Liquid phase separations by crystalline microporous coordination polymers
Katie A.
Cychosz
a,
Rashid
Ahmad
ab and
Adam J.
Matzger
*a
aDepartment of Chemistry and the Macromolecular Science and Engineering Program, University of Michigan, 930 North University Avenue, Ann Arbor, MI 48109-1055, USA. E-mail: matzger@umich.edu
bChemistry Division, Directorate of Science, PINSTECH, Nilore, Islamabad, Pakistan
First published on 17th June 2010
Abstract
Crystalline microporous coordination polymers (MCPs) are highly ordered, porous materials that have recently seen increasing attention in the literature. Whereas gas phase separations using MCPs have been extensively studied and reviewed, studies on applications in the liquid phase have lagged behind. This review details the work that has previously been reported on liquid phase separations using MCPs. Both enantioselective separations and separations of complex mixtures have been achieved using either adsorptive selectivities or size exclusion effects. Molecules that have been adsorbed include those as small as water to large organic dyes. In many cases, MCPs outperform their zeolite and activated carbon counterparts both kinetically and in efficiency of separation. The future outlook for the field is discussed in the context of current challenges in separations technologies.
 Katie Cychosz | Katie Cychosz received her BA degree in 2004 from Washington University in St. Louis. She is currently working toward her PhD at the University of Michigan with Adam J. Matzger, where she is studying the use of microporous coordination polymers as liquid phase adsorbents for complex systems including the desulfurization of transportation fuels. |
 Rashid Ahmad | Rashid Ahmad is working as Associate Professor of Chemistry, Department of Chemistry, Hazara University, Mansehra, Pakistan. Before joining Hazara University he was working as a Visiting Scientist in the Matzger Group, Department of Chemistry, University of Michigan. Previously, he worked in the Chemistry Division of PINSTECH, Pakistan, for ten years. In PINSTECH he contributed to the separation and pre-concentration of heavy metals from aqueous solutions using low cost sorbents. |
 Adam Matzger | Adam Matzger received his BA degree in 1992 from Oberlin College. His PhD was completed at the University of California at Berkeley in the group of K. Peter C. Vollhardt, where he conducted theoretical and experimental investigations of dehydrobenzoannulenes and phenylenes. He went on to postdoctoral work jointly with Nathan S. Lewis and Robert H. Grubbs at the California Institute of Technology, investigating a novel class of chemical sensors. In 2000, he joined the faculty at the University of Michigan at Ann Arbor, where he is now Professor of Chemistry and of Macromolecular Science and Engineering. His current research interests focus on organic materials in the solid state ranging from crystalline polymorphs to porous materials. |
Introduction
Adsorption represents a large and growing area of separations technologies impacting sectors ranging from pharmaceuticals and fine chemicals to the petrochemical industry. The market for sorbents is currently valued around $3 billion annually1 and virtually all of the sorbents used in these applications are from one of three general classes of materials: activated carbons (including carbon molecular sieves), zeolites (natural and synthetic), and metal/metalloid oxides including silica gel and activated alumina. Zeolites are crystalline materials with highly ordered pore structures whereas activated carbons, aluminas, and silicas are amorphous with variable pore size distributions. However, the key characteristic common to these materials is that all exhibit microporosity. A fourth category of adsorbents has the promise to revolutionize adsorption technologies; crystalline microporous coordination polymers (MCPs) are an exciting new class of highly ordered porous materials that combine the well-defined structural characteristics of zeolites with surface areas exceeding those of the best activated carbons.MCPs are referred to in the literature by a number of different names including metal–organic framework (MOF), porous coordination polymer (PCP), and porous coordination network (PCN), as well as by names which refer to the location where the material was originally synthesized such as Hong Kong University of Science and Technology (HKUST), Matériaux de l'Institut Lavoisier (MIL), Porphyrinic Illinois Zeolite Analogue (PIZA), and University of Michigan Crystalline Material (UMCM). These materials consist of metal ions or metal clusters assembled in a periodic fashion through organic ligands (referred to as linkers) resulting in an extended porous host structure. This style of assembly takes advantage of the directionality of the bonding between the metal atom and the linker and, in contrast to many zeolite preparations, avoids the need for a templating agent during synthesis. Like zeolites, very narrow pore size distributions can be obtained; however, this extremely high degree of control over pore size can be achieved over a much broader size range (3.5–34 Å are easily obtainable for MCPs). Compared to zeolites, metal oxides, or activated carbons, the dead volume or inaccessible space in MCPs is considerably diminished leading to the tremendous porosities and high surface areas observed. BET surface areas in excess of 5000 m2 g−1 and pore volumes reaching 2.3 cm3 g−1 have been obtained for MCPs.2 Furthermore, incorporation of functionality (e.g. halogen, nitrogen, sulfur, carboxy, cyano, nitro) on the organic linker, as well as the ability to select different metals, allows the electronic nature of the pore surface to be tuned, a feat very difficult to achieve in zeolites or activated carbons. Thus, the potential for using MCPs as sorbents for separations is considerable and indeed, as shown in Fig. 1, a range of molecules have been examined in the liquid phase. It is therefore surprising that as these novel sorbents head to market,3,4 the bulk of the investigations involve gas adsorption. This contrasts strongly with more established sorbents where applications to liquid phase separations are in fact more prominent. This chronology highlights the present progress in conducting liquid phase adsorptive separations with MCPs and prospects for future applications from an experimental perspective. Theoretical studies, although important in evaluation for future applications, are not covered.5,6
Liquid phase separations
The first reported use of MCPs as liquid phase adsorbents was the uptake of [Ru(2,2′-bipyridyl)3]Cl2 from methanol.7 Homochiral L-POST-1, which is a two-dimensional layer structure with chiral one-dimensional channels containing protonated pyridyl groups, was employed for the enantioselective adsorption of this racemic ruthenium complex. Analysis after adsorption revealed that 80% of the protons in the channels were exchanged with the metal complex and, additionally, 66% enantiomeric excess was obtained. This study marked the first instance of adsorption from the liquid phase and illustrates two concepts that are of considerable importance to the field: enantioselective separation and a chemical mechanism of adsorption.A hybrid organic–inorganic zeolite analogue composed of Cd2+ ions linked by quitenine was applied to the separation of racemic 2-butanol.8 When the MCP and 2-butanol were mixed under solvothermal conditions, only (S)-2-butanol was included, as verified using single crystal X-ray diffraction (Fig. 2). An enantiomeric excess of ∼98% was obtained. Racemic 2-methyl-1-butanol was also tested and again the S-isomer was selectively adsorbed demonstrating enantio-differentiation is possible with rather small molecules.
