completely perpendicular orientation. | ||
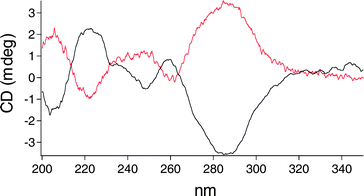 | ||
| Fig. 5 CD spectra of (2′R)-(P)-2-SAM (red) and (2′S)-(M)-3-SAM (black). | ||
Although the CD spectra indicate that the motor assembles on the gold film, photochemical and thermal isomerization processes of the rotary cycle cannot be assumed to work a priori. Irradiation of (2′S)-(P)-2-SAM (Hg lamp, λ > 280 nm or λmax = 365 nm) for 1 h under an inert N2 atmosphere generated no change in the CD spectrum of the sample. Note that irradiation of the SAMs in air was found to irreversibly diminish the intensity of the absorptions in the CD spectrum of the sample, implying that it had destroyed the motor-molecules. Previous studies on the photophysical behaviour of azobenzenes and stilbenes have shown that their photoisomerization was partially or completely suppressed in the solid state or when confined at interfaces.41–43 One study showed that although photoisomerization of azobenzenes was completely suppressed in densely packed monolayers, photoisomerization was restored in mixed monolayers comprising both an azobenzene-thiol and a ‘spacer’ thiol.42 The change in behavior was attributed to an additional ‘free-volume’ in the mixed monolayers that allowed the photoisomerization to occur. In an attempt to relieve steric congestion between the motor-molecules in the monolayer, mixed monolayers from a solution containing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dodecanethiol
1 dodecanethiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (2′S)-(P)-2 were prepared as noted above. Unfortunately, irradiation of the monolayer led to no changes in its CD spectrum, hinting that intermolecular crowding effects may not exclusively prevent photoisomerization. However, it cannot be assumed that the motor-molecules co-self-assemble with the dodecanethiol into a homogeneous monolayer rather phase-separate into domains, limiting the possible influence of mixing a second molecule into the layer.44 In any case, both surfaces showed no change under irradiation conditions that have been shown to produce geometric isomerization of the central overcrowded alkene in similar motors attached to gold nanoparticles31 or confined at the surface of silanated quartz.45,46 Assembly of 2 on thinner gold films (approximately 1.3 nm) gave monolayers that had a CD spectrum comprised of absorptions of both similar intensity and profile, suggesting that the available surface area for binding was unchanged. Unfortunately, this monolayer also remained unaffected by irradiation. In sharp contrast, preparation of gold nanoparticles protected with (2′R)-(M)-2 provided a surface-bound system that was able to undergo photoisomerization and thermal helix inversion as evident from the CD spectra corresponding to the four stages of the rotary cycle (S5†). The fact that 2 preserved its light-driven rotary cycle while grafted on the gold nanoparticles was encouraging because it showed that the parent motor was not intrinsically unable to function in the presence of a gold surface, although it was still unclear whether the malfunction was due to competing photophysical processes such as energy transfer, or to the rigidity of the monolayer.
(2′S)-(P)-2 were prepared as noted above. Unfortunately, irradiation of the monolayer led to no changes in its CD spectrum, hinting that intermolecular crowding effects may not exclusively prevent photoisomerization. However, it cannot be assumed that the motor-molecules co-self-assemble with the dodecanethiol into a homogeneous monolayer rather phase-separate into domains, limiting the possible influence of mixing a second molecule into the layer.44 In any case, both surfaces showed no change under irradiation conditions that have been shown to produce geometric isomerization of the central overcrowded alkene in similar motors attached to gold nanoparticles31 or confined at the surface of silanated quartz.45,46 Assembly of 2 on thinner gold films (approximately 1.3 nm) gave monolayers that had a CD spectrum comprised of absorptions of both similar intensity and profile, suggesting that the available surface area for binding was unchanged. Unfortunately, this monolayer also remained unaffected by irradiation. In sharp contrast, preparation of gold nanoparticles protected with (2′R)-(M)-2 provided a surface-bound system that was able to undergo photoisomerization and thermal helix inversion as evident from the CD spectra corresponding to the four stages of the rotary cycle (S5†). The fact that 2 preserved its light-driven rotary cycle while grafted on the gold nanoparticles was encouraging because it showed that the parent motor was not intrinsically unable to function in the presence of a gold surface, although it was still unclear whether the malfunction was due to competing photophysical processes such as energy transfer, or to the rigidity of the monolayer.