 | ||
| Fig. 2 Crystal structure of the cadmium hybrid inorganic–organic zeolite with (S)-2-butanol included in the pores (top) and a simplified picture of the framework showing the sites of the (S)-2-butanol in the chiral pores (bottom).8 Image copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. | ||
A third example of a chiral MCP capable of enantioselective adsorption is found in the separation of racemic trans-1,2-diaminocyclohexane using an ammonia-treated homochiral samarium bisphosphonate MCP.9 When employed in a chromatographic separation, enantio-enrichments of 13.6% S,S-1,2-diaminocyclohexane in the beginning fractions and 10.0% R,R-1,2-diaminocyclohexane in the ending fractions were observed. Although relatively modest enantioselectivities were obtained, this result, combined with the previous examples, indicates that with the design of new chiral MCPs, the potential for enantioselective separation is great and that industrially-relevant packed-bed adsorption is practical.
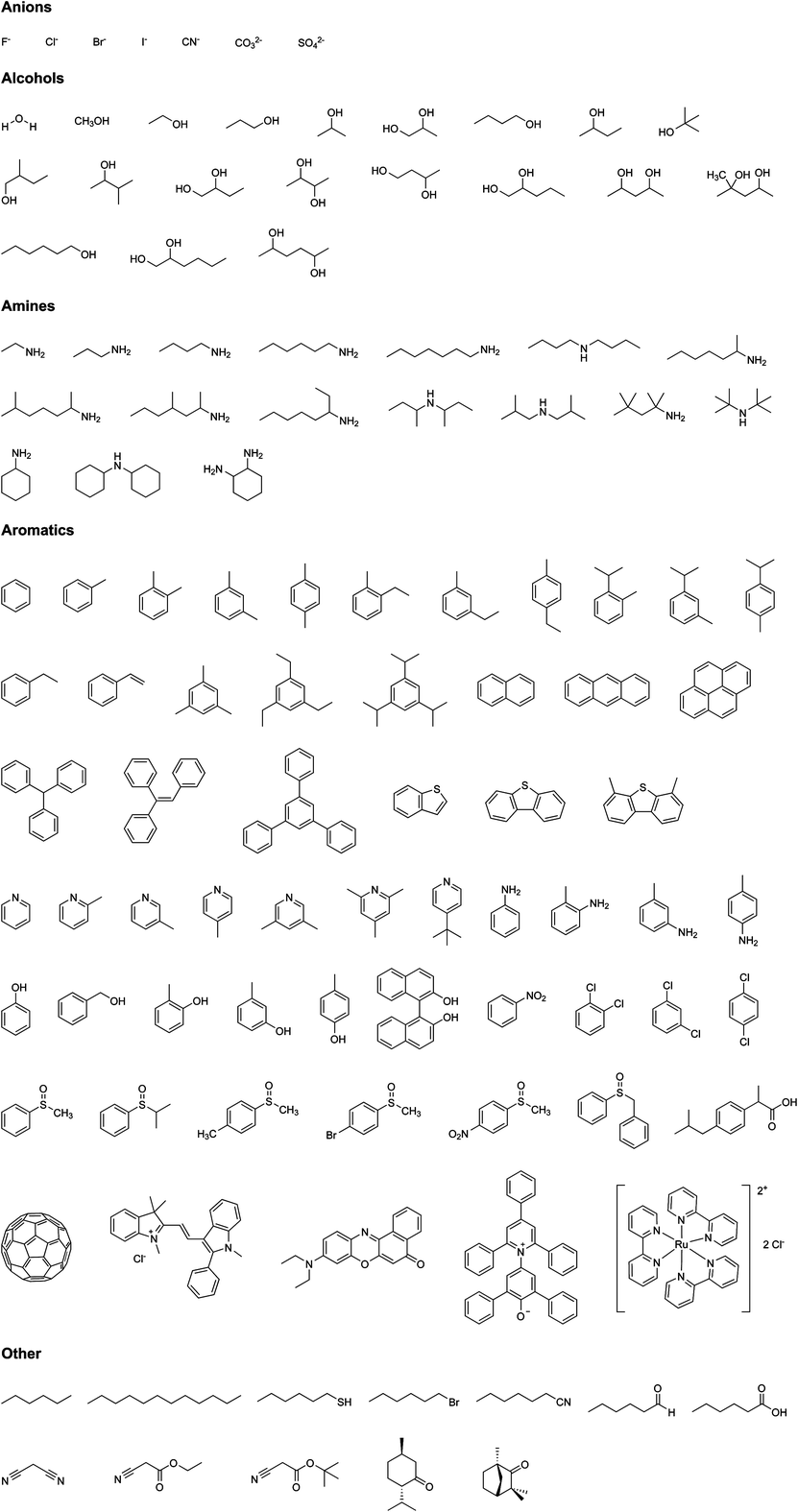
PIZA-1, which is comprised of ruffled cobalt(III) porphyrin cores connected by bridging trinuclear Co(II)-carboxylate clusters, demonstrated promise as a desiccant in the drying of the organic solvents benzene, toluene, and tetrahydrofuran.10 In comparison with zeolite 4A, PIZA-1 exhibited very good capacity and affinity for water and displayed rapid kinetics for the selective sorption of water from organic solvent. In fact, PIZA-1 acted as a better desiccant in one hour than zeolite 4A did in 24 h. Size and shape selectivity was also explored using a series of aromatic amines (to probe size selectivity) and both picolines and alcohols (to probe shape selectivity). In all cases, the smaller or less sterically bulky molecule was preferentially adsorbed (Fig. 3 shows all molecules tested). Similar behavior was observed in the related MCP PIZA-3, a manganese(III) porphyrin bridged by bent trinuclear manganese clusters.11 These studies revealed extraordinarily fast kinetics for guest inclusion and, indeed, the fast kinetics of guest diffusion into MCPs seems to be a general phenomenon that offers MCPs significant advantages over zeolites.4,12
 | ||
| Fig. 3 Analytes separated using PIZA-1. (a) Selective adsorption observed in alcohols, amines, and other functional groups. (b) Selective adsorption observed in linear and branched amines.10 Reprinted by permission from Macmillan Publishers Ltd: copyright 2002. | ||
Although PIZA-1 was found to preferentially adsorb polar solvents such as water, amines, and alcohols over n-hexane or other non-polar solvents, it was possible to “trick” PIZA-1 into adsorbing hexane from a pyridine–hexane mixture.10 After pyridine coordination with available metal sites in the structure, the sorption of the hydrophobic hexane guests inside the pores was observed. This is the first example of a post-synthetic chemical modification that alters the adsorption behavior of a material.
BOF-1, an MCP made of nickel macrocycles linked by 1,3,5-benzenetricarboxylate, was studied for the removal of methanol, ethanol, isopropanol, and benzyl alcohol from toluene.13,14 Benzyl alcohol had the largest binding constant of the molecules studied because of π–π interactions with the framework. However, BOF-1 had the largest molar capacity for methanol followed by ethanol, isopropanol, and benzyl alcohol in accord with a simple model of pore filling. In related work, a MCP composed of copper macrocycles and 1,3,5-benzenetricarboxylate was utilized for the removal of methanol, ethanol, and phenol from toluene.15 In a similar study, the separation of ethanol, phenol, pyridine, and benzene from isooctane was achieved using a structure consisting of nickel macrocycles linked by 2,2′-bipyridyl-5,5′-dicarboxylate.16 Ethanol and phenol were bound with the highest capacity and with the highest binding constant, indicating that this MCP favors adsorption of molecules that can form hydrogen bonds with the oxygen atoms exposed in the channels. These examples illustrate that designing a structure where specific functional groups decorate the channels is beneficial in order to favor adsorption of one organic molecule over another.17
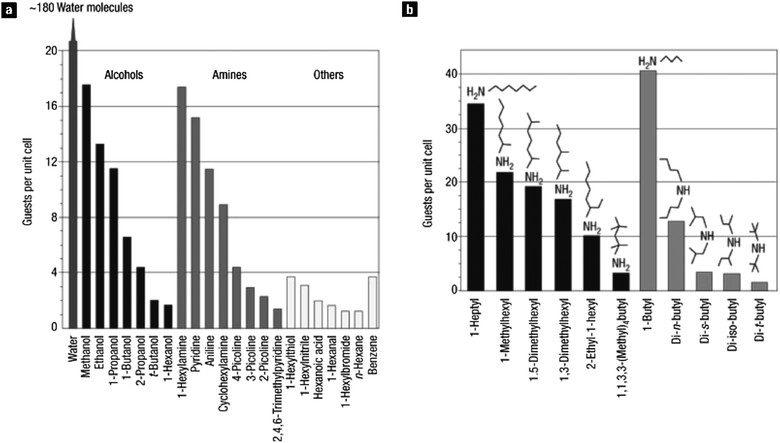
MOF-177, comprised of octahedral basic zinc carboxylate clusters linked by 1,3,5-(triscarboxyphenyl)benzene, was the first MCP to exceed the surface areas of the best activated carbons; moreover the extremely high surface area (4500 m2 g−1) of MOF-177 is accompanied by large pores leading to a pore volume of 1.59 cm3 g−1.18 The pore aperture is sufficient to allow free diffusion of C60 through macroscopic MOF-177 crystals as evidenced by Raman spectroscopy (Fig. 4a). To probe the limits of guest size inclusion, three polycyclic organic dyes: Astrazon Orange R, Nile Red, and Reichardt's dye in dichloromethane were adsorbed into MOF-177 (Fig. 4b). The uptake of Astrazon Orange R was more than 40 wt%, corresponding to 16 dye molecules in each unit cell. In the case of Nile Red, two molecules adsorbed in each unit cell. Due to the larger size of Reichardt's dye it was able to penetrate only the outer part of the crystal. These were the first demonstrations of the use of MCPs as sorbents for large molecules from solution and demonstrate the potential of this new class of materials for size selectivity in a regime not previously obtainable.
 | ||
| Fig. 4 (a) Inclusion of C60 in MOF-177 as evidenced by Raman spectroscopy of (A) bulk C60 (B) evacuated MOF-177 (C) a whole crystal impregnated with C60 and (D) a slice of the impregnated crystal. (b) Large organic dyes in MOF-177. The balls, with a diameter of 11 Å, fit into the pores of MOF-177.18 Reprinted by permission from Macmillan Publishers Ltd: copyright 2004. | ||
A chiral MCP (trinuclear nickel clusters linked by 1,3,5-benzenetricarboxylate templated by a bidentate resolved propan-1,2-diol bound to the metal center along with 3-picoline auxiliary ligands) was evaluated for the enantioselective sorption of the terpenes menthone and fenchone.19 Although menthone and fenchone were readily adsorbed, the enantiomers were not differentiated. However, in the case of binaphthol, a total uptake of 7.3 wt% was achieved with an enantiomeric excess of 8.3%. It was proposed that the terpenes, due to their smaller size, lack sufficient interaction with the internal helical surface for discrimination of the enantiomers whereas binaphthol has more appropriate dimensions. Again, these results demonstrate the potential for stereoselective adsorption from the liquid phase in MCPs and also illustrate that there are pore size and shape requirements that enhance the adsorption capacity and enantioselectivity of the MCP with the adsorbate.
A zinc MCP (octahedral basic zinc carboxylate clusters connected by 4,4′,4′′-tricarboxytriphenylamine) was able to selectively adsorb methanol, pyridine, dodecane, and benzene from isooctane.20 Methanol was adsorbed with the largest binding constant and highest capacity because, as previously described, there are hydrogen bonds that exist between methanol and the oxygen atoms exposed in the channels of the MCP. Large amounts of pyridine were also adsorbed and this was ascribed to favorable interactions between the benzyl groups in the structure and the guest, illustrating the range of noncovalent interactions that can be leveraged to increase binding affinity.
In another enantioselectivity study, a homochiral MCP was studied for the sorption of several substituted racemic thioether oxides.21 The MCP was made up of benzendicarboxylate and chiral lactate ligands coordinated to Zn2+ ions forming one dimensional chiral chains further linked by benzenedicarboxylate ligands to produce a three dimensional MCP. It was observed that the sulfoxides with smaller substituents such as H, Me and Br were adsorbed readily whereas the sulfoxides with larger substituents such as NO2 and CH2Ph were not able to diffuse into the MCP. This study reiterates two important points: MCPs have potential for the selective adsorption of enantiomers and size selective adsorption is possible with MCPs.