A plausible explanation for the absence of photochemical isomerization is energy transfer from the excited state of 2 to the gold substrate. Energy transfer from chromophores to nearby metal particles and films has been observed.47–52 For example, we showed that in a gold break junction reversibility of single molecule isomerization was blocked.53 Some reports have shown that photochemical and photophysical processes of chromophores are more severely quenched by gold films in comparison to gold nanoparticles. It has been reported that the quantum yield of trans–cis isomerization of azobenzenes was diminished by immobilization on gold colloids, and then further diminished by incorporation into SAMs on gold.42 Fluorescence from lissamine (a rhodamine dye) was visible when the dye was grafted to gold nanoparticles, yet completely quenched when grafted to gold films.52
Since the interaction of the gold layer with the excited state of the chromophores in the SAM should be distance dependent, increasing the length of the tethers of the chromophore to the metal surface should diminish the quenching, and restore the photoisomerization. We chose to explore (2′R)-(M)-3, which contains an additional 8 atoms of space between the motor and the surface (for synthesis and resolution see ESI†). CD analysis of a semi-transparent gold film after formation of a monolayer of (2′R)-(M)-3 showed a spectrum similar to (2′R)-(M)-2 (Fig. 6). Irradiation of the resulting SAM, (2′R)-(M)-3-SAM, with UV light (>365 nm) for 1 h under an inert N2 atmosphere gave inversion of the major absorptions in the CD spectrum (black to red, Fig. 6) analogous to the spectra obtained for the motor in solution and on Au nanoparticles, indicating successful completion of the first step of the rotary cycle: conversion of stable (2′R)-(M)-3-SAM to a monolayer comprised of predominantly unstable (2′R)-(P)-3-SAM. Moreover, subsequent heating of (2′R)-(P)-3-SAM at 70 °C for 2 h resulted in an almost complete regeneration of the original CD spectrum, which is consistent with a thermal induced helix inversion comprising a 180° rotation of the upper-half rotor relative to the lower-half stator, and hence, relative to the gold surface. A second irradiation (λmax = 365 nm) of the sample resulted in the inversion of the major bands in the CD spectrum of the monolayer. Heating the sample a second time regenerated the original CD spectrum completing a full 360° unidirectional rotation of the rotor relative to the gold surface. Similar results were obtained with a mixed monolayer prepared by the self-assembly of (2′R)-(M)-3 in the presence of 10 equivalents of 1-dodecanethiol (S6†). As before, the intensity of the absorptions in the CD spectrum of the mixed monolayer were weaker than was observed for (2′R)-(M)-3-SAM. Irradiation of the monolayer (>280 nm) required ∼1 h to reach its PSS, which is similar to what was observed for the dense SAM containing only motor 3 (S6†).
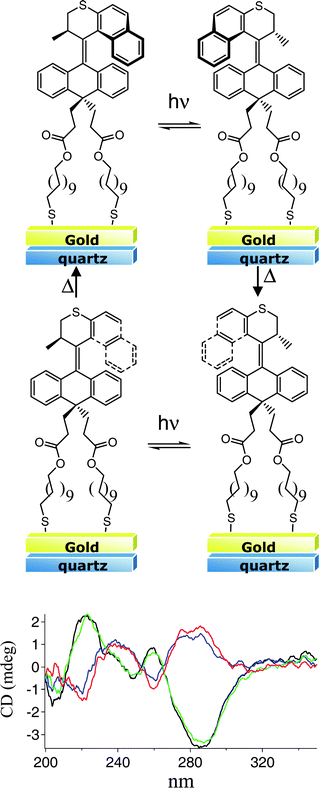 | ||
| Fig. 6 Irradiation of (2′R)-(M)-3-SAM results in a photochemical isomerization. The initial CD spectrum (black) shows an inversion of the signal as the enantiomer is generated (red). Thermal helix inversion restores the original spectrum (green). A second photochemical isomerization (blue) generates the second unstable enantiomer in the rotary cycle. Subsequent thermal helix inversion regenerates the original conformation of the motor. | ||
We have shown that self-assembly of motor-molecules 2 and 3 on semi-transparent gold surfaces allows the characterization and analysis of the substrate with CD spectroscopy, an invaluable technique in addressing the stages of the rotary cycle at the surface. Provided that the tethers between the chromophore and gold film are sufficiently long to minimize quenching of the excited state by the gold film, the molecular motor can undergo photochemically and thermally induced isomerizations that are consistent with a 360° rotary motion of the upper-half rotor relative to the gold surface. The ability to assemble functional unidirectional rotary motors on gold surfaces offers exciting opportunities in developing dynamic systems that allow for control of interfacial properties and movement by exploiting the collective motion of the nanomotors. The widespread availability and expertise in the preparation of SAMs on gold films and the ability to obtain atomically flat gold terraces expands the possible repertoire of experimental techniques to pattern, address, control and detect processes at the motor interface. The design parameters uncovered will be used to develop and assemble more advanced motors on metallic films.
Acknowledgements
Financial support from the Netherlands Foundation for Scientific Research (NWO-CW), ERC (advanced grant 227897) and NanoNed is gratefully acknowledged.Notes and references
-
D. S. Goodsell, The Machinery of Life, Springer, New York, 1994 Search PubMed
.
- P. Ball, Chem. World, 2010, 7, 56–60 Search PubMed
.
- V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348–3391 CrossRef CAS
.
- B. L. Feringa, J. Org. Chem., 2007, 72, 6635–6652 CrossRef CAS
.
- W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2006, 1, 25–35 CrossRef CAS
.
- V. Balzani, A. Credi and M. Venturi, ChemPhysChem, 2008, 9, 202–220 CrossRef
.
- J. Michl and E. C. H. Sykes, ACS Nano, 2009, 3, 1042–1048 CrossRef CAS
.
- M. A. Haidekker and E. A. Theodorakis, Org. Biomol. Chem., 2007, 5, 1669–1678 RSC
.
- J. J. de Jonge, M. A. Ratner, S. W. de Leeuw and R. O. Simonis, J. Phys. Chem. B, 2004, 108, 2666–2675 CrossRef CAS
.
- V. M. Rozenbaum, Phys. Rev. B, 1996, 53, 6240–6255 CrossRef CAS
.
- S. L. Gould, D. Tranchemontagne, O. M. Yaghi and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2008, 130, 3246 CrossRef CAS
.
- E. B. Winston, P. J. Lowell, J. Vacek, J. Chocholousova, J. Michl and J. C. Price, Phys. Chem. Chem. Phys., 2008, 10, 5188–5191 RSC
.
- R. D. Horansky, L. I. Clarke, E. B. Winston and J. C. Price, Phys. Rev. B, 2006, 74, 054306 CrossRef
.
- R. D. Horansky, L. I. Clarke, J. C. Price, T. A. V. Khuong, P. D. Jarowski and M. A. Garcia-Garibay, Phys. Rev. B, 2005, 72, 014302 CrossRef
.
- L. I. Clarke, D. Horinek, G. S. Kottas, N. Varaksa, T. F. Magnera, T. P. Hinderer, R. D. Horansky, J. Michl and J. C. Price, Nanotechnology, 2002, 13, 533–540 CrossRef CAS
.
- Y. Shirai, A. J. Osgood, Y. M. Zhao, K. F. Kelly and J. M. Tour, Nano Lett., 2005, 5, 2330–2334 CrossRef CAS
.
- A. V. Akimov, A. V. Nemukhin, A. A. Moskovsky, A. B. Kolomeisky and J. M. Tour, J. Chem. Theory Comput., 2008, 4, 652–656 CrossRef CAS
.
- S. Khatua, J. M. Guerrero, K. Claytor, G. Vives, A. B. Kolomeisky, J. M. Tour and S. Link, ACS Nano, 2009, 3, 351–356 CrossRef CAS
.
- R. Eelkema, M. M. Pollard, J. Vicario, N. Katsonis, B. S. Ramon, C. W. M. Bastiaansen, D. J. Broer and B. L. Feringa, Nature, 2006, 440, 163–163 CrossRef
.
- T. Hugel, N. B. Holland, A. Cattani, L. Moroder, M. Seitz and H. E. Gaub, Science, 2002, 296, 1103–1106 CrossRef
.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS
.
- X. L. Zheng, M. E. Mulcahy, D. Horinek, F. Galeotti, T. F. Magnera and J. Michl, J. Am. Chem. Soc., 2004, 126, 4540–4542 CrossRef CAS
.
- A. E. Baber, H. L. Tierney and E. C. H. Sykes, ACS Nano, 2008, 2, 2385–2391 CrossRef CAS
.
- P. Maksymovych, D. C. Sorescu, D. Dougherty and J. T. Yates, J. Phys. Chem. B, 2005, 109, 22463–22468 CrossRef CAS
.
- L. Gao, Q. Liu, Y. Y. Zhang, N. Jiang, H. G. Zhang, Z. H. Cheng, W. F. Qiu, S. X. Du, Y. Q. Liu, W. A. Hofer and H. J. Gao, Phys. Rev. Lett., 2008, 101, 197209-1–197209-4
.
- N. Koumura, R. W. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152–155 CrossRef CAS
.
- D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 174–179 CrossRef CAS
.
- S. P. Fletcher, F. Dumur, M. M. Pollard and B. L. Feringa, Science, 2005, 310, 80–82 CrossRef CAS
.
- S. Fournier-Bidoz, A. C. Arsenault, I. Manners and G. A. Ozin, Chem. Commun., 2005, 441–443 RSC
.
- M. M. Pollard, M. Klok, D. Pijper and B. L. Feringa, Adv. Funct. Mater., 2007, 17, 718–729 CrossRef CAS
.
- R. A. van Delden, M. K. J. ter Wiel, M. M. Pollard, J. Vicario, N. Koumura and B. L. Feringa, Nature, 2005, 437, 1337–1340 CrossRef CAS
.