As described above, a cadmium MCP was used to successfully resolve enantiomers of 2-butanol and 2-methyl-1-butanol. In a follow up study, the same group synthesized a new copper MCP (copper linked by N,N′-(2-pyridyl-(4-pyridyl methyl)-amine)) which crystallizes in a chiral space group.22 This material was able to separate racemic 2-butanol, with the S-isomer being adsorbed in the structure with an ee of 30%. While this structure does not lead to the high enantiomeric excesses previously reported, it is perhaps the first example of achiral building blocks forming a chiral structure capable of resolving racemic mixtures.
Not only can neutral molecules be separated using MCPs, but anions have also been separated from water using a luminescent porous framework comprised of terbium metal centers linked by mucic acid.23 I−, Br−, Cl−, F−, CN−, and CO32− were separated from aqueous solutions as evidenced by luminescence enhancement of the framework. However, SO42− and PO42− were not adsorbed because they were too large to fit inside the pores of the MCP. Adsorption was attributed to strong hydrogen bonding interactions between the anions and the OH groups on the mucic acid organic linkers. This example shows that size selective adsorption is possible for anions and that an MCP can be designed to enhance interactions between anions and the framework.
Another possible application for liquid phase adsorption in MCPs is shown by the use of a new copper MCP for the detection and adsorption of aromatic molecules in water.24 The MCP used consisted of hexanuclear Cu6S6 clusters connected by 5,6-diphenyl-4,5-dihydro-1,2,4-triazine-3-thiolate and was used to adsorb toluene (0.5%) from aqueous solution. The adsorption of toluene in this structure quenches the luminescence present in the desolvated material and a guest:host ratio of 3 toluene molecules per Cu6S6 cluster was obtained for the completely toluene saturated solid. The effect of other aromatic compounds on the luminescence was also investigated and it was determined that quenching was not affected by the presence of electron donor or acceptor groups (nitrobenzene, aniline). Intensities did vary, however, when o-, m-, and p-xylene were adsorbed, with less quenching observed for m- and p-xylene because of weakened π–π stacking interactions. This study offers another example of a luminescent MCP being used for detecting and adsorbing industrially and environmentally significant analytes.
Yet another important liquid phase separation application is that of drug delivery. Ibuprofen was loaded into the MCPs MIL-100 (trimers of chromium linked by 1,3,5-benzenetricarboxylate25) and MIL-101 (trimers of chromium linked by 1,4-benzenedicarboxylate26) from hexane.27 The drastically different pore sizes of the two materials lead to the adsorption of different amounts of ibuprofen, where MIL-101 adsorbs ∼4 times the amount of MIL-100. It was determined using solid-state 1H-NMR spectroscopy that ibuprofen exists as the deprotonated form within the MCPs. Further study28 was carried out using MIL-53 (Fe and Cr), infinite chains of octahedra formed by coordination of Fe3+ or Cr3+ by terephthalate and OH−.29,30 In this case, ibuprofen was again loaded from hexane, but existed as a neutral molecule with strong interactions between ibuprofen and the CO2H/OH groups of the framework. These examples serve to illustrate the potential of MCPs for a biomedical application.
A neutral, chiral nickel MCP (layers of nickel ions linked by L- or D-aspartic acid connected by 4,4′-bipyridine ligands) was used for the enantioselective adsorption of a variety of racemic diols, and enantiomeric excess values varying from 1.5–54% were obtained.31 Particularly notable was the fact that diols with similar chain lengths but differing separation between the hydroxyl groups gave different levels of enantioselection. For example, 1,3-butanediol was separated with an ee of 18%, but 1,2-butanediol was separated with an ee of only 5% due to different hydrogen bonding interactions of the molecules with the framework. It was also noted that for applications such as chiral adsorption, a match between the size and shape of the chiral guest and the pore of the framework is necessary in order to separate the enantiomers.
Microwave-synthesized MIL-101 was employed for the removal of benzene from aqueous solution and compared to activated carbon.32 MIL-101 adsorbed a larger amount of benzene from a 1000 ppm solution than activated carbon. Additionally, the rate of benzene adsorption was faster in MIL-101 than in activated carbon due to the large pore diameter. This is an example where MCPs outperform a material that is often used in industry for this application and indicates that MCPs will be excellent alternatives to commonly used sorbents.
Solutions of malonitrile, ethyl cyanoacetate, and cyano-acetic acid tert-butyl ester in benzene were used to test the adsorption of these molecules into an MCP containing octahedral Cd(II) centers linked by 1,3,5-benzene tricarboxylic acid tris[N(4-pyridyl)amide].33 This MCP adsorbed 2.9, 0.7, and 0.6 molecular stoichiometric amounts of malonitrile, ethyl cyanoacetate, and cyano-acetic acid tert-butyl ester per ligand of MCP, as determined using 1H-NMR spectroscopy. Malonitrile, the smallest compound tested, was adsorbed in the largest amount due to its small size. This size selectivity has implications for catalysis, as these small molecules are used in Knoevenagel reactions and in this example, malonitrile would make a better reactant than the other molecules tested due to its ability to access the channels of the MCP.
The homochiral MCP composed of chiral lactate ligands coordinated to Zn2+ ions described above was further studied as the first example of a chiral stationary phase in a chromatographic separation of racemic alkyl aryl sulfoxides.34 Electron withdrawing substituents on the sulfoxides reduced both the sorption constant and the enantiomeric excess of the separated racemic mixture. Electron donating substituents led to higher sorption constants but lower selectivity factors due to steric reasons. Enantiomeric excesses were highest for those sulfoxides that had the most appropriate size to fit well into the pores of the MCP, again emphasizing the key interplay of guest and pore shape in achieving optimal results.
HKUST-1 (copper paddlewheel metal clusters linked by 1,3,5-benzenetricarboxylate) is one of the earliest examples of a highly porous MCP35 and is a compound recently commercialized by BASF. HKUST-1 was used for the investigation of factors controlling liquid phase separations.36 When the sorption of mesitylene, 1,3,5-triethylbenzene, 1,3,5-triisopropylbenzene, triphenylmethane, triphenylethylene, and pyrene from hexane was examined, only mesitylene and triphenylmethane, which are of an appropriate size and flexibility to penetrate the pores, were adsorbed. In a competitive adsorption experiment, the adsorption of pairs of m-, o- and p-dichlorobenzene from hexane was tested. HKUST-1 selectively discriminated between the dichlorobenzene isomers, with a selectivity factor of 9.0 in favor of p-dichlorobenzene over o-dichlorobenzene. It was concluded that molecular recognition might be due to the interaction of the free electron pair on the chlorine atom with the Cu2+ sites and/or between the aromatic rings of the adsorbate and the linker moieties of the adsorbent. Steric effects were also ascribed to play a role in the differing affinities. This shows that HKUST-1 can be used as a size and shape selective sorbent and is another early example of competitive adsorption experiments in an MCP. Zeolites are often used for the separation of xylene isomers and this preliminary study with a similar system, dichlorobenzene, indicates that MCPs have the potential for similar isomer selectivity and separation.
The competitive adsorption of the C8 alkyl aromatic compounds ethylbenzene and all three isomers of xylenes from hexane was explored for the MCPs HKUST-1, MIL-47 (infinite chains of octahedra formed by coordination of V4+ by terephthalate and O2− to produce a structure with one dimensional pores37), and MIL-53(Al) (infinite chains of octahedra formed by coordination of Al3+ by terephthalate and OH− groups to produce a structure with one dimensional pores38) to probe the potential for isomer-selective adsorption.39 HKUST-1 was selective only for m-xylene over o-xylene. The other two sorbents were much better at separating the C8 alkyl aromatic compounds and had outstanding preferences for p-xylene over ethylbenzene. Unlike MIL-53, which did not discriminate between p- and m-xylene, MIL-47 was successful at separating these two isomers, with a selectivity of 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In pulse chromatographic experiments with MIL-47, separate peaks were obtained for ethylbenzene, m-xylene and p-xylene (Fig. 5). The selectivities in MIL-47 were attributed to a molecular packing effect inside the sterically confining environment of the pores. Rietveld refinement of powder X-ray diffraction patterns were used to locate the guests inside the pores of the framework and close π–π interactions between the p-xylene molecules were observed, suggesting that the selectivities in MIL-47 were not due to interactions with the framework, but rather were due to better packing of some isomers over others in the pores of the structure. This is the first example of an extremely industrially relevant separation application, as zeolites are often used for the separation of xylene isomers and these results illustrate that MIL-47 is also effective at separation of these isomers. The extraordinary properties of MIL-47 include its high uptake capacity for this application as well as its hydrophobic nature; both desirable properties for future selective sorbents.
1. In pulse chromatographic experiments with MIL-47, separate peaks were obtained for ethylbenzene, m-xylene and p-xylene (Fig. 5). The selectivities in MIL-47 were attributed to a molecular packing effect inside the sterically confining environment of the pores. Rietveld refinement of powder X-ray diffraction patterns were used to locate the guests inside the pores of the framework and close π–π interactions between the p-xylene molecules were observed, suggesting that the selectivities in MIL-47 were not due to interactions with the framework, but rather were due to better packing of some isomers over others in the pores of the structure. This is the first example of an extremely industrially relevant separation application, as zeolites are often used for the separation of xylene isomers and these results illustrate that MIL-47 is also effective at separation of these isomers. The extraordinary properties of MIL-47 include its high uptake capacity for this application as well as its hydrophobic nature; both desirable properties for future selective sorbents.
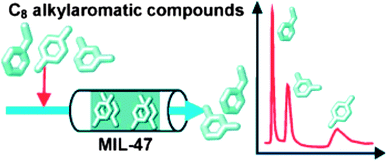 | ||
| Fig. 5 Schematic representation and experimental results of pulse chromatographic experiment using MIL-47 as a sorbent to separate ethylbenzene, m-xylene, and p-xylene.39 Image copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission. | ||
In a follow up study, the pore evacuation process of MIL-47 calcined at 573 K for different time intervals was studied in relation to the selective separation of p- and m-xylene.40 Maximal adsorption capacity of both isomers was achieved in the sample calcined for 21.5 h and the material was selective for the para isomer with selectivity ratios of 2 and 4.4 for concentrations of 0.02 and 0.14 M in hexane. It was also found that the presence of some residual terephthalic acid, incompletely removed post-synthesis, in the pores of MIL-47 slightly increased the selectivity between the two isomers. The higher selectivity is hypothesized to be due to one of two effects. First, MIL-47 is a flexible framework that has slightly different lattice parameters when incompletely evacuated which may lead to better packing of the para isomer. Alternatively, there is the possibility for specific interactions between xylene and the terephthalic acid guests in the pores. In either case, this indicates that MCP impregnants can be used to tune the selectivities of already existing MCPs to optimize the separation process. The investigation of selective batch adsorption of meta–para mixtures of disubstituted aromatics including ethyltoluene, dichlorobenzene, toluidine and cresol revealed that, like xylene, the para isomer of ethyltoluene and dichlorobenzene was adsorbed selectively. But this was not the case in toluidine and cresol, a result attributed to their hydrogen bond donor capabilities. This detailed study of MIL-47 reflects the large potential of MCPs for selective separation of isomeric mixtures that was previously possible exclusively with zeolites and demonstrates that other factors such as material activation are considerations when determining an optimal sorbent for an application.
In a similar study, the adsorption of C8 alkyl aromatic compounds from hexane on MIL-53, using batch and column adsorption techniques was explored and the results were compared with MIL-47.41 In batch adsorption the effective uptake of sorbates varied between 1 and 9 wt%. MIL-53 had a particular preference for the adsorption of o-xylene, but was not able to discriminate between m- and p-xylene and did not adsorb ethylbenzene. In the pulse chromatographic experiments ethylbenzene eluted first, m- and p-xylene came simultaneously and o-xylene appeared last. In breakthrough experiments, ethylbenzene breaks through after a very short time, then m-xylene, and, last, o-xylene. In competitive adsorption, the sorption capacity of o-xylene was 45 wt%, whereas for p-xylene it was around 5 wt%. MIL-53 was also used for the adsorption of ethyltoluene and cymene isomers and again adsorption of the ortho isomer was preferred. Rietveld refinement was employed to determine the location of the molecules within the MIL-53 pore (Fig. 6). It was found that the geometry of o-xylene allows for interaction between both methyl groups with the carboxylate groups in the structure, interactions which are not as prevalent for the other isomers, suggesting that this is the reason for the observed selectivities. In a comparison between MIL-47 and MIL-53 for adsorption of C8 alkyl aromatics, as mentioned earlier, MIL-47 preferred adsorption of p- and m-xylene over ethylbenzene and o-xylene and efficiently separated p- and m-xylene, in contrast to this study which showed that MIL-53 selectively adsorbs the ortho isomer and is unable to discriminate between the para and meta isomers. The two sorbents have almost identical pore topography indicating that the selectivities were instead due to the metals in the two sorbents leading to different polarization of the carboxylate groups. This leads to separation mechanisms for the two materials where, in MIL-47 efficient packing of the isomers within the pores leads to the observed selectivities whereas in MIL-53, interaction with the framework leads to the selectivity. The dissimilar selectivities for differing metal ions substituted in the same framework topology make it convenient to separate the isomer of interest from a mixture. This example illustrates how changing one of the building blocks of an MCP can drastically change adsorption capacities in the context of an industrially relevant separation example.