- M. M. Pollard, M. K. J. ter Wiel, R. A. van Delden, J. Vicario, N. Koumura, C. R. van den Brom, A. Meetsma and B. L. Feringa, Chem.–Eur. J., 2008, 14, 11610–11622 CrossRef CAS
.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS
.
- N. Koumura, E. M. Geertsema, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2000, 122, 12005–12006 CrossRef CAS
.
- M. M. Pollard, M. Lubomska, P. Rudolf and B. L. Feringa, Angew. Chem., Int. Ed., 2007, 46, 1278–1280 CrossRef CAS
.
- P. A. Dimilla, J. P. Folkers, H. A. Biebuyck, R. Harter, G. P. Lopez and G. M. Whitesides, J. Am. Chem. Soc., 1994, 116, 2225–2226 CrossRef CAS
.
- M. Wanunu, A. Vaskevich and I. Rubinstein, J. Am. Chem. Soc., 2004, 126, 5569–5576 CrossRef CAS
.
- I. Doron-Mor, Z. Barkay, N. Filip-Granit, A. Vaskevich and I. Rubinstein, Chem. Mater., 2004, 16, 3476–3483 CrossRef CAS
.
- C. Nuckolls, T. J. Katz, T. Verbiest, S. Van Elshocht, H. G. Kuball, S. Kiesewalter, A. J. Lovinger and A. Persoons, J. Am. Chem. Soc., 1998, 120, 8656–8660 CrossRef CAS
.
- N. Keegan, N. G. Wright and J. H. Lakey, Angew. Chem., Int. Ed., 2005, 44, 4801–4804 CrossRef CAS
.
- F. H. Quina and D. G. Whitten, J. Am. Chem. Soc., 1975, 97, 1602–1603 CrossRef CAS
.
- S. D. Evans, S. R. Johnson, H. Ringsdorf, L. M. Williams and H. Wolf, Langmuir, 1998, 14, 6436–6440 CrossRef CAS
.
- A. Manna, P.-L. Chen, H. Akiyama, T.-X. Wei, K. Tamada and W. Knoll, Chem. Mater., 2003, 15, 20–28 CrossRef CAS
.
- S. J. Stranick, A.
N. Parikh, Y. T. Tao, D. L. Allara and P. S. Weiss, J. Phys. Chem., 1994, 98, 7636–7646 CrossRef CAS
.
- J. Areephong, W. R. Browne, N. Katsonis and B. L. Feringa, Chem. Commun., 2006, 3090–3092 RSC
.
- G. London, G. T. Carroll, T. F. Landaluce, M. M. Pollard, P. Rudolf and B. L. Feringa, Chem. Commun., 2009, 1712–1714 RSC
.
- A. Adams, R. W. Rendell, W. P. West, H. P. Broida, P. K. Hansma and H. Metiu, Phys. Rev. B, 1980, 21, 5565 CrossRef CAS
.
- P. Andrew and W. L. Barnes, Science, 2000, 290, 785–788 CrossRef CAS
.
- H. Imahori, H. Norieda, Y. Nishimura, I. Yamazaki, K. Higuchi, N. Kato, T. Motohiro, H. Yamada, K. Tamaki, M. Arimura and Y. Sakata, J. Phys. Chem. B, 2000, 104, 1253–1260 CrossRef CAS
.
- E. Dulkeith, A. C. Morteani, T. Niedereichholz, T. A. Klar, J. Feldmann, S. A. Levi, F. van Veggel, D. N. Reinhoudt, M. Moller and D. I. Gittins, Phys. Rev. Lett., 2002, 89, 203002-1–203002-4
.
- K. G. Thomas and P. V. Kamat, Acc. Chem. Res., 2003, 36, 888–898 CrossRef CAS
.
- T. Kudernac, S. J. van der Molen, B. J. van Wees and B. L. Feringa, Chem. Commun., 2006, 3597–3599 RSC
.
- D. Dulic, S. J. van der Molen, T. Kudernac, H. T. Jonkman, J. J. D. de Jong, T. N. Bowden, J. van Esch, B. L. Feringa and B. J. van Wees, Phys. Rev. Lett., 2003, 91, 207402 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation of surfaces, UV-Vis spectrum of semi-transparent Au, additional CD spectra, synthesis of motors and characterization including 1H and 13C NMR spectra. See DOI: 10.1039/c0sc00162g |
| ‡ We note that, while all the motor molecules rotate in one direction, the number of rotations performed by each may vary. |
§ Piranha is a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 sulfuric acid 3 sulfuric acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 solution. Caution must be used in preparing and handling as it reacts violently with organic material. Mixing is very exothermic and must be performed slowly. H2O2 solution. Caution must be used in preparing and handling as it reacts violently with organic material. Mixing is very exothermic and must be performed slowly. |
| This journal is © The Royal Society of Chemistry 2010 |