 | ||
| Fig. 6 Structures of (a) o-xylene (b) m-xylene (c) p-xylene and (d) o-cymene packed in the pores of MIL-53 as determined using Rietveld refinement.41 Reprinted with permission from the American Chemical Society. Copyright 2008. | ||
The highest enantiomeric excesses using MCPs to date were achieved with a zinc-metallosalen based material.42 A racemic mixture of 2-butanol was efficiently separated due to selective inclusion of the R-isomer in the pores. Analysis revealed that an ee value of 99.8% was obtained. The R enantiomer of racemic 3-methyl-2-butanol was also selectively included with an ee of 99.6%. When the MCP was recycled and used for a second separation, the ee decreased to 91.6%. This work shows that it is possible to design an MCP to almost perfectly resolve small racemic alcohols.
MCPs have also been used for the separation of anions from methanol.43 MOF-76, consisting of terbium linked by 1,3,5-benzenetricarboxylate,44 was immersed in methanol containing varying amounts of F−, Cl−, Br−, CO32−, or SO42−. Adsorption of the ions led to a luminescence enhancement of the framework and it was hypothesized that the anions form hydrogen bonding interactions with the framework, therefore immobilizing them in the pores. Because of the potential for chemical interactions between guest anions and MCPs, separation of anions from solution is yet another attractive application for MCPs.
MCPs have demonstrated size-selective molecular inclusion from extremely large molecules to those containing a single benzene ring; molecular sieving of smaller molecules has seen less success. The selective sorption of water over methanol from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binary mixture was reported for a cubic MCP comprised of copper paddlewheel metal clusters linked by R-2-methylglutarate and containing 4,4′-bipyridine pillars.45 The selectivity for the adsorption of water over methanol was attributed to the narrow pore size (2.8 × 3.6 Å) of the MCP which blocks the entrance of the comparatively larger methanol molecule into the 1D channel. This behavior contrasts with that of PIZA-1, described above, as in that case water adsorption is driven by chemical effects (metal binding) as opposed to size selective effects. This study demonstrates that good selectivity can be achieved with coordination polymers in the small molecule regime putting MCPs in direct competition with small pore zeolites.
1 binary mixture was reported for a cubic MCP comprised of copper paddlewheel metal clusters linked by R-2-methylglutarate and containing 4,4′-bipyridine pillars.45 The selectivity for the adsorption of water over methanol was attributed to the narrow pore size (2.8 × 3.6 Å) of the MCP which blocks the entrance of the comparatively larger methanol molecule into the 1D channel. This behavior contrasts with that of PIZA-1, described above, as in that case water adsorption is driven by chemical effects (metal binding) as opposed to size selective effects. This study demonstrates that good selectivity can be achieved with coordination polymers in the small molecule regime putting MCPs in direct competition with small pore zeolites.
Selective adsorption of amines from acetonitrile was performed using a zinc-1,3,5-benzenetricarboxylate MCP prepared using an ultrasonic method.46 Adsorption was monitored using the fluorescence of the MCP and significant quenching was observed with ethylamine; this effect was attributed to interactions between the amine and the coordinatively unsaturated metal centers in the structure. n-Propylamine, n-butylamine, and aniline were also tested. In these cases, the amine was too large to diffuse into the framework and little to no quenching was seen. Again, this illustrates the size selectivity of MCPs for the separation of amines and their potential use in chemical sensing.
The performance of five different MCPs: HKUST-1, UMCM-150 (consisting of copper paddlewheel and trinuclear copper metal clusters linked by the reduced symmetry linker 3,4′,5-biphenyltricarboxylate47), MOF-505 (copper paddlewheel metal clusters linked by 3,3′,5,5′-biphenyltetracarboxylate ligands48), MOF-5 (basic zinc carboxylate clusters connected by benzenedicarboxylate ligands to form a three dimensional cubic structure49) and MOF-177 were evaluated for the removal of benzothiophene (BT), dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (DMDBT) from the fuel surrogate isooctane.50 Large capacities were observed for all three of the organosulfur compounds studied including uptakes of 48 wt% and 27 wt% for DBT and DMDBT in UMCM-150. For three of the MCPs studied, higher capacities were observed for DMDBT over the smaller DBT and BT. Adsorption capacity was hypothesized to depend on the size and shape of the pore of the MCP, with higher capacities correlating to more interactions of the organosulfur compound with the framework (Fig. 7). Benchmarking of these materials against the zeolite, Na(Y), demonstrated the far superior capacity of the MCPs for this application.
 | ||
| Fig. 7 Crystal structures of (a) MOF-177 (b) MOF-5 (c) UMCM-150 (d) HKUST-1 and (e) MOF-505 shown on the same size scale with dibenzothiophene represented in each pore to indicate the remaining free space.50 Reprinted with permission from the American Chemical Society. Copyright 2008. | ||
In a further study, the same MCPs were applied in breakthrough experiments for the removal of organosulfur compounds from diesel fuel.51 Even with the competition for adsorption sites in the presence of the high percentage of aromatic compounds present in the diesel, the MCPs still adsorb significant amounts of DBT and DMDBT from the fuel; this strongly contrasts with activated carbon, which is not very selective.51 For the adsorption of organosulfur compounds, this is a clear example of MCPs superiority over their zeolite and activated carbon counterparts and also demonstrates that pore size and shape, as well as electronics of the MCP, which are dictated by the chosen organic linker and metal, can drastically change the adsorption properties of the material to allow them to discriminate among similar molecules. Additionally, regeneration of the packed beds was easily accomplished under mild conditions. This study demonstrates that MCPs can excel in an industrial application where traditional sorbents have struggled to perform adequately. Diesel represents the most complex solvent matrix studied to date and the high selectivity and adsorption capacities of the MCPs even in the presence of many other components indicates the potential these materials have to target the separation of one compound when among many.
The separation of aromatic hydrocarbons from aliphatic solvents was achieved using an MCP comprised of zinc ions linked by (R,R)-(−)-N,N′-bis(3-tert-butyl-5-(4-ethynylpyridyl)salicylidene)-1,2-diaminocyclohexane.52 Evacuated crystals were suspended in mixtures of benzene–cyclohexane, toluene–cyclohexane, and toluene–n-heptane and analysis of the crystals after adsorption showed preferential uptake of the aromatic hydrocarbon in a molar ratio of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 98
3, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 94
2, and 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively. The MCP was also recycled and reused without loss of selectivity. Because benzene and cyclohexane have very similar boiling points and sizes, this separation has been difficult to achieve in industry, but this example shows that MCPs can be designed that can efficiently effect the separation of these very similar molecules.
6, respectively. The MCP was also recycled and reused without loss of selectivity. Because benzene and cyclohexane have very similar boiling points and sizes, this separation has been difficult to achieve in industry, but this example shows that MCPs can be designed that can efficiently effect the separation of these very similar molecules.
HKUST-1, specifically Basolite C300 produced by BASF, and MOF-5 were also utilized as stationary phases for liquid chromatographic separations.53 The size and shape selectivities of HKUST-1 were tested and it was found that in a mixture of benzene, naphthalene, and anthracene, the larger the aromatic compound, the longer the retention time due to greater interactions with the framework. Additionally, a mixture of benzene, naphthalene, and 1,3,5-triphenylbenzene was passed through the column and 1,3,5-triphenylbenzene was unretained due to size exclusion (Fig. 8). Again using HKUST-1, separation of ethyl benzene and styrene was very efficiently achieved. Unlike the previous case, here, separation is attributed to interactions between styrene and the copper of the framework by π-complexation, a conclusion further supported by attempting to use MOF-5 for the same separation. In this case, styrene and ethyl benzene co-elute due to the absence of coordinatively unsaturated metal sites in MOF-5. This study illustrates that MCPs have potential for use as HPLC stationary phases and are superior to polymers used for GPC because of their ability to separate molecules in a smaller, well-defined size regime. Furthermore, separations based on chemical interactions with the MCP may be useful for the separation of similarly sized molecules.
 | ||
| Fig. 8 Relative sizes of benzene, naphthalene, 1,3,5-triphenylbenzene, and HKUST-1 (top, common size scale) and the separation of benzene, naphthalene, and 1,3,5-triphenylbenzene in hexanes achieved using HKUST-1 (bottom).53 Adapted with permission from the American Chemical Society. Copyright 2009. | ||
Conclusions
MCPs have enormous potential for the adsorption of compounds from the liquid phase and can open a gateway to a completely new era in separation/purification sciences. In particular, their superior sorption behavior compared to classical sorbents has the potential to resolve several long standing problems in the energy-intensive field of separations. A large number of MCPs have been reported and characterized and their use for gas uptake has been extensively evaluated.54,55 The potential of comparatively few MCPs has been explored for uptake from the liquid phase in spite of the fact that data collected to date prove that these compounds have the potential to be selective sorbents and are kinetically viable for the adsorption of compounds from solution.The National Science Council Committee on Separation Science and Technology56 has identified six focus points for further sorption research: (1) Generating improved selectivity among solutes; (2) Concentrating solutes from dilute solutions; (3) Understanding and controlling interfacial phenomenon; (4) Increasing capacity and speed of separation systems; (5) Developing improved process configurations for separation equipment and; (6) Improving energy efficiency in separation. MCPs have the potential to address all of these points and, in some cases, have already exceeded the state of the art of other sorbent classes. It is clear that MCPs act as exquisitely size and shape selective adsorbents and effectively capture molecules from dilute solutions. The ability to change both the metal and the organic linker, with a knowledge of structure derived from crystallography, offers a path towards rationally tailoring interfacial properties. In many cases, MCPs have been shown to outperform benchmark materials such as zeolites and activated carbons by exhibiting higher capacities or more favorable kinetics in certain applications.
Although MCPs have been shown to outperform other microporous materials in liquid phase adsorption applications, it is important to note that issues such as cost and stability of materials have not been taken into account. To date, the cost to make many of the MCPs reported in this review prohibits large-scale production of the material. Further research on inexpensive, easily scalable MCPs that exhibit the stability of zeolites and activated carbons to liquid phase conditions is of the utmost importance. Additionally, separations are highly dependent on the loading of the adsorbents and very few reported examples address this issue. Further elucidation of the effect of loading on MCP performance is also necessary before MCPs can make a mark on important industrial applications.
To date, two main areas have been the focus of liquid phase separations by MCPs: separation of enantiomers and the separation of mixtures of chemically different compounds. Within these two areas, two main mechanisms for separation have been prominently observed: size/shape selective separations and chemical separations. In some cases, both mechanisms work together to afford efficient separation of the desired molecules. Going forward with this knowledge, one can imagine the possibility for design of an MCP for a specific separation that would take advantage of either the potential for chemical interactions between the framework and the molecules to be separated or the potential to design a structure with a specific pore size necessary to afford the separation of molecules that themselves are very close in size. With new MCP designs, chiral separations with enantiomeric excesses exceeding those that have previously been observed are possible. With further research and effort, MCPs are poised to become leaders in the liquid phase separation of compounds for applications that are very relevant to industry worldwide. Further study is necessary both in the engineering of optimal process configurations and the design of new materials with higher capacities and affinities specifically for industrial applications such that the potential for energy efficiency can be fully realized.
Notes and references
- S. Deng, Sorbent Technology, in Encyclopedia of Chemical Processing, ed. S. Lee, Taylor & Francis Group, New York, 2006, vol. 5 Search PubMed.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2009, 131, 4184–4185 CrossRef CAS.
- A. U. Czaja, N. Trukhan and U. Müller, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626–636 RSC.
- D. Dubbeldam, C. J. Galvin, K. S. Walton, D. E. Ellis and R. Q. Snurr, J. Am. Chem. Soc., 2008, 130, 10884–10885 CrossRef CAS.
- X. Y. Bao, L. J. Broadbelt and R. Q. Snurr, Mol. Simul., 2009, 35, 50–59 CrossRef CAS.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS.
- R. Xiong, X. You, B. F. Abrahams, Z. Xue and C. M. Che, Angew. Chem., Int. Ed., 2001, 40, 4422–4425 CrossRef CAS.
- O. R. Evans, H. L. Ngo and W. Lin, J. Am. Chem. Soc., 2001, 123, 10395–10396 CrossRef CAS.
- M. E. Kosal, J. H. Chou, S. R. Wilson and K. S. Suslick, Nat. Mater., 2002, 1, 118–121 CrossRef CAS.
- K. S. Suslick, P. Bhyrappa, J. H. Chou, M. E. Kosal, S. Nakagaki, D. W. Smithenry and S. R. Wilson, Acc. Chem. Res., 2005, 38, 283–291 CrossRef CAS.
- F. Stallmach, S. Gröger, V. Künzel, J. Kärger, O. M. Yaghi, M. Hesse and U. Müller, Angew. Chem., Int. Ed., 2006, 45, 2123–2126 CrossRef CAS.
- M. P. Suh, J. W. Ko and H. J. Choi, J. Am. Chem. Soc., 2002, 124, 10976–10977 CrossRef CAS.
- H. J. Choi and M. P. Suh, J. Am. Chem. Soc., 2004, 126, 15844–15851 CrossRef CAS.
- J. W. Ko, K. S. Min and M. P. Suh, Inorg. Chem., 2002, 41, 2151–2157 CrossRef CAS.
- E. Y. Lee and M. P. Suh, Angew. Chem., Int. Ed., 2004, 43, 2798–2801 CrossRef CAS.
- For analogous pore decoration with different metals applied to gas selectivity see: S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 Search PubMed.
- H. K. Chae, D. Y. Siberio-Pérez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523–527 CrossRef CAS.
- D. Bradshaw, T. J. Prior, E. J. Cussen, J. B. Claridge and M. J. Rosseinsky, J. Am. Chem. Soc., 2004, 126, 6106–6114 CrossRef CAS.
- E. Y. Lee, S. Y. Jang and M. P. Suh, J. Am. Chem. Soc., 2005, 127, 6374–6381 CrossRef CAS.
- D. N. Dybtsev, A. L. Nuzhdin, H. Chun, K. P. Bryliakov, E. P. Talsi, V. P. Fedin and K. Kim, Angew. Chem., Int. Ed., 2006, 45, 916–920 CrossRef CAS.
- Y. M. Song, T. Zhou, X. S. Wang, X. N. Li and R. G. Xiong, Cryst. Growth Des., 2006, 6, 14–17 CrossRef.
- K. L. Wong, G. L. Law, Y. Y. Yang and W. T. Wong, Adv. Mater., 2006, 18, 1051–1054 CrossRef CAS.
- Y. Bai, G. J. He, Y. G. Zhao, C. Y. Duan, D. B. Dang and Q. J. Meng, Chem. Commun., 2006, 1530–1532 RSC.
- G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour and I. Margiolaki, Angew. Chem., Int. Ed., 2004, 43, 6296–6301 CrossRef CAS.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef CAS.
- P. Horcajada, C. Serre, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, Angew. Chem., Int. Ed., 2006, 45, 5974–5978 CrossRef CAS.
- P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS.
- C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS.
- T. R. Whitfield, X. Q. Wang, L. M. Liu and A. J. Jacobson, Solid State Sci., 2005, 7, 1096–1103 CrossRef CAS.
- R. Vaidhyanathan, D. Bradshaw, J. N. Rebilly, J. P. Barrio, J. A. Gould, N. G. Berry and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2006, 45, 6495–6499 CrossRef CAS.
- S. H. Jhung, J. H. Lee, J. W. Yoon, C. Serre, G. Férey and J. S. Chang, Adv. Mater., 2007, 19, 121–124 CrossRef CAS.
- S. Hasegawa, S. Horike, R. Matsuda, S. Furukawa, K. Mochizuki, Y. Kinoshita and S. Kitagawa, J. Am. Chem. Soc., 2007, 129, 2607–2614 CrossRef CAS.
- A. L. Nuzhdin, D. N. Dybtsev, K. P. Bryliakov, E. P. Talsi and V. P. Fedin, J. Am. Chem. Soc., 2007, 129, 12958–12959 CrossRef CAS.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS.
- L. Alaerts, F. Thibault-Starzyk, E. Séguin, J. F. M. Denayer, P. A. Jacobs and D. E. De Vos, Stud. Surf. Sci. Catal., 2007, 170, 1996–2003.
- K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281–284 CrossRef CAS.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem.–Eur. J., 2004, 10, 1373–1382 CrossRef CAS.
- L. Alaerts, C. E. A. Kirschhock, M. Maes, M. A. van der Veen, V. Finsy, A. Depla, J. A. Martens, G. V. Baron, P. A. Jacobs, J. E. M. Denayer and D. E. De Vos, Angew. Chem., Int. Ed., 2007, 46, 4293–4297 CrossRef CAS.
- L. Alaerts, M. Maes, P. A. Jacobs, J. F. M. Denayer and D. E. De Vos, Phys. Chem. Chem. Phys., 2008, 10, 2979–2985 RSC.
- L. Alaerts, M. Maes, L. Giebeler, P. A. Jacobs, J. A. Martens, J. F. M. Denayer, C. E. A. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2008, 130, 14170–14178 CrossRef CAS.
- G. Li, W. B. Yu, J. Ni, T. F. Liu, Y. Liu, E. H. Sheng and Y. Cui, Angew. Chem., Int. Ed., 2008, 47, 1245–1249 CrossRef CAS.
- B. Chen, L. Wang, F. Zapata, G. Qian and E. B. Lobkovsky, J. Am. Chem. Soc., 2008, 130, 6718–6719 CrossRef CAS.
- N. L. Rosi, J. Kim, M. Eddaoudi, B. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS.
- B. Chen, Y. Ji, M. Xue, F. R. Fronczek, E. J. Hurtado, J. U. Mondal, C. Liang and S. Dai, Inorg. Chem., 2008, 47, 5543–5545 CrossRef CAS.
- L. G. Qiu, Z. Q. Li, Y. Wu, W. Wang, T. Xu and X. Jiang, Chem. Commun., 2008, 3642–3644 RSC.
- A. G. Wong-Foy, O. Lebel and A. J. Matzger, J. Am. Chem. Soc., 2007, 129, 15740–15741 CrossRef CAS.
- B. Chen, N. W. Ockwig, A. R. Millward, D. S. Contreras and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4745–4749 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- K. A. Cychosz, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 6938–6939 CrossRef CAS.
- K. A. Cychosz, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2009, 131, 14538–14543 CrossRef CAS.
- G. Li, C. F. Zhu, X. B. Xi and Y. Cui, Chem. Commun., 2009, 2118–2120 RSC.
- R. Ahmad, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2009, 25, 11977–11979 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS.
- D. Zhao, D. Yuan and H. Zhou, Energy Environ. Sci., 2008, 1, 222–235 RSC.
- Separation and Purification: Critical Needs and Opportunities; National Research Council; National Academy Press, Washington, DC, 1987 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |